Abstract
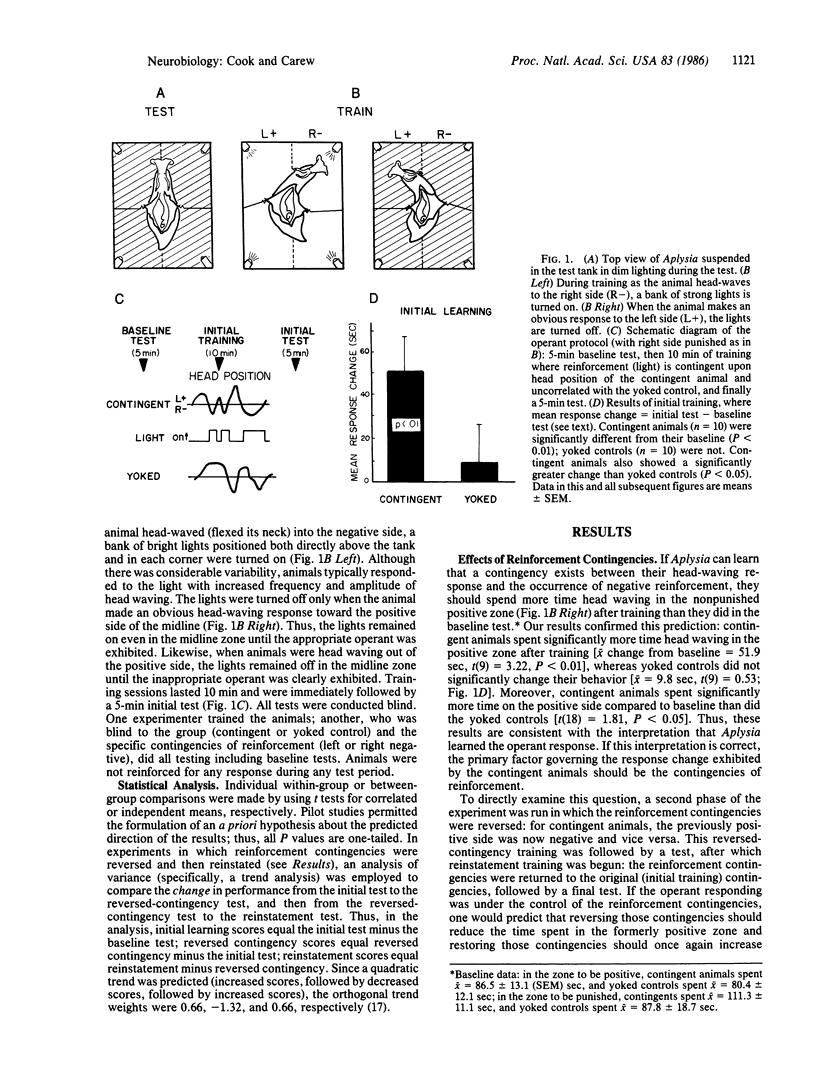
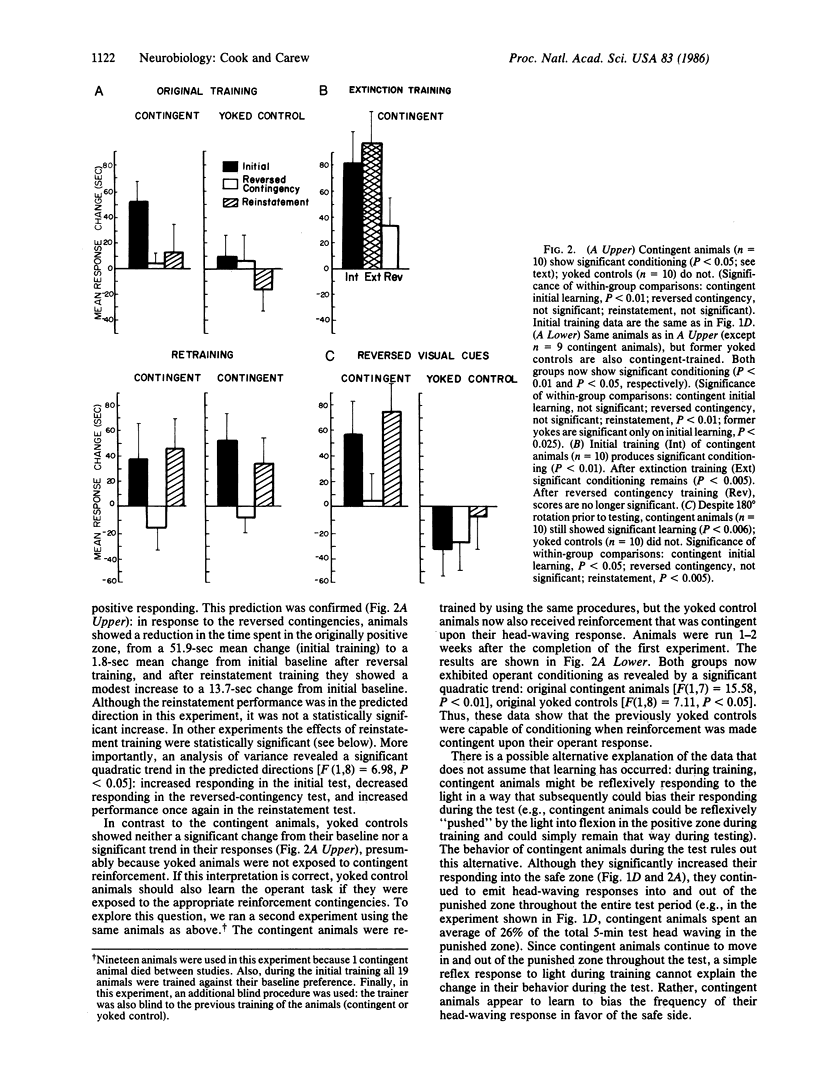
Head waving is a naturally occurring behavior that Aplysia use to explore their environment. Aplysia can be operantly trained to modify their head-waving response, increasing the amount of head waving on one side of their body in order to terminate the presentation of an aversive strong light. Acquisition of the operant response is rapid, within 10 min. Two observations indicate that the operant conditioning is under the control of the contingencies of reinforcement: (i) contingent reinforcement significantly elevates operant responding, reversing the contingencies significantly reduces operant performance, and reinstating the contingencies significantly reinstates operant responding; and (ii) yoked controls do not acquire the operant response, yet these same animals readily learn when reinforcement is made contingent upon their responses. Finally, internally derived cues (e.g., proprioceptive or reafferent) appear to play a predominant role in acquiring the operant response. Since progress has been made in understanding the cellular basis of classical conditioning in Aplysia, this demonstration of operant conditioning in a response system that is well-suited for a cellular analysis provides a preparation in which it is possible both to analyze the cellular mechanisms of operant conditioning and to address the theoretical question of the relationship between classical and operant conditioning on a mechanistic level.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L. Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science. 1984 Nov 30;226(4678):1037–1045. doi: 10.1126/science.6093258. [DOI] [PubMed] [Google Scholar]
- Alkon D. L. Learning in a marine snail. Sci Am. 1983 Jul;249(1):70-4, 76-8, 80-4. doi: 10.1038/scientificamerican0783-70. [DOI] [PubMed] [Google Scholar]
- Audesirk T. E., Alexander J. E., Jr, Audesirk G. J., Moyer C. M. Rapid, nonaversive conditioning in a freshwater gastropod. I. Effects of age and motivation. Behav Neural Biol. 1982 Dec;36(4):379–390. doi: 10.1016/s0163-1047(82)90782-8. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Hawkins R. D., Kandel E. R. Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science. 1983 Jan 28;219(4583):397–400. doi: 10.1126/science.6681571. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Walters E. T., Kandel E. R. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci. 1981 Dec;1(12):1426–1437. doi: 10.1523/JNEUROSCI.01-12-01426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. J., Gelperin A. Rapid taste-aversion learning by an isolated molluscan central nervous system. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6204–6206. doi: 10.1073/pnas.77.10.6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T. J., Alkon D. L. Retention of an associative behavioral change in Hermissenda. Science. 1978 Sep 29;201(4362):1239–1241. doi: 10.1126/science.694512. [DOI] [PubMed] [Google Scholar]
- Davis W. J., Gillette R. Neural correlate of behavioral plasticity in command neurons of Pleurobranchaea. Science. 1978 Feb 17;199(4330):801–804. doi: 10.1126/science.622572. [DOI] [PubMed] [Google Scholar]
- Forman R. R. Leg position learning by an insect. I. A heat avoidance learning paradigm. J Neurobiol. 1984 Mar;15(2):127–140. doi: 10.1002/neu.480150206. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Abrams T. W., Carew T. J., Kandel E. R. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983 Jan 28;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- Hening W. A., Walters E. T., Carew T. J., Kandel E. R. Motorneuronal control of locomotion in Aplysia. Brain Res. 1979 Dec 28;179(2):231–253. doi: 10.1016/0006-8993(79)90441-4. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Learning, using natural reinforcements, in insect preparations that permit cellular neuronal analysis. J Neurobiol. 1980 Jul;11(4):323–354. doi: 10.1002/neu.480110402. [DOI] [PubMed] [Google Scholar]
- Jahan-Parwar B., Fredman S. M. Control of pedal and parapodial movements in Aplysia. I. Proprioceptive and tactile reflexes. J Neurophysiol. 1978 May;41(3):600–608. doi: 10.1152/jn.1978.41.3.600. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Abrams T., Bernier L., Carew T. J., Hawkins R. D., Schwartz J. H. Classical conditioning and sensitization share aspects of the same molecular cascade in Aplysia. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):821–830. doi: 10.1101/sqb.1983.048.01.085. [DOI] [PubMed] [Google Scholar]
- Kupfermann I., Carew T. J. Behavior patterns of Aplysia californica in its natural environment. Behav Biol. 1974 Nov;12(3):317–337. doi: 10.1016/s0091-6773(74)91503-x. [DOI] [PubMed] [Google Scholar]
- London J. A., Gillette R. Functional roles and circuitry in an inhibitory pathway to feeding command neurones in Pleurobranchaea. J Exp Biol. 1984 Nov;113:423–446. doi: 10.1242/jeb.113.1.423. [DOI] [PubMed] [Google Scholar]
- Mpitsos G. J., Collins S. D., McClellan A. D. Learning: a model system for physiological studies. Science. 1978 Feb 3;199(4328):497–506. doi: 10.1126/science.622551. [DOI] [PubMed] [Google Scholar]
- Rescorla R. A., Solomon R. L. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol Rev. 1967 May;74(3):151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Schwarz M., Susswein A. J. A neural pathway for learning that food is inedible in Aplysia. Brain Res. 1984 Mar 5;294(2):363–366. doi: 10.1016/0006-8993(84)91051-5. [DOI] [PubMed] [Google Scholar]
- Susswein A. J., Schwarz M. A learned change of response to inedible food in Aplysia. Behav Neural Biol. 1983 Sep;39(1):1–6. doi: 10.1016/s0163-1047(83)90535-6. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science. 1983 Jan 28;219(4583):405–408. doi: 10.1126/science.6294834. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Slow depolarization produced by associative conditioning of Aplysia sensory neurons may enhance Ca2+ entry. Brain Res. 1983 Nov 28;280(1):165–168. doi: 10.1016/0006-8993(83)91186-1. [DOI] [PubMed] [Google Scholar]
- Woollacott M., Hoyle G. Neural events underlying learning in insects: changes in pacemaker. Proc R Soc Lond B Biol Sci. 1977 Jan 14;195(1120):395–415. doi: 10.1098/rspb.1977.0017. [DOI] [PubMed] [Google Scholar]



