Abstract
The time it takes new drugs to penetrate the market is shrinking. David Kao suggests how drug companies’ techniques could be used to improve safety surveillance systems
Institutions responsible for monitoring drug safety have been criticised widely after the withdrawal of drugs such as rofecoxib because of safety concerns. An estimated 20 million patients received prescriptions for rofecoxib over five years before the drug was withdrawn, and events attributable to rofecoxib may number in tens to hundreds of thousands.1 Regulatory bodies such as the US Food and Drug Administration have simultaneously been under pressure to reduce drug approval times to ensure timely availability of new drugs. However, concerns have been expressed that deadlines for approving drugs have reduced the focus on safety.2 3 4
New efficiencies in drug marketing exacerbate the problem because rapid adoption of new drugs can quickly expose large numbers of patients to unknown risks. Here, I review trends in drug approval times in the United States, the mechanism by which this has been achieved, and concerns raised by this approach. I then discuss an example of the speed with which a new product may be adopted once approved and suggest improvements to drug safety surveillance systems.
Trends in drug approval
Faced with staffing and budget limitations on drug approval, the US Congress passed the Prescription Drug User Fee Act in 1992. This authorised the FDA to collect fees from drug manufacturers and use the revenue to hire additional staff to review drugs and improve its administrative infrastructure. In return, the FDA established goals of reviewing 90% of priority new drug applications within six months and 90% of standard new drug applications within 12 months. As a result, the mean review time in the US decreased from 33.6 months during 1979-86 to 16.1 months during 1997-2002.5 The act has been renewed three times since 1992, and revenues collected from industry now account for 43% of the FDA budget for drug oversight.6 Similarly, the European Agency for the Evaluation of Medicinal Products receives 75% of its funding in this manner, and the United Kingdom’s Medicines and Healthcare Products Regulatory Agency is entirely funded by user fees.7 8 9
Critics have voiced concern over the dependence of regulatory agencies on drug companies for operational funding.2 10 Although drug approval times have decreased overall, the relation between this trend and subsequent postmarketing safety problems is less clear.7 Drug withdrawal rates have not increased overall since the US act, but a recent study showed that drugs approved in the US in the two months before the mandated deadline are more likely than those approved at other times to be withdrawn for safety reasons, to carry a subsequent black box warning, or to have at least one dosage voluntarily discontinued by the manufacturer. Because drugs approved more than three months before or just after the deadline were not associated with the same degree of subsequent safety problems, the authors concluded that the deadlines may increase the likelihood of unexpected safety problems.4
Deficits in postmarketing safety monitoring may, in turn, prevent these safety concerns from being discovered for years after approval. Depending on the success of the product, this can mean widespread exposure of patients to risk. Rapid acceptance of new products without concomitant improvements in postmarketing surveillance can only exacerbate this risk.
Sitagliptin launch
Merck’s novel treatment for hyperglycaemia, sitagliptin, provides a good example of the speed with which companies can now get new products to market. The FDA approved sitagliptin 3.8 years after the drug’s discovery; this compares with five years for rofecoxib and an industry average of 14.2 years between 1990 and 1999. Once the drug was approved Merck began a multifaceted marketing campaign that capitalised on sitagliptin being a new class of drug (oral dipeptyl peptidase-IV inhibitor). Within 48 hours, education forums were delivered, sales representatives had their first doctor contacts, and broadcasts were made by webcast and satellite to thousands of healthcare providers and investigators. The product website was functional within 90 minutes of approval, and within eight days, Merck had reached 70% of target doctors and made first deliveries of sitagliptin to pharmacies. Within 14 days, discussions were completed with managed care organisations covering around 188 million patients or 73% of the insured US population.
The company also formed a partnership with the American Diabetic Association to produce educational materials and patient guidebooks and to train diabetes educators with the goals of prompting undiagnosed patients to seek testing and to identify cases in which treatment goals are not being met.11 Since about a third of diabetic patients are undiagnosed and half of diagnosed patients are not meeting treatment goals, these strategies increase the potential market. Emphasis on the versatility of the drug as both single and add-on treatment also makes the drug marketable to nearly any patient with type 2 diabetes.
The success of this campaign was evident quickly. One month after FDA approval, one survey found that sitagliptin accounted for 14% of all new prescriptions given for type 2 diabetes drugs by primary care doctors. Among endocrinologists, 20% of new prescriptions for type 2 diabetes were for sitagliptin, compared with 22% for exenatide, 19% for rosiglitazone, and 16% for metformin.12 Merck estimates that by the end of 2007, three million prescriptions had been written, and sitagliptin had become the second most prescribed branded oral hypoglycaemic drug in the US. Merck reports a steady increase in sales of sitagliptin from $42m (£28m; €33m) in the final quarter of 2006 to $272m in the first quarter of 2008, putting sitagliptin on course for $1bn in annual sales in its second year of release.13
Marketing motivations and methods
The time required to bring a new product to market in the US has been decreasing for the past 20 years.14 For drug manufacturers, rapid deployment and adoption are necessitated by shifts in industry economics and public demand. Companies continually face the threat of loss of revenue because of expiring patents, and administrative and legal costs of withdrawn or suspended products can add millions of dollars to companies’ annual expenditures.11 15 16 Spending on drug research and development is aimed primarily at developing novel products to replace these losses, ideally with new classes of drugs.
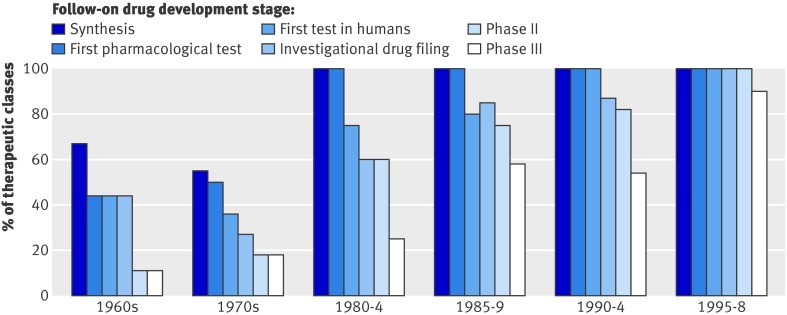
The importance of developing new products and the advantage of being the first in a class is reflected by the decrease in time between approval of new class drugs and follow-on drugs over the past 30 years. Most drug classes now have at least one competitor in advanced stages of development at the time that the first drug in the class is approved, and more follow-on drugs are in development at the time that the first in the class is approved than ever before (figure). Furthermore, the mean and median interval of marketing exclusivity have decreased from highs of 8.2 and 10.2 years respectively (n=9 drugs) during the 1960s to 1.8 and 1.2 years from 1995-8 (n=18 drugs, P<0.0001 and P=0.0005, respectively).5 Once FDA approval is received, companies must act quickly to establish dominance before a competitor becomes available.
Proportion of therapeutic classes with at least one follow-on drug in development at time the first in class was approved5
Recent years have seen major changes in drug marketing strategies facilitated by the evolution of mass media and prompted by changes in the relationships between manufacturers, doctors, and patients. Doctors are more aware of the effect of marketing on prescription practices, and concerns regarding conflicts of interest have led to changes in institutional policy in many settings, making traditional marketing to doctors more challenging.17 These changes have resulted in marketing budgets being moved to campaigns directed at funders and, where permitted, consumers.11 In countries such as the UK, where direct advertising to patients is prohibited, the public may still access internet resources designed for patients in other countries.
Public health initiatives regarding disease awareness and education have become important vehicles for increasing public demand for drugs, and engagement of healthcare funders is also a priority to ensure availability and coverage of costly new products for patients. The success of these strategies has been magnified by new mass media technologies, and companies are capitalising on their favourable cost-benefit ratio. This is exemplified by Merck’s discussion in their 2006 and 2007 annual business briefings of the efficiency of techniques such as video detailing and of their transition to multichannel marketing campaigns using web, video, and customisable, unbranded education resources to engage potential customers.11 These techniques enable drug companies to reach more potential customers faster than ever before. In most cases, this is not harmful and can benefit many patients in need of new treatment options. Nevertheless, improved infrastructure for postmarketing surveillance will be crucial to minimise risk to users of these new products.
Monitoring drug safety
Many groups have criticised the FDA for the time taken to publicise suspected risks associated with approved products like rofecoxib. As a result, the Federal Drug Administration Amendments Act of 2007 included a 50% increase in fees collected in association with the Prescription Drug Users Fee Act, with $29.2m designated specifically for surveillance of drug safety. FDA postmarket surveillance relies heavily on the Adverse Event Reporting System, which collects spontaneous reports from manufacturers, providers, and consumers that are reviewed by scientists at the Center for Drug Evaluation and Research. This database is publicly available and updated quarterly, although it is difficult to extract meaningful datasets and the data are of variable quality. The World Health Organization Collaborating Centre for International Drug Monitoring, also called the Uppsala Monitoring Centre, integrates reports collected by similar systems in 83 member countries into a single database called Vigibase, which in June 2007 contained about 3.87 million cases.18 Vigibase is not publicly available, although it is possible to purchase extracts of this database for about $2500 (€1600) per drug entity and reports of analyses are sent periodically to member pharmacovigilance centers.
Most adverse event reporting systems rely on voluntary reporting of clinical observations and therefore are sensitive to many biases. Lack of associated data on usage also makes it difficult to determine the true frequency of adverse events identified, and observers have called for development of an active postmarketing surveillance system wherein cohorts of patients are followed after starting a new treatment.19 Nations with centralised healthcare systems have some established infrastructure to support such systems, but most use a much smaller volume of drugs than the US. The initial draft of the FDA’s five year drug safety plan discusses acquiring data from organisations that have prescribing information such as drug sales databases and the Centers for Medicare and Medicaid Services to generate higher quality utilisation and longitudinal outcomes data, with the goal of building a database of 100 million patients by 2012.20 It has been estimated that with a dataset of this size, the increased cardiovascular risk associated with rofecoxib could have been detected independently after two to three months rather than as a result of ongoing clinical trials several years after approval.21 In addition, the FDA can now require drug companies to participate in postmarketing safety surveillance, greatly expanding potential resources for monitoring drug safety. Still, these improvements are expected to take several years, and the $29.3m budget allocated for developing drug safety monitoring represents only 1% of that spent on advertising and 0.1% of total annual drug sales in the US.19 20
Suggestions
Emerging marketing techniques use several methods that might also be used to engage a wider audience in monitoring drug safety. The 2007 FDA Amendments Act includes some examples such as the requirement that television advertisements must instruct patients experiencing negative side effects to report their symptoms to the FDA.22 Other techniques might also prove useful. For example, public health initiatives sponsored by drug companies have helped providers to raise disease awareness. Expanding this collaboration to include campaigns dedicated to drug safety surveillance could provide healthcare professionals with the marketing expertise and infrastructure to track utilisation patterns. Merck has described a shift towards a “value based partnership”’ marketing model that builds product loyalty by rewarding customers with individual benefits in the form of convenience and improved health outcomes.11 Maximising the direct benefit to individual patients and providers from participating in drug safety surveillance programmes might similarly increase voluntary involvement. Reporting a single adverse event provides little direct benefit to the reporting individual, but the development of tools for managing prescriptions, which in turn communicate with broad utilisation monitoring systems, provides patients and doctors with personal incentives to take an active role in drug safety monitoring by giving a useful service in return for their participation. A multifaceted approach to postmarketing surveillance of drug safety involving all stakeholders in healthcare including providers, payers, regulatory agencies, and patients seems likely to hold the most promise for maximising the benefits of pharmaceutical advancements while minimising unknown risks.
I thank Jerome Kassirer and Ralph Horwitz for their help in revision of this manuscript.
Contributors and sources: DPK is a fellow in cardiovascular medicine at the University of Colorado Health Sciences Center and prepared this manuscript as a postdoctoral research fellow in the Center for Biomedical Informatics Research at Stanford University. In addition to published medical literature, this manuscript was prepared using budgets, financial data, and briefings which are publicly available. This article is based on a presentation at Stanford University Department of Medicine Grand Rounds in February 2007.
Funding: DPK was supported through NIH grant 2 T15 LM007033-24 for graduate training in informatics at Stanford University.
Competing interests: None declared.
Cite this as:BMJ 2008;337:a2591
References
- 1.Krumholz HM, Ross JS, Presler AH, Egilman DS. What have we learnt from Vioxx? BMJ 2007;334:120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Union of Concerned Scientists. Voices of scientists at FDA: protecting public health depends on independent science. 2006www.ucsusa.org/assets/documents/scientific_integrity/FDA-Survey-Brochure.pdf.
- 3.Okie S. What ails the FDA? N Engl J Med 2005;352:1063-6. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter D, Zucker EJ, Avorn J. Drug-review deadlines and safety problems. N Engl J Med 2008;358:1354-61. [DOI] [PubMed] [Google Scholar]
- 5.DiMasi JA, Paquette C. The economics of follow-on drug research and development. Pharmacoeconomics 2004;22(suppl 2):1-14. [DOI] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services. Budget in brief FY, 2007. Washington, DC: DHHS, 2007.
- 7.Berndt ER, Gottschalk AHB, Philipson TJ, Strobeck MW. Industry funding of the FDA: effecs of PDUFA on approval time and withdrawal rates. Nature Rev Drug Disc 2005;4:545-54. [DOI] [PubMed] [Google Scholar]
- 8.Vilas-Boas IM, Tharp CP. The drug approval process in the US, Europe, and Japan. J Manag Care Pharm 1997;3:459-65. [Google Scholar]
- 9.Medicines and Healthcare Products Regulatory Agency. Corporate plan 2008-2013. www.mhra.gov.uk/home/groups/comms-sp/documents/publication/con014980.pdf.
- 10.Horton R. Lotronex and the FDA: a fatal erosion of integrity. Lancet 2001;357:1544-5. [DOI] [PubMed] [Google Scholar]
- 11.Securities and Exchange Commission. Merck & Co. Ex-99.3: annual business briefing presentations. www.secinfo.com/dsvr4.ug4b.b.htm.
- 12.Berkrot B. Data show strong launch of new Merck diabetes drug. Reuters 2006. Nov 21. www.reuters.com/article/healthNews/idUSN2029409020061121.
- 13.Merck. Merck reports first quarter 2008 results. Press release, 21 April 2008. http://media.corporate-ir.net/media_files/irol/73/73184/1Q08_Release.pdf.
- 14.DiMasi JA. New drug development in the United States from 1963 to 1999. Clin Pharmacol Ther 2001;69:286-96. [DOI] [PubMed] [Google Scholar]
- 15.Pfizer. 2006 financial report. http://media.pfizer.com/files/annualreport/2006/financial/financial2006.pdf.
- 16.Hong SH, Shepherd MD, Scoones D, Wan TT. Product-line extensions and pricing strategies of brand-name drugs facing patent expiration. J Manag Care Pharm 2005;11:746-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan TA, Rothman DJ, Blank L, Blumenthal D, Chimonas SC, Cohen JJ, et al. Health industry practices that create conflicts of interest: A policy proposal for academic medical centers. JAMA 2000;295:429-33. [DOI] [PubMed] [Google Scholar]
- 18.Uppsala Monitoring Centre. Report from the WHO Collaborating Centre for International Drug Monitoring: activities July 2007 to June 2007. www.who-umc.org/graphics/14865.pdf.
- 19.McClellan M. Drug safety reform at the FDA—pendulum swing or systematic improvement? N Engl J Med 2007;356:1700-2. [DOI] [PubMed] [Google Scholar]
- 20.Food and Drug Administration. Prescription Drug User Fee Act (PDUFA IV) drug safety five year plan, 2008. www.fda.gov/cder/pdufa/PDUFA_IV_5yr_plan_draft.pdf.
- 21.Platt R. Using health plan data to improve post-marketing safety. Institute of Medicine symposium on future of drug safety, 2007. www.iom.edu/Object.File/Master/41/443/Platt%20slides.pdf.
- 22.United States Congress. Food and Drug Administration Amendments Act of 2007. HR 3580. www.fda.gov/oc/initiatives/hr3580.pdf.