Abstract
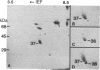
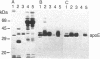
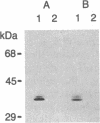
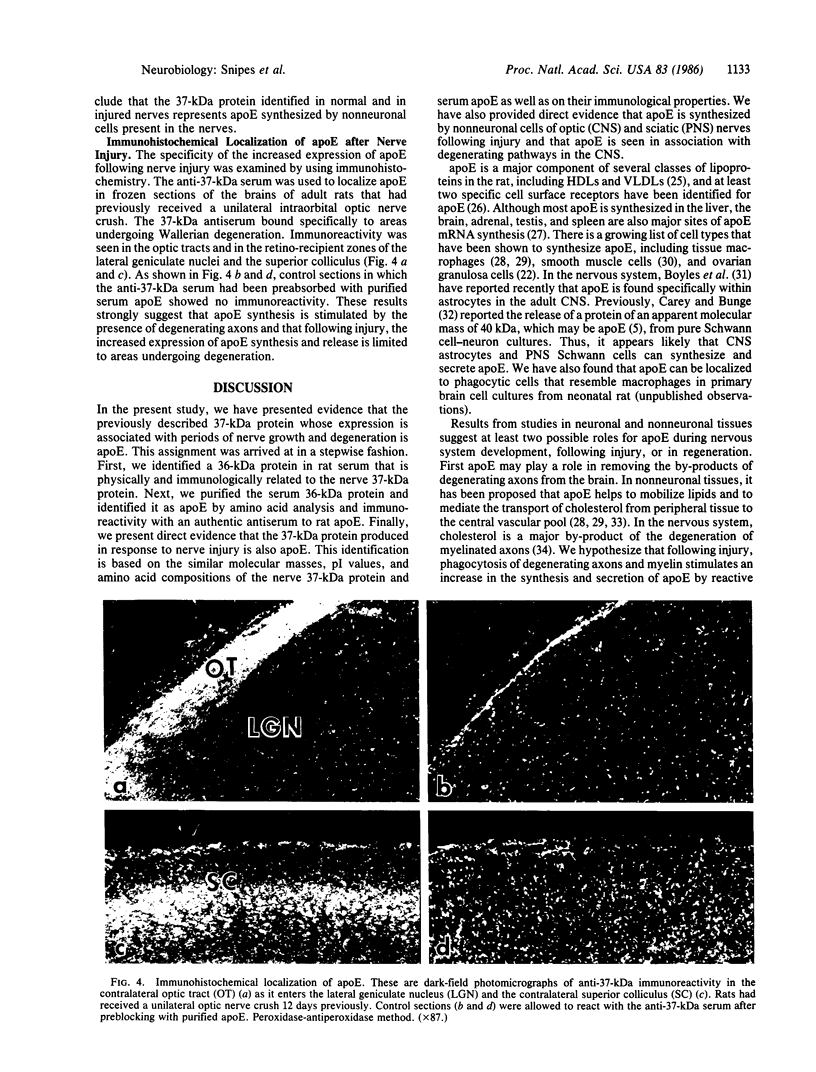
Nerve trauma initiates significant changes in the composition of proteins secreted by nonneuronal cells. The most prominent of these proteins is a 37-kDa protein, whose expression correlates with the time course of nerve development, degeneration, and regeneration. We now report that the 37-kDa protein is apolipoprotein E (apoE). We produced a specific antiserum against the 37-kDa protein isolated from previously crushed nerves. This antiserum recognizes a 36-kDa protein in rat serum that we have purified and identified as apoE. The anti-37-kDa antiserum also recognizes apoE on electrophoretic transfer blots of authentic samples of high and very low density lipoproteins. The nerve 37-kDa protein comigrates with apoE by two-dimensional electrophoresis, shares a similar amino acid composition, and reacts with an antiserum against authentic apoE. The purified apoE specifically blocks the immunoprecipitation of [35S]methionine-labeled 37-kDa protein synthesized by nonneuronal cells. Thus, on the basis of its molecular mass, isoelectric point, amino acid composition, and immunological properties, we conclude that the 37-kDa protein is apoE. We also used light microscopic immunohistochemistry to localize apoE following nerve injury. In rats with optic nerve lesions, the 37-kDa antiserum bound specifically to the degenerating optic tracts and to the retino-recipient layers of the lateral geniculate nucleus and the superior colliculus. We propose that apoE is synthesized by phagocytic cells in response to nerve injury for the purpose of mobilizing lipids produced as a consequence of axon degeneration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Brown M. S., Ho Y. K., Havel R. J., Goldstein J. L. Mouse macrophages synthesize and secrete a protein resembling apolipoprotein E. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7545–7549. doi: 10.1073/pnas.78.12.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Brown M. S. Independent pathways for secretion of cholesterol and apolipoprotein E by macrophages. Science. 1983 Feb 18;219(4586):871–873. doi: 10.1126/science.6823554. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Ho Y. K., Brown M. S., Bilheimer D. W., Anderson R. G., Goldstein J. L. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982 Aug 25;257(16):9788–9795. [PubMed] [Google Scholar]
- Boulard C., Lecroisey A. Specific antisera produced by direct immunization with slices of polyacrylamide gel containing small amounts of protein. J Immunol Methods. 1982;50(2):221–226. doi: 10.1016/0022-1759(82)90228-9. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carey D. J., Bunge R. P. Factors influencing the release of proteins by cultured Schwann cells. J Cell Biol. 1981 Dec;91(3 Pt 1):666–672. doi: 10.1083/jcb.91.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan W. M., Fawcett J. W., O'Leary D. D., Stanfield B. B. Regressive events in neurogenesis. Science. 1984 Sep 21;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Driscoll D. M., Getz G. S. Extrahepatic synthesis of apolipoprotein E. J Lipid Res. 1984 Dec 1;25(12):1368–1379. [PubMed] [Google Scholar]
- Driscoll D. M., Schreiber J. R., Schmit V. M., Getz G. S. Regulation of apolipoprotein E synthesis in rat ovarian granulosa cells. J Biol Chem. 1985 Jul 25;260(15):9031–9038. [PubMed] [Google Scholar]
- Elshourbagy N. A., Liao W. S., Mahley R. W., Taylor J. M. Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc Natl Acad Sci U S A. 1985 Jan;82(1):203–207. doi: 10.1073/pnas.82.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidez L. I., Swaney J. B., Murnane S. Analysis of rat serum apolipoproteins by isoelectric focusing. I. Studies on the middle molecular weight subunits. J Lipid Res. 1977 Jan;18(1):59–68. [PubMed] [Google Scholar]
- Goldstein J. L., Helgeson J. A., Brown M. S. Inhibition of cholesterol synthesis with compactin renders growth of cultured cells dependent on the low density lipoprotein receptor. J Biol Chem. 1979 Jun 25;254(12):5403–5409. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Jen G., Thach R. E. Inhibition of host translation in encephalomyocarditis virus-infected L cells: a novel mechanism. J Virol. 1982 Jul;43(1):250–261. doi: 10.1128/jvi.43.1.250-261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983 May 24;737(2):197–222. doi: 10.1016/0304-4157(83)90001-1. [DOI] [PubMed] [Google Scholar]
- McLean J. W., Fukazawa C., Taylor J. M. Rat apolipoprotein E mRNA. Cloning and sequencing of double-stranded cDNA. J Biol Chem. 1983 Jul 25;258(14):8993–9000. [PubMed] [Google Scholar]
- Müller H. W., Gebicke-Härter P. J., Hangen D. H., Shooter E. M. A specific 37,000-dalton protein that accumulates in regenerating but not in nonregenerating mammalian nerves. Science. 1985 Apr 26;228(4698):499–501. doi: 10.1126/science.3983637. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Politis M. J., Pellegrino R. G., Oaklander A. L., Ritchie J. M. Reactive glial protein synthesis and early disappearance of saxitoxin binding in degenerating rat optic nerve. Brain Res. 1983 Aug 29;273(2):392–395. doi: 10.1016/0006-8993(83)90870-3. [DOI] [PubMed] [Google Scholar]
- Royer G. P., Schwartz W. E., Liberatore F. A. Complete hydrolysis of polypeptides with insolubilized enzymes. Methods Enzymol. 1977;47:40–45. doi: 10.1016/0076-6879(77)47006-x. [DOI] [PubMed] [Google Scholar]
- Skene J. H., Shooter E. M. Denervated sheath cells secrete a new protein after nerve injury. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4169–4173. doi: 10.1073/pnas.80.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekrishna K., Jones C. E., Guetzow K. A., Prasad M. R., Joshi V. C. A modified method for amino acid analysis with ninhydrin of Coomassie-stained proteins from polyacrylamide gels. Anal Biochem. 1980 Mar 15;103(1):55–57. doi: 10.1016/0003-2697(80)90235-3. [DOI] [PubMed] [Google Scholar]
- Swaney J. B., Braithwaite F., Eder H. A. Characterization of the apolipoproteins of rat plasma lipoproteins. Biochemistry. 1977 Jan 25;16(2):271–278. doi: 10.1021/bi00621a018. [DOI] [PubMed] [Google Scholar]
- Vaughn J. E., Hinds P. L., Skoff R. P. Electron microscopic studies of Wallerian degeneration in rat optic nerves. I. The multipotential glia. J Comp Neurol. 1970 Oct;140(2):175–206. doi: 10.1002/cne.901400204. [DOI] [PubMed] [Google Scholar]
- Zannis V. I., Kurnit D. M., Breslow J. L. Hepatic apo-A-I and apo-E and intestinal apo-A-I are synthesized in precursor isoprotein forms by organ cultures of human fetal tissues. J Biol Chem. 1982 Jan 10;257(1):536–544. [PubMed] [Google Scholar]