Abstract
It is generally assumed that proprioceptive feedback plays a crucial role in limb posture and movement. However, the role of afferent signals from extraocular muscles (EOM) in the control of eye movement has been a matter of continuous debate. These muscles have atypical sensory receptors in several species and it has been proposed that they are not supported by stretch reXexes. We recorded electromyographic activity of EOM during passive rotations of the eye in sedated rats and squirrel monkeys and observed typical stretch reXexes in these muscles. Results suggest that there is a similarity in the reXexive control of limb and eye movement, despite substantial differences in their biomechanics and sensory receptors. Like in some limb skeletal muscles, the stretch reflex in EOM in the investigated species might be mediated by other length-sensitive receptors, rather than muscle spindles.
Keywords: Motor control, Sensorimotor integration, Eye movement, Proprioception, Electromyogram
Introduction
Sensory signals from muscle spindles are often assumed to play a major role in the stretch reflex (SR) and in the regulation of muscle activity (Matthews 1981). However, these receptors are either absent, rare, or atypical in some muscles. For example, few muscle spindles were found in jaw-openers (Lennartsson 1979). Nevertheless, these muscles do have functional phasic and tonic SRs (Ostry et al. 1997), implying that SRs might be mediated by non-spindle receptors.
Similarly, spindles are found inconsistently in extraocular muscles (EOM) across species (Maier et al. 1974). They are absent in rat EOM, sparse, absent or diminutive in EOM of non-human primates (Maier et al. 1974) and present in EOM of humans (Merrillees et al. 1950). Although afferent signals evoked by EOM stretching are transmitted to the central nervous system (Cooper et al. 1953; Fillenz 1955; Donaldson and Long 1980; Donaldson 2000), some studies have failed to demonstrate an effect of these signals on EOM motoneurons (McCouch and Adler 1932; Keller and Robinson 1971; Carpenter 1988). This has led to the conclusion that EOM have no SRs (Keller and Robinson 1971). On the other hand, electromygraphic (EMG) studies in humans have revealed SR responses in EOM (Breinin 1957; Sears et al. 1959; Matsuo 2002). To our knowledge, direct EMG recording of EOM responses to muscle stretch in animals has not been examined, particularly in species with very few, atypical, or no spindles. Here we report investigation of EMG responses to stretch in New World monkeys and rats.
Methods
Experimentally naive male adult squirrel monkeys (Saimiri spp., n = 2) and Sprague–Dawley rats (n = 2) were used in the present experiment. All animal use was in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center. All animals were healthy adult subjects.
Squirrel monkeys
Any surgical procedures (e.g., cutting nerves or EOM tendons) that could permanently alter the oculomotor system were excluded. The animals fully recovered and behaved as usual a day after the experiment.
The monkeys were first lightly anesthetized with ketamine hydrochloride (20 mg/kg, i.m.) as a pre-anesthetic agent. Following tracheal intubation, halothane-nitrous oxide anesthesia was used (1–2% halothane; 75% nitrous oxide) as a general anesthetic and vital signs were monitored throughout the experiment. Fluids were delivered through the saphenous vein (for details see Nudo et al. 1992, 2003).
Halothane anesthesia was begun immediately after intubation and maintained during the entire duration of surgical procedures involved in the electrode positioning. The animals were placed in the stereotaxic frame and the orbicularis oculi muscle was retracted so that the anterior eye surface was exposed allowing direct observation of the eyes. Two wires were used to record bipolar EMG activity (Fig. 1) from the lateral rectus muscle in monkey 1 and 2. In monkey 2, after testing SR responses in this muscle, a second pair of wires was used to simultaneously record EMG activity from the antagonist muscle (medial rectus). At each recording site, one EMG electrode was a soft, Teflon insulated, multi-stranded wire attached at the frontal insertion of the selected muscle and sutured to its fascia. The other, single-wired electrode (100 μm diameter) was gently guided along the wall of the orbit and inserted into the belly of the muscle. The rigid wire was used because it allowed us to pierce the conjunctiva and also provided resistance when it reached the posterior wall of the orbit (where the belly of the EOM is located). A nylon suture on the conjunctiva at the cantus was used to stabilize the electrode's position. This was done in order to minimize mechanical or/and electrical artifacts that could result from passive rotations of the eye.
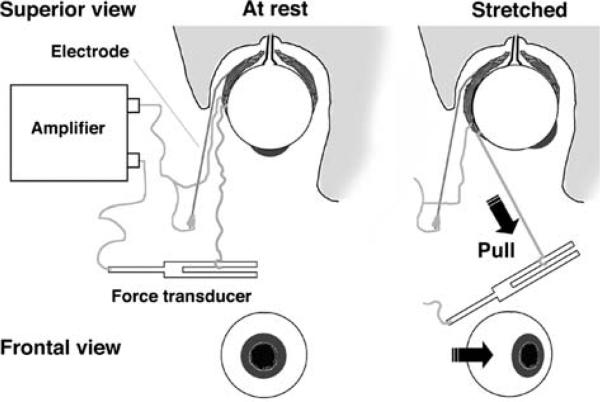
Fig. 1.
Experimental setup: a soft, multi-stranded wire electrode was positioned at the frontal attachment of the lateral rectus muscle and sutured to its fascia. Another wire (100 μm diameter, 1 mm tip without insulation, beveled at ~45°) was gently guided along the wall of the orbit and inserted into the belly of the medial rectus muscle. A polypropylene wire (7.0; 0.5 metric; Prolene, Ethicon) was tied between the frontal muscle attachment and a force transducer. The transducer was pulled manually to rotate the eyeball back and forth in the medio-lateral direction, thus producing cycles of muscle lengthening and shortening, while the force and EMG activity were recorded
Following the surgical procedures, the halothane was replaced with both ketamine hydrochloride (20 mg/cc diluted in saline and delivered at a rate of approximately 15 mg/kg/h i.v.) and diazepam (0.1 mg/cc diluted in saline; approximately 0.01 mg/kg/h i.v.). The rate of drug infusion was carefully adjusted to obtain stable spontaneous EMG signals, as these signals were attenuated at deeper sedated states of the animal. EMG signals were amplified (A-M systems), filtered (50–750 Hz), and digitized (1.5 kHz) using an Instru-Net Network system (GW Instruments). The background noise was removed from the EMG signals. Signals were then rectified and the linear envelope (cut-off frequency 40 Hz, Wiener optimal filter) was obtained to modulate the original EMG signal while preserving the high frequency structure unchanged.
To produce passive rotations of the eyeball, a polypropylene suture (7.0; 0.5 metric; Prolene, Ethicon) was tied between the frontal attachment of lateral rectus muscle and a force transducer. While the force and EMG activity were recorded, the transducer was pulled manually to rotate the eyeball back and forth in the medio-lateral direction, thus producing cycles of muscle lengthening and shortening with pauses of about 1–2 s between stretches. Before the onset of each cycle, the tension on the thread was released so that the eye was in a neutral position and the force recorded with the transducer was zero. After the end of pulling, the transducer was moved back until the tension was released again so that the eyeball returned to about the same neutral position passively due to the elasticity of stretched muscles and the connective tissues of the eye. The eye remained in a neutral position 0.5–2 s before the next stretch-release cycle. The amplitude of eye rotations in sequential cycles remained relatively constant (about 25–30°).
Sprague-Dawley rats
In two rats, no gas anesthesia was used. Instead, a combination of ketamine (100 mg/ml) and xylazine (20 mg/ml) was administered (i.p.) as needed to maintain a stable anesthetic state. Similar techniques as described for squirrel monkeys were used for EMG and force recording.
Results
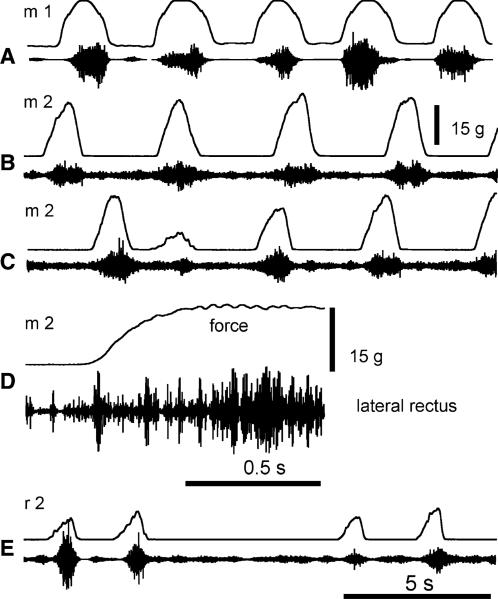
During the experiment, no artificial electrical signals (e.g., following small motion of EMG electrodes) that could be mistakenly interpreted as EMG signals were evoked in response to eye rotation. Beyond a certain level of anesthesia, neither were EMG responses evoked. The force resulting from passive resistance of the eye to this rotation did not exceed several grams, which was much below the force resulting from active muscle resistance when regular SR responses were observed (see below). In monkeys (Fig. 2a, b), SR responses could occur at any time during the 1 h experiment provided that level of anesthesia was mild enough to allow occasional spontaneous EMG activity in the EOM. Once this state was achieved, the anesthesia could be maintained for a long period of time at a level when nearly no spontaneous or light-evoked eye movements occurred but SR responses of the muscles to passive eye rotations occurred regularly (Fig. 2a, b). In monkeys, EMG SR responses appeared when overall eye resistance to passive rotation exceeded several grams, and this resistance increased up to about 30 g with further rotation. EMG responses dropped comparatively sharply when muscle shortening began, implying that EMG responses were also velocity-dependent (phasic SR). Figure 2c shows that SR response amplitude varied with the stretch extent. In the first cycle of eye rotation, the experimenter produced a standard-amplitude stretch, a smaller stretch in the second cycle and returned to the standard-amplitude stretches in two subsequent cycles, resulting in graded SR responses in terms of EMG signals and forces. When stretches were prolonged (Fig. 2d), the increased EMG activity and tension persisted during the plateau of the stretch, implying that the SR responses had a tonic component. SR responses of the lateral rectus could also be observed in rats (Fig. 2e).
Fig. 2.
Stretch-evoked EMG activity of extraocular muscles Examples of stretch-evoked activity in 2 monkeys, m1 and m2 a–d and a rat, r2 e. The upper trace indicates the force applied to produce eye rotation (the upward deviation in the force trace resembles stretching of the lateral rectus muscle). The lower trace shows stretch-evoked EMG signals from this muscle. In a, d, the experimenter produced rhythmical eye rotations of about the same amplitude. In c, in the first cycle of eye rotation, the experimenter produced a regular-amplitude stretch, a smaller stretch in the second cycle and returned to regular-amplitude stretches in two subsequent cycles, resulting in respective modifications of SR responses in terms of EMG signals and forces. In d, the stretch was not reversed during about half a second, resulting in a prolongation of the stretch-related activity. e Rat stretch-evoked EMG responses were comparable to what was found in monkeys. Stretch reflex could be evoked repetitively and the amplitude of the response modulated with the intensity of the external force applied to rotate the eye
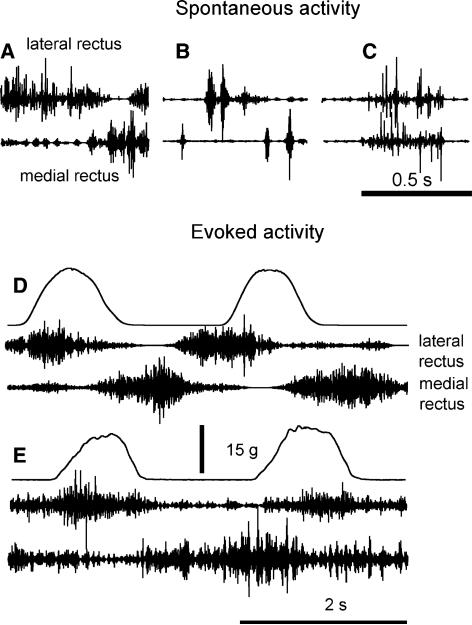
In monkey 2, after testing SR in the lateral rectus, we inserted EMG electrodes into the medial rectus in order to see how EMG activity of the two antagonist muscles was coordinated during spontaneous or stretch-evoked activity. The two muscles could spontaneously be activated in isolation or combination. For example (Fig. 3a), spontaneous EMG activity in the lateral rectus was suppressed when a burst of EMG activity occurred in the antagonist, medial rectus muscle (a reciprocal pattern). The muscles could spontaneously generate alternating bursts of EMG activity (another reciprocal pattern; Fig. 3b). Although reciprocal patterns of spontaneous activity were most common, the two muscles could occasionally be activated simultaneously (co-activation; Fig. 3c). Although the muscles were co-active, the spikes in the EMG bursts of these muscles were not synchronized, implying that there was no “cross talk” between EMG signals from the two muscles. Such observations taken together with those of reciprocal activation of the two muscles show that each pair of our EMG electrodes was sufficiently selective to avoid picking up signals from muscles on the opposite site of the eyeball.
Fig. 3.
Activation pattern of two antagonist EOM. a, b Examples of spontaneous reciprocal activity in the lateral and medial rectus. c Example of spontaneous co-activation of the lateral and medial rectus. d, e Examples of stretch-evoked EMG activity in the lateral and medial rectus muscles in the first and second cycles of passive eye rotations, in monkey 2
Figure 3d and e show EMGs of the two antagonist muscles in monkey 2, when passive eye rotations were made. An important consideration in our experimental setup is that eye rotations resulting in stretching of the lateral rectus were initiated by pulling the thread via the force transducer (see Fig. 1). When the pulling ceased, the viscous-elastic forces of the oculomotor plant resulted in comparatively slow return of the eye to the initial position. This movement was not reflected in the force recording since the thread between the eye and force transducer was slackened when the pulling stopped. Thus the duration of the eye movement reversal (when the medial rectus was lengthened) outlasted the duration of the force decrease in each cycle. This can explain why the EMG bursts in the medial rectus resulting from lengthening at the reversal phase of eye rotation continued some time after the force signal returned to zero. In Fig. 3d, SR responses of two muscles were observed in the first cycle. The eye rotation also evoked a reciprocal pattern of activation in the two muscles. Thereby, the second EMG burst in the lateral rectus became desynchronized with the stretch phase in the second cycle. However, the onset of EMG bursts in the medial rectus remained related to the onset of stretch phase for this muscle in both cycles. A similar mixed SR and reciprocal response is shown in Fig. 3e, except that the two EMG bursts in the lateral rectus remained SR-related in both cycles, like in most cases when the EMG activity of only this muscle was recorded in the same monkey (Fig. 2b). One can also note that two of several bursts in the EMG activity in the medial rectus (Fig. 3e) were generated during the stretch phases for this muscle, in both cycles. Taken together, these examples imply that, like in the skeletal neuromuscular system, EOM SR responses can be modified in terms of threshold and magnitude.
Discussion
In the present study, through direct recording of EMG activity from EOM in sedated animals, we found regular responses of these muscles to stretch, resembling those described in skeletal muscles.
It is known that stretching eye muscles evokes a sensory stimulus that could produce a synchronized response in neck or jaw muscles (Donaldson 2000; Ruskell 1999). It could be suggested that our electrodes were not sufficiently selective and picked up EMG signals from non-extraocular muscles—a “cross talk” phenomenon. This phenomenon is usually avoided by using bipolar EMG recording with appropriately selected inter-electrode distance. If present, cross talk can be recognized by the occurrence of synchronized spikes in EMG signals of two muscles and by the absence of occurrences when one muscle is silent while the other is active. If our EMG electrodes were insufficiently selective and picked up “non-specific” activity of surrounding face, jaw or neck muscles, then cross talk would have been observed between the EMG signals recorded from the two closest, lateral and medial extraocular muscles. In our experiments, both spontaneous and stretch-evoked EMG signals from these two muscles could occur separately, reciprocally or simultaneously without any sign of synchronized spikes (see Fig. 3). Thus, our data show that our bipolar EMG recordings were sufficiently selective to exclude picking up responses of non-extraocular muscles. In addition, reflex reactions of non-extraocular muscles to eye rotations are reported to occur when the latter are combined with some other stimulation such as passive rotation of the head (Donaldson 2000), which was not the case in our experiments—our animals were anesthetized and immobilized in a stereotaxic frame. Therefore, it is also doubtful that reflex responses of neck or jaw muscles to eye rotations occurred in our animals.
It is equally unlikely that the signals we recorded were high-frequency electrical artifacts resulting from shifts of the electrodes within and on the surface of the muscle following the eye rotation. If present, such artifacts could be seen at any level of anesthesia of the animal, which was not the case in the present study. As previously stated, the EMG signal was clearly absent at deeper anesthetic states and no modulation of the amplitude of the background noise signal could be seen during eye rotation. In addition, movement artifacts in EMG recordings would have had low-frequency content that would have been removed by filtering.
Because the present study was conducted in sedated animals, we cannot rule out the possibility that the SR is suppressed in awake animals. If so, this may reconcile apparently opposite findings of polysynaptic responses to EOM stretch in the oculomotor nucleus of sedated cats (Tomlinson and Schwarz 1977) and the absence of stretch-related responses in cells of abducens nucleus in awake macaques (Keller and Robinson 1971). Alternatively, because motoneurons were not identiWed as such in the latter study, the cells being recorded may not have been associated with the muscle in which the SR was tested. In this regard, according to our observations, SR could not be evoked unless the anesthesia was sufficiently relaxed. This finding suggests that the reflex would be present, although centrally modulated in terms of threshold and amplitude (see below) in the awake animal. To clarify this issue, testing the presence of SRs in awake animals is necessary. Nevertheless, our results do document the presence of SR pathways in the EOM system, i.e., pathways that have not, until now, been revealed in these species.
It can also be suggested that the SR nature of the observed EMG patterns could be confirmed if the stretch-related responses were abolished by EOM deafferentation. Although interesting, such an experiment faces theoretical and technical challenges. Indeed, the pathway of EOM afferents has been a matter of extensive debate (for review see Donaldson 2000). When these afferent signals leave the EOM through the oculomotor nerves, they become intermixed with motor fibers (Cooper et al. 1955). It is not yet clear if all afferent fibers then transfer to the ophthalmic branch of the trigeminal nerve (Spencer and Porter 1988; Porter and Donaldson 1991; Ruskell 1999). Thus, it is debatable whether or not the dissection of the ophthalmic branch of the trigeminal ganglion, an invasive surgery targeting deep structures, would abolish the reflex.
Sensory receptors involved in the SR
A critical question emerging from our findings is determining which receptors support the SR. Rat EOM do not have spindles (Maier et al. 1974) and preliminary data from our group suggest that EOM of squirrel monkeys also lack typical spindles (Taylor et al. 2005). It is possible that EOM possess specialized length receptors specifically adapted to their unique environment, relative to limb skeletal muscles. The structure and organization of other receptors, the palisades, found in EOM have been compared to spindles and it appears that palisades might be well positioned to signal eye rotation (Donaldson 2000). However, to date, the physiological role of these receptors remains obscure. Our finding of SR responses of EOM that have few, if any, spindles but which do have palisades (Ruskell 1978, 1999; Eberhorn et al. 2005) strengthens the possibility that palisades play a role in transmitting signals related to muscle length, possibly polysynaptically, to EOM motoneurons.
Possible role of the SR in the control of eye movements
The importance of proprioceptive feedback for limb posture and movement has been regularly emphasized since Sherrington (1906). In limb muscles, proprioceptive feedback may compensate for effects of changeable limb geometry and variable external forces. On the other hand, it has been argued that proprioceptive feedback is unnecessary for the EOM since these muscles are subjected to relatively constant inertial and gravitational loads (Ruskell 1999).
There, however, exists a common functional requirement for both limb and EOM systems that seemingly cannot be met in the absence of proprioceptive feedback. Like limb positions, eye positions can be stabilized for extended periods. A saccadic eye movement would evoke substantial muscle resistance to the rapid deviation from the previously stabilized position, unless the stabilizing mechanisms are reset toward the position to which the eye moves (Von Holst and Mittelstaedt 1950/1973). This posture-movement problem is solved in the limb motor system by central shifts in SR thresholds (Asatryan and Feldman 1965). In this way, the initial position appears to be deviated from the newly specified stable position, so that the system not only eliminates the resistance but uses the position-stabilizing mechanisms to support movement to a final position (Ostry and Feldman 2003).
In the limb muscle-reflex system, resetting of position-stabilizing mechanisms is manifested by shifts of the static force–length characteristic in spatial (muscle length or joint angle) coordinates. Such shifts have been shown to occur during voluntary arm movements in humans (Asatryan and Feldman 1965; Archambault et al. 2005). It has also been shown in decerebrated cats that different descending and reflex systems shift the static force–length characteristic of hind limb muscles by changing their SR thresholds (Matthews 1959; Feldman and Orlovsky 1972; Nichols and Steeves 1986; Capaday et al. 1995; Archambault et al. 2005). Strikingly similar, in terms of shape, force–length characteristics were recorded in EOM of intact humans (Collins et al. 1975). Moreover, these researchers also showed that the force–length characteristic was shifted along the length coordinate when the subject changed the gaze direction. Since EMG activity of EOM was not recorded by Collins and collaborators (1975), it remains unclear whether or not the repositioning of force–length characteristics resulted from shifts in the SR threshold. It seems unlikely that such repositioning of characteristics resulted from changing in the tonic EMG activity: in striated muscles (to which EOM belong), an increase in tonic stimulation results not only in repositioning but also in an increase of the slope (stiffness) of force–length characteristics (Rack and Westbury 1969), whereas, in the study by Collins et al. (1975) the slope of such characteristics remained the same regardless of the shift. Therefore, one can suggest that the repositioning of EOM force–length curves (and thus shifts in gaze) can also be accomplished by central resetting of SR thresholds and that movements in both limb and ocular motor systems could be controlled in a similar manner. Our experiments were not designed to test this suggestion for the oculomotor system but it would be interesting to address this issue in specifically designed experiments.
In conclusion, proprioceptive feedback likely plays a much more essential role in eye movements than has been suggested previously. Our data do support the role of EOM afferent signals in ocular motor control (Lewis et al. 1994). Control of eye movement could thus be achieved by central shifts in the SR thresholds, as suggested for the control of limb skeletal muscles (Asatryan and Feldman 1965). These results may also have clinical relevance, as acute development of post-traumatic strabismus may result from deWcits in regulation of SR threshold in the EOM, caused by altered or lost proprioceptive signals.
Acknowledgments
The authors wish to thank James Rengel, Abderraouf Belhaj-Saif and Paul D. Cheney for their help and expertise for data collection. Numa Dancause is supported by a fellowship from the Canadian Institutes of Health Research (CIHR). Randolph J. Nudo is supported by National Institutes of Health (NIH)-National Institute of Neurological Disorders and Stroke Grant NS30853, NIH-National Institute on Deafness and Other Communication Disorders Grant HD02528. Anatol G. Feldman is supported by CIHR and the Natural Sciences and Engineering Research Council of Canada (NSERC).
Contributor Information
Numa Dancause, Department of Neurology, University of Rochester Medical Center, 601 Elmwood Ave, Box 673, Rochester, NY 14642, USA Numa_Dancause@urmc.rochester.edu.
Michael D. Taylor, Landon Center on Aging, Kansas University Medical Center, Kansas City, KS 66160, USA
Erik J. Plautz, Department of Molecular and Integrative Physiology, Kansas University Medical Center, Kansas City, KS 66160, USA Landon Center on Aging, Kansas University Medical Center, Kansas City, KS 66160, USA.
Jeffery D. Radel, Occupational Therapy Education, Kansas University Medical Center, Kansas City, KS 66160, USA
Thomas Whittaker, Department of Neurology, Kansas University Medical Center, Kansas City, KS 66160, USA.
Randolph J. Nudo, Department of Molecular and Integrative Physiology, Kansas University Medical Center, Kansas City, KS 66160, USA Landon Center on Aging, Kansas University Medical Center, Kansas City, KS 66160, USA.
Anatol G. Feldman, Department of Physiology, University of Montreal, Montreal, QC H3S 2J4, Canada
References
- Archambault PS, Mihaltchev P, Levin MF, Feldman AG. Basic elements of arm postural control analyzed by unloading. Exp Brain Res. 2005;164:225–241. doi: 10.1007/s00221-005-2245-6. [DOI] [PubMed] [Google Scholar]
- Asatryan DG, Feldman AG. Functional tuning of nervous sytem with control of movement or maintenace of steady posture-I. Mechanographic analysis of the work of the joint on execution of a postural task. Biofizika. 1965;5:837–846. [PubMed] [Google Scholar]
- Breinin GM. Electromyographic evidence for ocular muscle proprioception in man. AMA Arch Ophthalmol. 1957;57:176–180. doi: 10.1001/archopht.1957.00930050184003. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Comeau F. Differential effects of a flexor nerve input on the human soleus H-reflex during standing versus walking. Can J Physiol Pharmacol. 1995;73:436–449. doi: 10.1139/y95-056. [DOI] [PubMed] [Google Scholar]
- Carpenter RHS. Movements of the eyes. Pion; London: 1988. [Google Scholar]
- Collins CC, O'Meara D, Scott AB. Muscle tension during unrestrained human eye movements. J Physiol. 1975;245:351–369. doi: 10.1113/jphysiol.1975.sp010850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Daniel PM, Whitteridge D. Nerve impulses in the brainstem of the goat; short latency responses obtained by stretching the extrinsic eye muscles and the jaw muscles. J Physiol. 1953;120:471–490. doi: 10.1113/jphysiol.1953.sp004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Daniel PM, Whitteridge D. Muscle spindles and other sensory endings in the extrinsic eye muscles; the physiology and anatomy of these receptors and of their connexions with the brain-stem. Brain. 1955;78:564–583. doi: 10.1093/brain/78.4.564. [DOI] [PubMed] [Google Scholar]
- Donaldson IM. The functions of the proprioceptors of the eye muscles. Philos Trans R Soc Lond B Biol Sci. 2000;355:1685–1754. doi: 10.1098/rstb.2000.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson IM, Long AC. Interactions between extraocular proprioceptive and visual signals in the superior colliculus of the cat. J Physiol. 1980;298:85–110. doi: 10.1113/jphysiol.1980.sp013069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhorn AC, Horn AK, Fischer P, Buttner-Ennever JA. Proprioception and palisade endings in extraocular eye muscles. Ann NY Acad Sci. 2005;1039:1–8. doi: 10.1196/annals.1325.001. [DOI] [PubMed] [Google Scholar]
- Feldman AG, Orlovsky GN. The influence of different descending systems on the tonic stretch reflex in the cat. Exp Neurol. 1972;37:481–494. doi: 10.1016/0014-4886(72)90091-x. [DOI] [PubMed] [Google Scholar]
- Fillenz M. Responses in the brainstem of the cat to stretch of extrinsic ocular muscles. J Physiol. 1955;128:182–199. doi: 10.1113/jphysiol.1955.sp005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller EL, Robinson DA. Absence of a stretch reflex in extraocular muscles of the monkey. J Neurophysiol. 1971;34:908–919. doi: 10.1152/jn.1971.34.5.908. [DOI] [PubMed] [Google Scholar]
- Lennartsson B. Muscle spindles in the human anterior digastric muscle. Acta Odontol Scand. 1979;37:329–333. doi: 10.3109/00016357909004704. [DOI] [PubMed] [Google Scholar]
- Lewis RF, Zee DS, Gaymard BM, Guthrie BL. Extraocular muscle proprioception functions in the control of ocular alignment and eye movement conjugacy. J Neurophysiol. 1994;72:1028–1031. doi: 10.1152/jn.1994.72.2.1028. [DOI] [PubMed] [Google Scholar]
- Maier A, DeSantis M, Eldred E. The occurrence of muscle spindles in extraocular muscles of various vertebrates. J Morphol. 1974;143:397–408. doi: 10.1002/jmor.1051430404. [DOI] [PubMed] [Google Scholar]
- Matsuo K. Stretching of the Mueller muscle results in involuntary contraction of the levator muscle. Ophthal Plast Reconstr Surg. 2002;18:5–10. doi: 10.1097/00002341-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. A study of certain factors influencing the stretch reflex of the decerebrate cat. J Physiol (Lond) 1959;147:547–564. doi: 10.1113/jphysiol.1959.sp006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Muscle spindles: their messages and their fusimotor supply. In: Brooks VB, editor. Handbook of physiology. Section 1. The nervous system, vol. II, motor control, part 2. American Physiological Society; Bethesda: 1981. pp. 189–228. [Google Scholar]
- McCouch GP, Adler FH. Extraocular reflexes. Am J Physiol. 1932;100:78–88. [Google Scholar]
- Merrillees NC, Sunderland S, Hayhow W. Neuromuscular spindles in the extraocular muscles in man. Anat Rec. 1950;108:23–30. doi: 10.1002/ar.1091080103. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Steeves JD. Resetting of resultant stiffness in ankle flexor and extensor muscles in the decerebrate cat. Exp Brain Res. 1986;62:401–410. doi: 10.1007/BF00238859. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992;12:2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Larson D, Plautz EJ, Friel KM, Barbay S, Frost SB. A squirrel monkey model of poststroke motor recovery. ILAR J. 2003;44:161–174. doi: 10.1093/ilar.44.2.161. [DOI] [PubMed] [Google Scholar]
- Ostry DJ, Feldman AG. A critical evaluation of the force control hypothesis in motor control. Exp Brain Res. 2003;153:275–288. doi: 10.1007/s00221-003-1624-0. [DOI] [PubMed] [Google Scholar]
- Ostry DJ, Gribble PL, Levin MF, Feldman AG. Phasic and tonic stretch reflexes in muscles with few muscle spindles: human jaw-opener muscles. Exp Brain Res. 1997;116:299–308. doi: 10.1007/pl00005757. [DOI] [PubMed] [Google Scholar]
- Porter JD, Donaldson IM. The anatomical substrate for cat extraocular muscle proprioception. Neuroscience. 1991;43:473–481. doi: 10.1016/0306-4522(91)90309-c. [DOI] [PubMed] [Google Scholar]
- Rack PM, Westbury DR. The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol. 1969;204:443–460. doi: 10.1113/jphysiol.1969.sp008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL. The fine structure of innervated myotendinous cylinders in extraocular muscles of rhesus monkeys. J Neurocytol. 1978;7:693–708. doi: 10.1007/BF01205145. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. Extraocular muscle proprioceptors and proprioception. Prog Retin Eye Res. 1999;18:269–291. doi: 10.1016/s1350-9462(98)00029-9. [DOI] [PubMed] [Google Scholar]
- Sears ML, Teasdall RD, Stone HH. Stretch effects in human extraocular muscle; an electromyographic study. Bull Johns Hopkins Hosp. 1959;104:174–178. [PubMed] [Google Scholar]
- Sherrington CS. On the proprioceptive system, especially in its reflex aspect. Brain. 1906;29:467–482. [Google Scholar]
- Spencer RF, Porter JD. Structural organization of the extraocular muscles. Rev Oculomot Res. 1988;2:33–79. [PubMed] [Google Scholar]
- Taylor MD, Dancause N, Feldman AG, Radel JD, Wright DE, Nudo RJ. Sensory receptors of the extraocular muscles in the squirrel monkey. Society for Neuroscience Abstracts. Prog # 744.17. 2005.
- Tomlinson RD, Schwarz DW. Response of oculomotor neurons to eye muscle stretch. Can J Physiol Pharmacol. 1977;55:568–573. doi: 10.1139/y77-079. [DOI] [PubMed] [Google Scholar]
- Von Holst E, Mittelstaedt H. Daz reafferezprincip. Wechselwirkungen zwischen Zentralnerven-system und Peripherie. Naturwissenschaften. 1950/1973;37:467–476. 1950. [Google Scholar]; Martin Rt., editor. The collected papers of Erich bon Holst. University of Miami Press; Coral Gables: The behavioral physiology of animals and man. pp. 139–173. The reafference principle. [Google Scholar]