Abstract
D-serine administration has been shown to be effective for the treatment of schizophrenia symptoms. However, D-serine has to be administered at high doses in order to observe clinical effects. This is in large part due to D-serine undergoing oxidation by D-amino acid oxidase (DAAO) before it reaches the brain. Consequently, co-administration of D-serine with a DAAO inhibitor has been suggested as a way to lower the dose of D-serine required to treat schizophrenia. During the characterization of DAAO inhibitors as potential drugs, inhibitors are evaluated in rodents for their ability to increase plasma D-serine levels after oral co-administration. Current HPLC-based methodologies to measure D-serine in plasma are time consuming and are not amenable to concomitant analysis of multiple samples. We report the characterization of a 96-well-format assay to monitor D-serine in plasma that greatly expedites analysis time. The assay involves the use of strong cation exchange solid phase extraction (SPE) to isolate D-serine from plasma followed by quantitation of D-serine using the DAAO catalyzed reaction. Plasma D-serine determination using this assay could also be used as pharmacodynamic marker and as biomarker.
Keywords: D-serine, solid phase extraction, D-amino acid oxidase, schizophrenia
D-serine is an endogenous allosteric activator of the NMDA1 receptor. In multiple clinical studies, D-serine administration has been shown to be effective at treating the positive, negative and cognitive deficits of schizophrenia [1; 2; 3]. In order to observe clinical effects, however, D-serine had to be administered at high doses (2 g per day po) multiple times per day (TID or BID). One reason for the high and frequent dose is that D-serine undergoes oxidation by D-amino acid oxidase (DAAO), a flavoenzyme expressed in the liver, kidney, and brain. Only a fraction of the administered D-serine is thought to cross the blood brain barrier and act on the presumed target, the NMDA receptor. One additional issue with D-serine therapy is that the products of D-serine oxidation, hydroxy pyruvate and hydrogen peroxide, have been associated with nephrotoxicity [4; 5]. In order to address these problems, co-administration of D-serine with a DAAO inhibitor has been suggested to lower the dose of D-serine required to treat schizophrenia symptoms and also to prevent unwanted side effects caused by the DAAO-catalyzed reaction [6]. Early results using this approach have been promising: oral co-administration of D-serine with a prototype DAAO inhibitor, 5-chloro-benzo[d]isoxazol-3-ol (CBIO), significantly enhanced plasma and brain levels of D-serine in rats compared to D-serine alone [6]. Further, co-administration of CBIO with D-serine normalized prepulse inhibition deficits in ddy mice similar to the normalization observed when using 10-fold higher doses of D-serine alone [7]. Drug-like DAAO inhibitors with acceptable pharmacokinetics and toxicity profiles are being sought as a novel therapeutic for patients with schizophrenia [8]. In the early preclinical characterization of these new drug candidates inhibitors are evaluated in rodents for their ability to increase plasma D-serine levels after oral co-administration. Plasma D-serine could also be a useful pharmacodynamic marker to establish dose and a biomarker of drug effect once DAAO inhibitors are in the clinic.
Currently there are two HPLC-based methods to measure D-serine in plasma. One involves D-serine extraction, derivatization using N-tert-butyloxycarbonyl –L-cysteine and o-phthaldialdehyde followed by HPLC separation with a C18 column and detection of fluorescent signal of derivatized D-serine [9]. The other method was originally implemented with rat brain microdialysis samples using two HPLC columns in tandem involving derivatization with 4-fluoro-7-nitro-2, 1, 3-benzoxadiazole (NBD-F), separation of the derivatized amino acids in an ODS column followed by a chiral column separation and fluorimetric detection [10]. Both methods allow for the separation of D-serine from other amino acids present in plasma including L- serine. These methods require 40 – 70 min chromatographic separations [9; 10] and they are not amenable to concomitant analysis of multiple samples. Consequently, analyses of D-serine time profiles in plasma after co-administration with DAAO inhibitors is time consuming. We report the characterization of a new 96-well-format assay to monitor D-serine in plasma thus greatly expediting analysis time. The assay involves the use of strong cation exchange solid phase extraction (SPE) to isolate D-serine from plasma followed by quantitation of D-serine using the DAAO catalyzed reaction.
Materials and Methods
Chemicals
5-chloro-benzo[d]isoxazol-3-ol (CBIO) and 4H-thieno [3, 2-b] pyrrole-5-carboxylic acid (TPC) were bought from Maybridge and Chembridge respectively.
Animals
CD1 mice (6 – 8 wk old, Sprague Dawley, Harlan) were dosed orally with D-serine (30 mg/kg) ± compounds. Animals were sacrificed 0.5, 1, 2, 3 and 6 h after dosing and blood was collected by cardiac puncture bleeds. Plasma was prepared and frozen at −80 °C until use.
Cation Exchange SPE
D-serine was added to normal mouse plasma at different concentrations to generate a standard curve. Plasma (50 μL) containing D-serine or plasma from D-serine treated animals was diluted in 200 μL 0.03 N HCl (pH 1.5). Even though protein denaturation must have occurred to some extent at pH 1.5, we did not observe precipitation or turbidity. Acidified samples were then added to a cation exchange resin (BioRad AG 50W-X4, 200 μL resin equilibrated with 0.03 N HCl) in deep well spin plates (Harvard Apparatus). Minicolumns were centrifuged for 2 min at 200 × g. The resin was washed twice with 250 μL 0.03 N HCl and 3 times with 250 μL 100 mM Tris, pH 8.5. D-serine was eluted with 2 times 250 μL 100 mM Tris, pH 13.
D-serine Measurement
An aliquot (50 μL) of the D-serine eluant from the cation exchange SPE was used as the source of D-serine in the DAAO catalyzed reaction. The DAAO assay is an enzyme coupled assay that was adapted from a procedure that has been reported previously [11]. Briefly, the reaction is carried out in Tris buffer (pH 8.5, 100 mM) in the presence of flavin adenine dinucleotide (10 μM). The peroxide formed from the oxidation of D-serine in the presence of porcine DAAO (34 units/mg, Sigma) was made to react with horse radish peroxidase (0.01 mg/ml, Sigma) in the presence of Amplex Red (50 μM, Invitrogen) to make resorufin. Fluorescence of resorufin (ex/em 530/590) was monitored in a SpectraMax Gemini XS fluorimeter for 1 h at 5 min intervals. D-serine concentrations were determined by interpolation from the standard curve of fluorescence vs. D-serine concentration.
Results and Discussion
D-serine can be extracted from plasma using SPE and quantified using a standard DAAO enzymatic assay
The carboxylic acid of D-serine (pKa 2.1) is approximately 80% in the neutral form at pH 1.5 and the amino acid molecule acquires a net positive charge of 0.8 due to the protonated amine. When passed through a strong cation exchange column, positively charged D-serine binds to the resin while neutral molecules and acidic molecules do not bind and are removed through stepwise washes at higher pH values. The pH of the mobile phase was increased first to pH 8.5 and then to pH 13. The pH of the eluant changed gradually but D-serine eluted in a small volume between pH 2 and 9 immediately after the pH of the mobile phase was changed to pH 13 (Table 1).
Table 1.
Binding and elution of D-serine from cation exchange resina
| Mobile Phase | pH of mobile phase | Measured pH of eluate | D-serine concentration (uM) |
|---|---|---|---|
| Flow Through | 1.5 | 1.5 | 0 |
| Rinse 1 | 1.5 | 1.5 | 0 |
| Rinse 2 | 1.5 | 1.5 | 0 |
| Eluent 1 | 8.5 | 2 | 0 |
| Eluent 2 | 8.5 | 2 | 0 |
| Eluent 3 | 8.5 | 2 | 0.6 |
| Eluent 4 | 13 | 7 | 13 |
| Eluent 5 | 13 | 9 | 29 |
| Eluent 6 | 13 | 12 | 0 |
Mouse plasma was spiked with D-serine (50 μM) and applied to BioRad AG 50W-X4 cation exchange resin (2 ml wet volume) previously equilibrated at pH 1.5. Eluate fractions (2 ml) were collected and their pH measured. The pH of the eluate was then adjusted to 8.5 and the concentration of D-serine in the eluate was determined using the DAAO enzyme coupled assay.
The eluant containing D-serine was then used as substrate in the DAAO catalyzed reaction. D-serine was the limiting reagent in the enzyme coupled reaction where a high concentration of DAAO was used. Plasma exhibited background fluorescence of about 1500 RFUs (Fig 1A) possibly due to compounds that fluoresce and/or D-amino acids that are endogenously produced or of exogenous origin. The levels of several D-amino acids in mouse serum have been reported previously and they range from not detectable to 6 μM [12]; these concentrations would give a low fluorescence background. Consequently, the higher background that is observed must come largely from other compounds that fluoresce that are not D-amino acids. In any case, background fluorescence was determined from control animals every time an experiment was carried out. This background was then subtracted from the plasma fluorescence obtained from animals that had been dosed with D-serine. Increasing concentrations of added D-serine in plasma that went through acidification, SPE and the DAAO-catalyzed reaction gave increasing fluorescence in a linear fashion in the 4 – 250 μM range (Fig. 1A). Fluorescence was then correlated to the concentrations of D-serine added to plasma to generate a standard curve, which was subsequently used to determine unknown concentrations of D –serine in plasma by interpolation. Since DAAO is specific for the D-isomer of serine, no separation of L and D enantiomers was necessary. It is important to note that the SPE/DAAO procedure works effectively only when the DAAO inhibitor does not carry positive charges at pH 1.5; this is the case with known DAAO inhibitors including CBIO. If a DAAO inhibitor present in plasma were to co-elute with D-serine during the SPE step, it would inhibit DAAO in the second step and compromise the determination of D-serine concentrations. In fact, during earlier experiments when using methanol instead of SPE to extract D-serine from plasma, D-serine did not separate from DAAO inhibitors which were found to inhibit DAAO in the second step of the procedure (data not shown). Consequently, it will be important to demonstrate that any DAAO inhibitor being evaluated using this assay does not co-elute with D-serine during SPE. When CBIO, a prototype DAAO inhibitor, was added to plasma at 100 μM (about 10,000-fold Ki) with D-serine and the samples were subsequently subjected to SPE/DAAO analysis, values obtained for D-serine were identical to those from samples that had not been spiked with DAAO inhibitor (Fig. 1A). The results are in accordance with the expectation that cation exchange resins do not bind to compounds without a positive charge. The above results were obtained from end-point measurements of fluorescence after 1 h incubation when all D-serine was expected to have been oxidized. In order to ensure that the reaction had indeed reached an equilibrium stage, SPE/DAAO analyses were carried out at various time points. The reaction was complete within the first 15 min of incubation (Fig. 1B). Further, the rate of oxidation of D-serine obtained from plasma samples without CBIO was the same as that of D-serine from samples with CBIO added and subsequently removed by SPE. In a control experiment, when CBIO was not removed by SPE, oxidation of D-serine was completely inhibited (data not shown). The results indicated that 1 h incubation was more than sufficient for the DAAO reaction to reach equilibrium and that SPE removed CBIO from D-serine. There was a slight gradual decrease in the fluorescence at 40 – 60 min (Fig 1B) possibly due to resorufin degradation so fluorescence measurements are best carried out at 20 – 30 min.
Fig. 1.
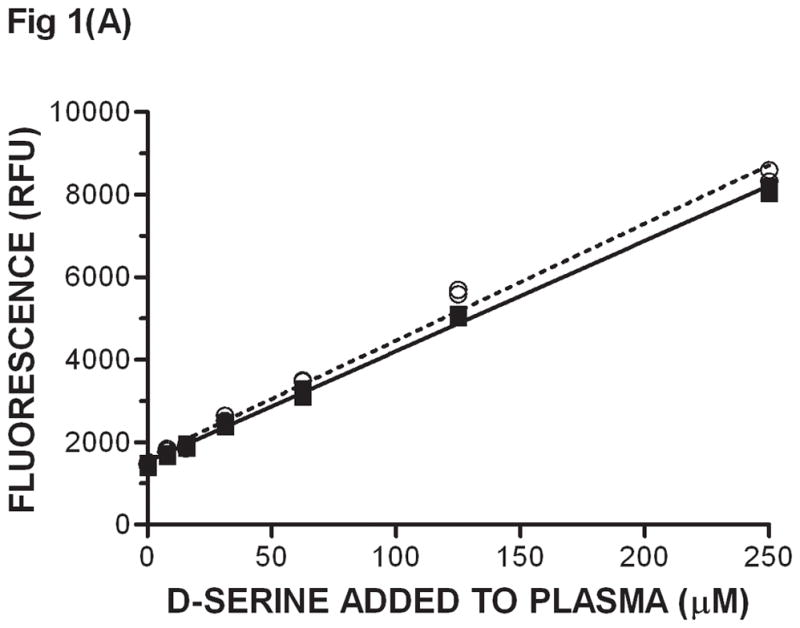
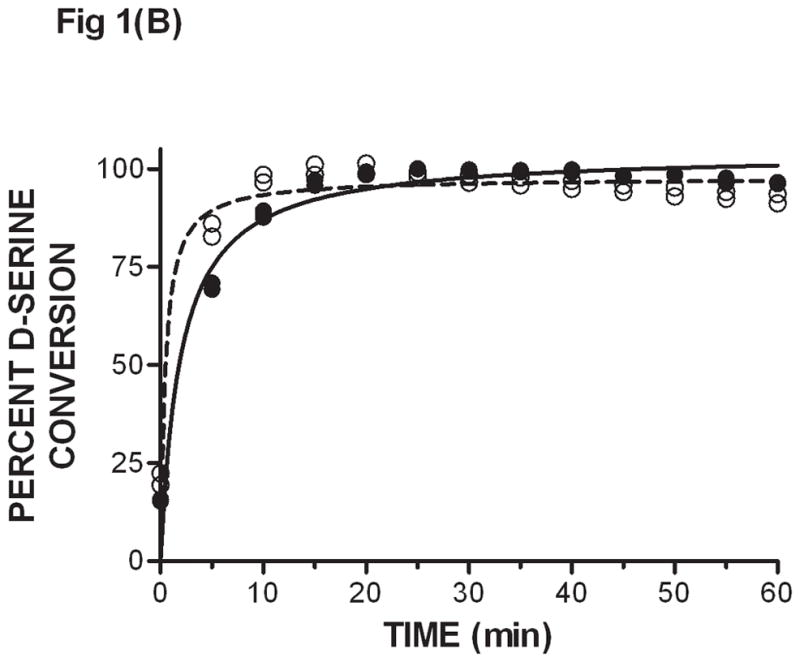
Extraction of D-serine from plasma by SPE and subsequent determination of D-serine levels using the DAAO enzyme coupled assay - (A) D-serine at various concentrations was added to plasma in the presence of 100 μM CBIO (filled squares, solid line) or 1 % DMSO vehicle (empty circles, dashed line). D-serine was separated from CBIO by solid phase extraction. Subsequently, D-serine concentrations were determined using the DAAO-catalyzed conversion of D-serine to fluorescent resorufin in the DAAO enzyme coupled assay (materials and methods). Fluorescence readings (n = 2) in relative fluorescent units (RFU) were obtained from end point measurements after the DAAO reaction was allowed to go completion. Single measurements are shown. A fluorescence (RFU) vs. D-serine (μM) standard curve was constructed with each experiment. (B) Kinetic trace of resorufin resulting from D-serine oxidation. D-serine (250 μM) ± CBIO (100 μM) were added to plasma, and SPE/DAAO analysis was used to monitor D-serine concentrations at different time points during the DAAO-catalyzed oxidation. D-serine only: empty circles, dashed line. D-serine + CBIO: filled circles, solid line. Data points at each concentration are duplicates.
Resin and samples can be loaded into a 96-well plate and processed concomitantly within 2 hrs. A comparable analysis of 96 samples in sequential HPLC assays would take approximately 3 days assuming non-stop use of the instrument.
D-serine levels in plasma can be monitored ex-vivo
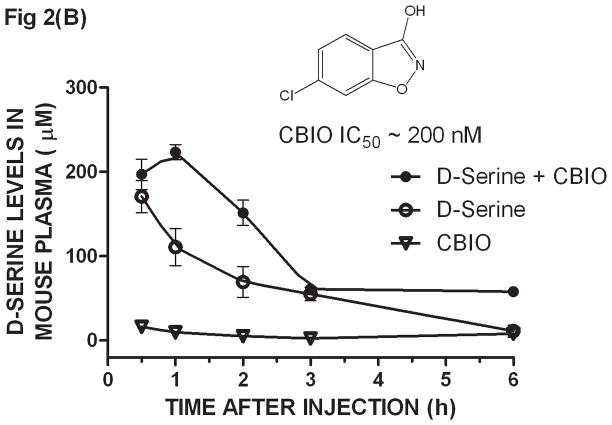
Mice were given D-serine (326 μmoles/kg, po) and their plasma collected at 0.5, 1, 2, 3 and 6 h after dosing. An aliquot (50 μL) of each plasma sample was subjected to SPE/DAAO to determine D-serine levels. The first time point at 0.5 h showed the highest D-serine plasma concentration; the maximum time of drug concentration (Tmax) was likely reached at an earlier time point. The concentration of D-serine gradually decreased to the lowest concentration at 6 h (Fig. 2A). The estimated half life of D-serine in mouse plasma was approximately 50 min. D-serine levels were the same as background levels when CBIO was administered in the absence of D-serine suggesting that the effect of DAAO inhibitors on plasma D-serine if any, would be largely on D-serine of exogenous origin.
Fig. 2.
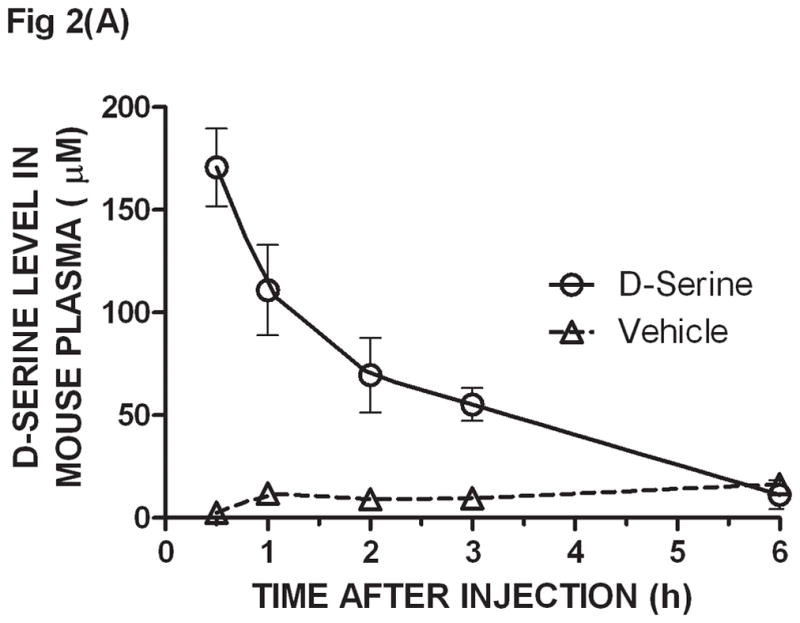
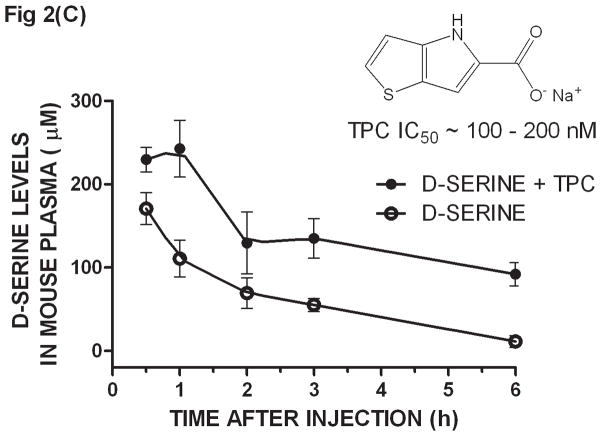
Ex-vivo determination of plasma D-serine by the SPE/DAAO method after oral administration of D-serine ± DAAO inhibitors - CD1 mice were given D-serine (326 μmoles/kg, po) ± DAAO inhibitors CBIO (180 μmoles/kg) or TPC (160 μmoles/kg). Plasma was collected at 0.5, 1, 2, 3, and 6 hours after dosage. D-serine was extracted from plasma by SPE and its levels determined by the DAAO reaction: (A) D-serine alone, (B) CBIO alone, D-serine ± CBIO and (C) D-serine ± TPC. IC50 values for CBIO [6] and TPC [13] were averaged from those listed in the literature. Each data point is the average of data from 3 separate mice; error bars correspond to ± S.E.M. D-serine standard curves were constructed for each experiment.
When dosing D-serine (326 μmole/kg po) plus CBIO (180 μmoles/kg, po), the D-serine curve showed a similar maximum concentration to that observed when using D-serine alone but the overall curve was shifted to the right confirming that co-administration of a DAAO inhibitor increases exposure to D-serine (Fig. 2B). The shape of the curves and the timing and concentrations reached were similar to those previously reported using the HPLC methodology to determine plasma D-serine levels [6]. Another DAAO inhibitor, 4H-thieno [3, 2-b] pyrrole-5-carboxylic acid (TPC) [13], was also dosed (160 μmoles/kg, po) with D-serine and assayed by SPE/DAAO. TPC also showed that the presence of the inhibitor in vivo shifted the exposure of D-serine in plasma (Fig. 2C). The SPE/DAAO assay is currently being used in our laboratory to evaluate the effect of newly synthesized DAAO inhibitors on D-serine levels in plasma after oral co-administration with D-serine.
In summary, we have developed a new method, SPE/DAAO, based on SPE isolation of D-serine from plasma followed by quantitation of D-serine using the DAAO-catalyzed reaction. The assay is aimed at drug development and not intended for diagnostic purposes. D-serine concentrations were linear with fluorescence in the 4 – 250 μM (fig 1A), well within the expected concentration ranges of plasma D-serine after D-serine administration (±) DAAO inhibitors (fig 2B and fig 2C). The method could be readily adapted to the determination of other exogenously added DAAO substrates such as D-Ala or D- proline as well as to other biological matrices such as brain or peripheral tissues from different species. The new method offers substantial time savings over the traditional HPLC methods currently employed.
Acknowledgments
This work has been partially funded by NIH (Grant R01MH091387; TT)
Footnotes
Abbreviations Used: BID, (bis in die) twice a day; CBIO, 5-chloro-benzo[d]isoxazol-3-ol; DAAO, D-amino acid oxidase; HPLC, High pressure liquid chromatography; NBD-F 4-fluoro-7-nitro-2,1,3-benzoxadiazole; NMDA, N-methyl-D-aspartate; ODS, octadecyl silane; RFU, relative fluorescent units; SPE, solid phase extraction; TID, (ter in die) three times a day; TPC, 4H-thieno[3,2-b]pyrrole-5-carboxylic acid
References
- 1.Tsai G, Yang P, Chung LC, Lange N, Coyle JT. D-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 1998;44:1081–9. doi: 10.1016/s0006-3223(98)00279-0. [DOI] [PubMed] [Google Scholar]
- 2.Heresco-Levy U, Javitt DC, Ebstein R, Vass A, Lichtenberg P, Bar G, Catinari S, Ermilov M. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005;57:577–85. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Kantrowitz JT, Malhotra AK, Cornblatt B, Silipo G, Balla A, Suckow RF, D’Souza C, Saksa J, Woods SW, Javitt DC. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121:125–30. doi: 10.1016/j.schres.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams RE, Lock EA. Sodium benzoate attenuates D-serine induced nephrotoxicity in the rat. Toxicology. 2005;207:35–48. doi: 10.1016/j.tox.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Chung SP, Sogabe K, Park HK, Song Y, Ono K, Abou El-Magd RM, Shishido Y, Yorita K, Sakai T, Fukui K. Potential cytotoxic effect of hydroxypyruvate produced from D-serine by astroglial D-amino acid oxidase. J Biochem. 2010;148:743–53. doi: 10.1093/jb/mvq112. [DOI] [PubMed] [Google Scholar]
- 6.Ferraris D, Duvall B, Ko YS, Thomas AG, Rojas C, Majer P, Hashimoto K, Tsukamoto T. Synthesis and biological evaluation of D-amino acid oxidase inhibitors. J Med Chem. 2008;51:3357–9. doi: 10.1021/jm800200u. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto K, Fujita Y, Horio M, Kunitachi S, Iyo M, Ferraris D, Tsukamoto T. Co-administration of a D-amino acid oxidase inhibitor potentiates the efficacy of D-serine in attenuating prepulse inhibition deficits after administration of dizocilpine. Biol Psychiatry. 2009;65:1103–6. doi: 10.1016/j.biopsych.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Ferraris DV, Tsukamoto T. Recent Advances in the Discovery of D-Amino Acid Oxidase Inhibitors and their Therapeutic Utility in Schizophrenia. Curr Pharm Des. 2011;17:103–11. doi: 10.2174/138161211795049633. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto A, Nishikawa T, Oka T, Takahashi K, Hayashi T. Determination of free amino acid enantiomers in rat brain and serum by high-performance liquid chromatography after derivatization with N-tert.-butyloxycarbonyl-L-cysteine and o-phthaldialdehyde. J Chromatogr. 1992;582:41–8. doi: 10.1016/0378-4347(92)80300-f. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima T, Kawai J, Imai K, Toyo’oka T. Simultaneous determination of D-and L-serine in rat brain microdialysis sample using a column-switching HPLC with fluorimetric detection. Biomed Chromatogr. 2004;18:813–9. doi: 10.1002/bmc.394. [DOI] [PubMed] [Google Scholar]
- 11.Brandish PE, Chiu CS, Schneeweis J, Brandon NJ, Leech CL, Kornienko O, Scolnick EM, Strulovici B, Zheng W. A cell-based ultra-high-throughput screening assay for identifying inhibitors of D-amino acid oxidase. J Biomol Screen. 2006;11:481–7. doi: 10.1177/1087057106288181. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto A, Nishikawa T, Konno R, Niwa A, Yasumura Y, Oka T, Takahashi K. Free D-serine, D-aspartate and D-alanine in central nervous system and serum in mutant mice lacking D-amino acid oxidase. Neurosci Lett. 1993;152:33–6. doi: 10.1016/0304-3940(93)90476-2. [DOI] [PubMed] [Google Scholar]
- 13.Smith SM, Uslaner JM, Yao L, Mullins CM, Surles NO, Huszar SL, McNaughton CH, Pascarella DM, Kandebo M, Hinchliffe RM, Sparey T, Brandon NJ, Jones B, Venkatraman S, Young MB, Sachs N, Jacobson MA, Hutson PH. The behavioral and neurochemical effects of a novel D-amino acid oxidase inhibitor compound 8 [4H-thieno [3,2-b]pyrrole-5-carboxylic acid] and D-serine. J Pharmacol Exp Ther. 2009;328:921–30. doi: 10.1124/jpet.108.147884. [DOI] [PubMed] [Google Scholar]