Abstract
Absorption of drugs from subcutaneous tissue depends on several factors, including tissue perfusion at the administration site. Tissue perfusion can be manipulated by e.g. application of local heat. This may subsequently alter the rate or amount of absorption of drugs from a subcutaneous depot. The aim of the present study was to investigate if increased tissue perfusion after controlled local heating can change the absorption of subcutaneously administered short-acting insulin (Actrapid®, 100IU/ml). Thirteen healthy Caucasian males participated in two randomized experimental sessions; one session with locally applied controlled heat at the injection site, and a control session without local heat application. Tissue perfusion (blood flow) was monitored with Laser Doppler Imaging, and blood samples were taken to assess the levels of glucose and insulin. Local heat application at the site of insulin injection significantly enhanced tissue perfusion by approximately 145%. However, no correlation was found between insulin absorption and tissue perfusion. Based on our findings, it was concluded that tissue perfusion is not the rate-limiting factor in the absorption of high-concentration short-acting insulin from a subcutaneous depot. It is suggested that dissociation of insulin hexamers into dimers and monomers is a major rate limiting factor to the absorption.
Keywords: Local heat, skin perfusion, insulin, Actrapid®, subcutaneous depot, absorption
Introduction
Oral drug delivery is one of the most desirable and preferred methods of administering therapeutic agents for their systemic effects [1]. However, protein or peptide drugs are subject to high enzymatic degradation in the gastrointestinal tract resulting in poor bioavailability [2]. Other routes of administration such as intranasal, buccal, transdermal and intraocular have been explored with varying degrees of success [3]. However, subcutaneous administration of protein (e.g. insulin) and peptide drugs is still the most common route. Insulin is one of the most commonly-used drugs for type I diabetes, and is typically injected subcutaneously. Following subcutaneous administration, it forms a depot and is absorbed gradually but passively into the systemic circulation [4, 5]. Absorption of drugs from subcutaneous tissue depends on several factors, such as the quantity and composition of the connective tissue, capillary density, vascular permeability, and the rate of vascular perfusion [6]. Control of these factors is important to ensure a stable and safe pharmacokinetic profile following the subcutaneous administration of a given drug [4]. It is possible to manipulate the absorption amount or rate by applying heat, which can potentially alter tissue perfusion and vascular permeability [7]. Several studies have demonstrated that the insulin absorption rate from an injection site is related to ambient temperature [5, 7, 8]. The effect of ambient temperature on insulin has been studied by Koivisto [9, 10] who found that warm temperature was associated with 3-5 folds higher insulin absorption most likely due to elevated skin temperature and blood flow. The effect of sauna on insulin absorption from a subcutaneous injection site has also been investigated by his group and others [9, 11]. The published findings support the idea that changes in hemodynamic, body fluid volume and blood flow distribution during external heating (saunas and steam baths) may affect proteins like insulin pharmacokinetic parameters such as absorption, distribution and elimination [12]. Individual differences in endogenous skin temperature at normal room temperature have also shown an influence on insulin absorption, whereas higher skin temperature is correlated with higher serum insulin levels following subcutaneous administration [13]. However, documentation on the effects of local controlled heat on insulin pharmacokinetics remains scant [14].
To the best of our knowledge, there is no systematic study on effect of local controlled heat applied at the injection site on cutaneous blood flow and subsequent changes in insulin absorption kinetics. Hence, the aim of the present study was to test whether locally applied controlled heat has the potential to accelerate local tissue perfusion and alter the absorption profile of short-acting insulin (Actrapid®, used as a model) from a subcutaneous depot in healthy male volunteers.
Material and methods
Subjects and study design
Fourteen healthy non-smoking Caucasian male volunteers (18-40 years) were screened for this study. Initial screening involved a complete health check by a physician in order to only recruit healthy volunteers with a Body Mass Index (BMI) < 30 kg/m2, and plasma glucose levels < 6.0 mmol/L, medical conditions that could potentially interfere with the experiment or increase the risk for the subject in relation to the experiment, being on a prescriptive medicine, having known allergies, hereditary diseases, infectious or dermatological conditions, participation in a medical experiment 30 days prior to this experiment, and alcohol or medical abuse were excluded from participation. Following the screening, 13 volunteers participated in the study. A written informed consent was obtained from all participants. The study protocol was approved by the local ethics committee (N-20100066). The study was conducted in accordance with the Declaration of Helsinki, the guidelines of Good Clinical Practice (GCP), and was performed at the Center for Basic and Clinical Research (CCBR) and C4Pain, Aalborg, Denmark.
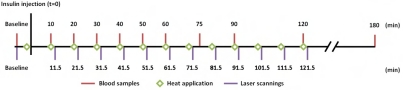
The randomized controlled cross-over study consisted of two experimental sessions of with or without application of local controlled heat. The subjects fasted for at least eight hours prior to each experimental session. The timeline for the experimental setup is shown in Figure 1. All experiments were done in a room with a constant temperature (23-24°C).
Figure 1.
Schematic overview of the study design and time point of the measurements.
Blood sampling and analysis
At the beginning of each experimental session, subjects had an intravenous cannula (BD VenflonTM 18 GA, 1.2 × 32 mm, NJ, USA) inserted in either cubital vein. Blood samples were taken in collecting tubes (S-Monovette®, 92 × 11 mm, 4.5 mL, Nümbrecht, Germany) at 10, 20, 30, 40, 50, 60, 75, 90, 120 and 180 minutes after insulin injection (Figure 1). At each time point, two blood samples (2 × 4.5 mL) were taken for further analysis.
Blood samples were analyzed for three parameters: plasma C-peptide, total plasma insulin and blood glucose. Blood glucose levels were measured immediately from blood samples by HemoCue Glucose 201 (HemoCue, Inc., CA, USA). For the two other parameters, plasma samples were sent frozen to Steno Diabetes Center A/S (Gentofte, Denmark) for analyzing C-peptide and total plasma insulin by AutoDELFIA C-peptide kit and AutoDELFIA Insulin kit, respectively. The range of measurement for plasma C-peptide and plasma insulin was 10-6000 and 3-1000 (pmol/L), respectively. Detection limits for plasma C-peptide and plasma insulin were 5 and 3 (pmol/L), respectively.
To calculate exogenous insulin levels, C-peptide values are used to correct for endogenous insulin by use of the endogenous insulin:C-peptide ratio [15, 16]:
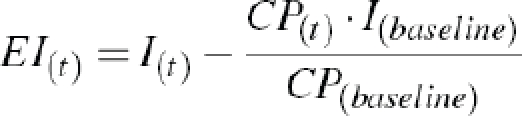 |
where EI(t), I(t) and CP(t) is the exogenous insulin, total insulin concentration and C-peptide concentration, respectively, at time point (t) after injection of insulin. I(baseline) and CP (baseline) are the endogenous insulin concentration and the concentration of C-peptide, respectively, at baseline before insulin injection.
Skin temperature recording
Skin temperature was measured prior to each laser scanning with an infrared thermometer (Radiant TH03F Infrared Thermometer, Med-scope Ltd, United Kingdom). The thermometer was placed medial to the injection site and just inferior of the umbilicus outside the scanning area. The skin temperature was monitored in an attempt to keep a constant temperature of 32±2°C, corresponding to normal skin temperature [17]. In case of deviations the abdomen was either covered with a blanket or left exposed for a few minutes.
Laser doppler imaging (LDI)
Local skin perfusion was monitored using Laser Doppler Imaging (LDI), which works based on the Doppler principle [18, 19]. LDI allows assessment of the movement of red blood cells in the most superficial skin layers (1-1.5 mm) [20, 21] and generates a result in arbitrary units. The generated result reflects the blood flow at a proportional rate, allowing relative comparisons, for example changes in blood flow induced by local heating relative to a baseline or control measurement [19, 22].
Scanning to obtain skin perfusion was performed with a Moor Laser Doppler Imager (Moor Instruments, Devon, United Kingdom). In this study the scanner setup was defined as a bandwidth of 0.25-15 kHz and resolution of 116×70 pixels with a scan speed of 4 ms/pixel at a distance of 20 cm from the skin surface.
The scanning area, called the region of interest (ROI), was defined as a 6.0 × 6.0 cm area, which allowed a safety margin around the 3.0 × 3.0 cm thermode (see below), when applied in the center of the ROI. The laser scanner was adjusted and calibrated to scan the ROI. At baseline and following every heat application, a laser scanning was performed. Laser scans were stored on a hard disk for off-line analysis of the profile and local changes of skin perfusion (moor LDI software, v. 5.3). ROI (6.0 × 6.0 cm area) was used for data analysis. Subjects were asked to keep their eyes closed while wearing special protective goggles during laser scanning.
Heat application
The Medoc PATHWAY Pain & Sensory Evaluation System (Medoc Ltd. Advanced Medical Systems, Ramat Yishai, Israel) was used for local controlled heat application. The specific stimulation parameters were adjusted using the PATHWAY software (v. 4.4). The stimulation program was manually configured before initiation of the experiments.
A 3.0 × 3.0 cm ATS thermode was utilized. The contact probe was kept in touch with the abdominal skin. Controlled heat (43°C for 60 sec) [23] was delivered in each heat session, before the injection of insulin and subsequently every 10 minutes following the injection for up to 120 minutes. Baseline temperature of the thermode was adjusted to 32°C and the heating rate toward the destination temperature was set to 2° C/sec, to prevent a sensation of burning [17]. The area of heat application can be seen in Figure 2.
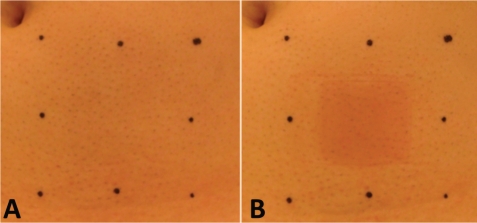
Figure 2.
Photographs depicting the ROI after definition by use of a template. A) typical image taken without heating at the injection site. B) typical image immediately after heating (43°C for 60 sec).
Insulin injection
Actrapid® Penfill® (Novo Nordisk A/S) was used in the present study. Actrapid® is a rapid-acting insulin formulation with a short duration of action. Based on the product information leaflet, the effect on blood glucose levels occurs after 30 minutes, the maximal plasma concentration is reached in 102 minutes, and the half-life is 122 minutes in man. One mL Actrapid® contains 100 IU human insulin in sterile water plus glycerol, metacresol and zinc to assure appropriate isotonic, preservative and stabilizing properties, respectively.
In both experimental sessions, 0.05 IU of insulin per kg body weight was injected in the center of the ROI (Figure 2) at the time point of 0. Due to a limitation of the injection pen (Penfill®), which could only adjust at intervals of 1 IU, doses were rounded down to an even unit, e.g. a subject with a weight of 75 kg who should receive a dose of 3.75 IU, received 3 IU insulin. The dose was set to 0.05 IU/kg which is within the safe range in healthy humans [13].
Safety
The general condition of the subjects was monitored throughout the experiment in order to detect any potential episodes of hypoglycemia during the experiments. Furthermore, beside blood glucose was measured immediately following each blood sampling. If the blood glucose values decreased below 2.2 mmol/L for more than 30 minutes, a glass of lemonade containing dissolved dextrose was given to the subject.
After each experimental session, subjects received a carbohydrate-rich meal and were monitored for another 30 minutes to ensure reestablishment of blood glucose levels before they were discharged.
Statistical analysis
Data acquired from laser scans, skin temperature measurements, blood glucose, total plasma insulin and plasma C-peptide, were transferred to SigmaPlot (v. 11.0, Systat Software, Inc.) for further statistical analysis. Significance level and power level were set to 5% and 80%, respectively. Data were presented as Mean ± Standard Deviation (SD) unless otherwise stated.
Baseline data obtained from skin temperature, blood glucose, total plasma insulin, and plasma C-peptide measurements from control and heat sessions were analyzed in a paired t-test. The same parameters and exogenous insulin levels were subsequently processed by a Two-Way Repeated Measures ANOVA, with the two factors being session type and time. A Holm-Sidak post hoc test was utilized to address individual group differences.
The laser scanning baseline data from control and heat sessions were analyzed in a paired t-test. Subsequently, data were normalized to baseline, noting percentage changes from baseline. The normalized data was processed by a Two-Way Repeated Measures ANOVA, with the two factors of session type and time.
Results
The present study was carried out in 13 healthy Caucasian male volunteers. The characteristics of the subjects are shown in Table 1. None of the subjects experienced episodes of hypoglycemia or other side effects.
Table 1.
Participants information from the screening session. Values are given as Mean±SD. BMI: Body Mass Index (BMI)
| Subjects (n=13) | |
|---|---|
| Age (years) | 23.2±2.4 |
| Weight (kg) | 79.9±11.7 |
| Height (m) | 1.84±0.07 |
| BMI (kg/m2) | 23.6±3.0 |
| Blood Glucose (mmol/L) | 4.8±0.4 |
Skin temperature recording
Skin temperature at the site of insulin injection was monitored with an infrared thermometer every 10 minutes for the first two hours of each experimental session to ensure that the variations in skin temperature (if any) remained at 32±2°C. No significant differences were observed between the two sessions or over time in each session.
Blood flow measurements
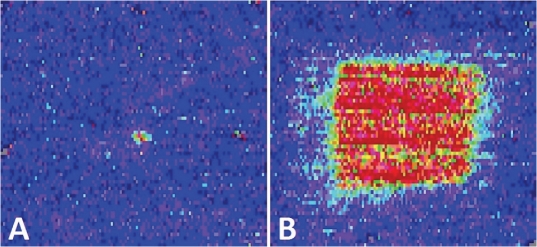
Changes in cutaneous blood flow were monitored with LDI prior to insulin injection, and subsequently every 10 minutes throughout the first two hours of each experimental session. Typical results of laser scans obtained with LDI for both control and heat sessions are shown in Figure 3.
Figure 3.
Typical laser scan results of the ROI from a single subject at time point 31.5 min at control session (A) and following the application of local controlled heat (B) heat session.
Statistical analysis of normalized data revealed a significant difference between control and heat sessions at all time points (p<0.001). Within the heat session, significant differences in blood flow were observed between time point 81.5 min and the two time points 11.5 min (p<0.001) and 31.5 min (p<0.001), respectively. No significant differences were found in blood flow between any time points in the control session. The mean percentage change from baseline was 1.7±1.8% for control sessions and 145.6±6.6% for heat sessions. This indicates an approximately 145% increase in cutaneous blood flow from baseline during heat application based on the ROI.
Blood sample measurements
Blood samples were analyzed for 3 parameters: total plasma insulin, plasma C-peptide, and blood glucose. Total plasma insulin and C-peptide levels were measured to estimate the absorption of exogenous insulin.
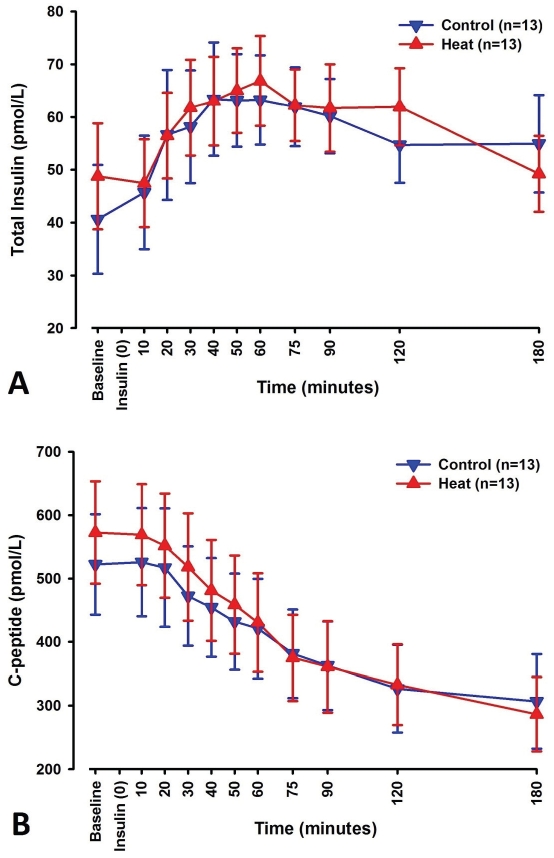
No differences were found between control and heat sessions for baseline levels of total plasma insulin. A significant difference was observed over time (p<0.001), while no difference was found between control and heat sessions.
C-peptide did not differ in baseline levels between control and heat sessions. Significant changes were observed over time (p<0.001), with no significant differences between the two sessions.
C-peptide levels showed a general tendency to decrease throughout the experiment, while total plasma insulin levels peaked within the first hour of the experiment. Figure 4 shows total plasma insulin and C-peptide levels over time.
Figure 4.
Line-scatter graphs of total insulin levels (A) Cpeptide levels (B) measured over time in blood samples collected at the designated time points. Blue graphs depict changes seen in control sessions, while red graphs depict changes seen in heat sessions. Data points noted are mean values of 13 subjects with standard error of the mean.
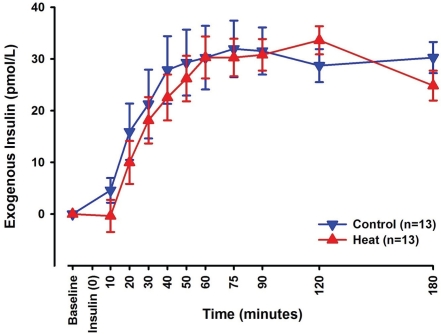
Exogenous insulin was calculated as previously described. Significant changes were seen over time for the exogenous insulin (p<0.001). In addition, a statistically significant interaction was observed between session type and time (p<0.05). No significant difference in exogenous insulin was seen between control and heat sessions (Figure 5).
Figure 5.
Line-scatter graph of exogenous insulin levels calculated from total insulin and C-peptide levels measured in all blood samples collected at designated time points. The blue graph depicts changes seen in control sessions, while the red graph depicts changes seen in heat sessions. Data points noted are mean values of all 13 subjects with standard error of the mean.
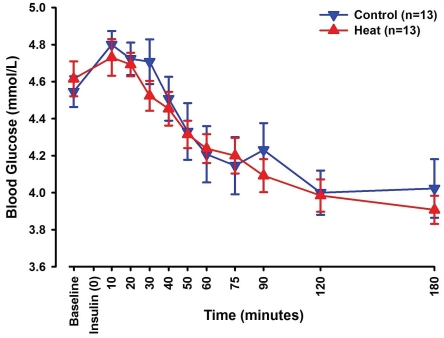
Blood glucose levels changed significantly over time (p<0.001), while no differences were observed between the control and the heat sessions (Figure 6).
Figure 6.
Line-scatter graph of blood glucose levels measured in the blood samples collected at designated time points. The blue graph depicts changes seen in control sessions, while the red graph depicts changes seen in heat sessions. Data points noted are mean values of 13 subjects with standard error of the mean.
Discussion
The present study investigated the effect of skin perfusion on the absorption of subcu-taneously administered short-acting insulin (Actrapid®). The applied local controlled heat (43°C for 60 sec) significantly elevated tissue perfusion but did not lead to any significant increase in the absorption of insulin.
In the early 80's, Koivisto reported on the effect of sauna-induced accelerated insulin absorption from subcutaneous tissue [9, 10]. Different methodological dissimilarities between the present study and those studies exist and may suggest that an intense systemic temperature elevation affects the absorption of subcutaneously administered drugs differently from controlled local heat on the injection site. In addition, diversity in concentration of injected insulin may influence the results and lead to a discrepancy between different studies. A notable difference in absorption rate exists between different concentrations of insulin preparations. Human insulin in a concentration of 100 IU/mL is absorbed less efficiently and slower than human insulin in a lower concentration e.g. 10 IU/mL. This is most likely due to increased hexamer formation, at higher insulin concentrations, which is inversely correlated to the absorption rate [15, 24]. With the use of insulin at the high concentration of 100 IU/mL, the majority of the injected insulin is present in its hexameric form. Dissociation of hexamers into dimers and monomers is required before absorption of insulin into the circulation [16]. Thereby, dissociation of hexamers into dimers and monomers probably constitutes a greater barrier to the absorption of insulin, than does limited tissue perfusion. The dissociation of hexamers to dimers and monomers is a thermosensitive process [4]. Polaschegg used human insulin in a concentration of 100 IU/mL to investigate the influence of insulin preparation temperature on the absorption rate [25] and found no difference in the absorption rate between preparation temperatures of 8°C and 37°C, suggesting that even higher temperatures are needed to significantly increase the dissociation rate of 100 IU/mL human insulin. In the present study, the insulin preparation had accommodated to 19-21 °C overnight, which fits into the conditions used in Polaschegg's study. In heat sessions, the injection site was heated to 43°C for 1 minute every 10 minutes for the first two hours of the experiment. The temperature of the injected insulin is unlikely to reach 43°C, as epidermis and dermis serve as isolating layers and it is unlikely that locally applied heat (43°C) has influenced the dissociation rate of insulin. As the slow dissociation rate provides the delayed onset of action of 100 IU/mL Actrapid®, and this process is not influenced by heat, the dissociation rate will be independent of the tissue perfusion. Thus, this provides a possible explanation as to the lack of heat induced accelerated insulin absorption observed in our study.
Tissue perfusion was monitored with LDI, which allowed assessment of blood flow in the most superficial layers of the skin. Additionally, abdominal skin temperature was measured and controlled. Skin temperature affects cutaneous blood flow, and thus it was desirable to keep skin temperature at the same level between control and heat sessions. In our study, no significant fluctuations were seen in skin temperature between control and heat sessions. Controlled local heat induced a significant increase in cutaneous blood flow, which is in accordance with our previous findings [23, 26]. Increased blood flow, however, did not accelerate the absorption of subcutaneously administered insulin presumably due to methodological limitations e.g. the type of insulin utilized or the applied heat pattern. In both control and heat sessions, there was a minor tendency for blood flow to decline over time which is in agreement with our previous finding that repeated application of mild heat can maintain the elevated tissue perfusion with minimum fluctuations [23].
Enhanced drug absorption, through increased tissue perfusion, depends on several factors for instance the anatomy of the vessel plexuses in the skin. The organization of cutaneous micro-circulation has been described in two plexus of arterioles: an upper horizontal plexus located in the papillary dermis just below the epidermis, and a lower horizontal plexus located in the deep dermis at the border of the underlying subcutaneous tissue [27]. Each plexus of arterioles gives rise to various networks of capillaries. Vessels of the lower horizontal plexus are generally thicker and less abundant than those of the upper horizontal plexus. In the present study, insulin is injected subcutaneously. Thus, the majority of the insulin absorption is thought to happen through vessels of the lower horizontal plexus and not the upper plexus. Application of heat increases the perfusion and permeability of both plexuses, but due to the anatomy, there is probably more potential for heat-induced increased drug absorption into vessels of the upper plexus, as the total surface area of these vessels is larger. Therefore, heat presumably has more potential as an enhancer in drug delivery systems, where drugs are absorbed by vessels in the upper horizontal plexus, such as transdermal drug delivery. We have recently shown that application of mild controlled heat (every 5 min for 30 min) significantly enhanced the delivery of nicotine from a patch [26].
In summary, the present study showed that controlled local heat did not accelerate the absorption of short-acting insulin (Actrapid®) from a subcutaneous depot. This finding suggests that tissue perfusion is not the rate-limiting factor in the absorption of high-concentration short-acting insulin. Instead, dissociation of insulin hexamers into dimers and monomers is assumed to constitute a greater barrier to absorption.
The influence of heat on the rate of absorption should be investigated further in order to validate controlled local heat application as a method in subcutaneous drug delivery. Insulin analogues serve this purpose ideally, as they are only present on absorbable forms, whereby dissociation should not constitute a limiting factor.
Acknowledgments
The authors are grateful to the staff at CCBR and C4Pain, Aalborg for their kind collaboration and excellent assistance.
The present study received financial support from the Center for Sensory-Motor Interaction (SMI), Aalborg University, Denmark.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Kimura T, Higaki K. Gastrointestinal transit and drug absorption. Biol Pharm Bull. 2002;25:149–164. doi: 10.1248/bpb.25.149. [DOI] [PubMed] [Google Scholar]
- 2.Woodley JF. Enzymatic barriers for GI peptide and protein delivery. Crit Rev Ther Drug Carrier Syst. 1994;11:61–95. [PubMed] [Google Scholar]
- 3.Malik DK, Baboota S, Ahuja A, Hasan S, Ali J. Recent advances in protein and peptide drug delivery systems. Curr Drug Deliv. 2007;4:141–151. doi: 10.2174/156720107780362339. [DOI] [PubMed] [Google Scholar]
- 4.Chen JW, Christiansen JS, Lauritzen T. Limitations to subcutaneous insulin administration in type 1 diabetes. Diabetes Obes Metab. 2003;5:223–233. doi: 10.1046/j.1463-1326.2003.00266.x. [DOI] [PubMed] [Google Scholar]
- 5.Guerci B, Sauvanet JP. Subcutaneous insulinpharmacokinetic variability and glycemic variability. Diabetes Metab. 2005;31:4S7–4S24. doi: 10.1016/s1262-3636(05)88263-1. [DOI] [PubMed] [Google Scholar]
- 6.Scheindlin S. Transdermal drug delivery: PAST, PRESENT, FUTURE. Mol Interv. 2004;4:308–312. doi: 10.1124/mi.4.6.1. [DOI] [PubMed] [Google Scholar]
- 7.Vanakoski J, Seppala T. Heat exposure and drugs. A review of the effects of hyperthermia on pharmacokinetics. Clin Pharmacokinet. 1998;34:311–322. doi: 10.2165/00003088-199834040-00004. [DOI] [PubMed] [Google Scholar]
- 8.Koivisto VA, Fortney S, Hendler R, Felig P. A rise in ambient temperature augments insulin absorption in diabetic patients. Metabolism. 1981;30:402–405. doi: 10.1016/0026-0495(81)90122-0. [DOI] [PubMed] [Google Scholar]
- 9.Koivisto VA. Sauna-induced acceleration in insulin absorption. Br Med J. 1980;281:621–622. doi: 10.1136/bmj.281.6240.621-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koivisto VA. Influence of heat on insulin absorption: different effects on amorphous and soluble insulins. Acta Diabetol Lat. 1983;20:175–178. doi: 10.1007/BF02624918. [DOI] [PubMed] [Google Scholar]
- 11.Cuppers HJ, Berchtold P, Berger M. Sauna-induced acceleration in insulin absorption. Br Med J. 1980;281:307. doi: 10.1136/bmj.281.6235.307-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hull W. Heat-Enhanced Transdermal Drug Delivery: A Survey Paper. The journal of applied research in clinical and experimental therapeutics. 2002;2:1–9. [Google Scholar]
- 13.Sindelka G, Heinemann L, Berger M, Frenck W, Chantelau E. Effect of insulin concentration, subcutaneous fat thickness and skin temperature on subcutaneous insulin absorption in healthy subjects. Diabetologia. 1994;37:377–380. doi: 10.1007/BF00408474. [DOI] [PubMed] [Google Scholar]
- 14.Raz I, Weiss R, Yegorchikov Y, Bitton G, Nagar R, Pesach B. Effect of a local heating device on insulin and glucose pharmacokinetic profiles in an open-label, randomized, two-period, one-way crossover study in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Clin Ther. 2009;31:980–987. doi: 10.1016/j.clinthera.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Home PD, Barriocanal L, Lindholm A. Comparative pharmacokinetics and pharmacodynamics of the novel rapid-acting insulin analogue, insulin aspart, in healthy volunteers. Eur J Clin Pharmacol. 1999;55:199–203. doi: 10.1007/s002280050618. [DOI] [PubMed] [Google Scholar]
- 16.Kaku K, Matsuda M, Urae A, Irie S. Pharmacokinetics and pharmacodynamics of insulin aspart, a rapid-acting analog of human insulin, in healthy Japanese volunteers. Diabetes Res Clin Pract. 2000;49:119–126. doi: 10.1016/s0168-8227(00)00151-0. [DOI] [PubMed] [Google Scholar]
- 17.Siekmann H. Recommended maximum temperatures for touchable surfaces. Appl Ergon. 1990;21:69–73. doi: 10.1016/0003-6870(90)90076-a. [DOI] [PubMed] [Google Scholar]
- 18.Eun HC. Evaluation of skin blood flow by laser Doppler flowmetry. Clin Dermatol. 1995;13:337–347. doi: 10.1016/0738-081x(95)00080-y. [DOI] [PubMed] [Google Scholar]
- 19.Choi CM, Bennett RG. Laser Dopplers to determine cutaneous blood flow. Dermatol Surg. 2003;29:272–280. doi: 10.1046/j.1524-4725.2003.29042.x. [DOI] [PubMed] [Google Scholar]
- 20.Wright CI, Kroner CI, Draijer R. Non-invasive methods and stimuli for evaluating the skin's microcirculation. J Pharmacol Toxicol Methods. 2006;54:1–25. doi: 10.1016/j.vascn.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Lima A, Bakker J. Noninvasive monitoring of peripheral perfusion. Intensive Care Med. 2005;31:1316–1326. doi: 10.1007/s00134-005-2790-2. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JM, Kellogg DL., Jr Thermoregulatory and thermal control in the human cutaneous circulation. Front Biosci (Schol Ed) 2:825–853. doi: 10.2741/s105. [DOI] [PubMed] [Google Scholar]
- 23.Gazerani P, Arendt-Nielsen L. Cutaneous vasomotor reactions in response to controlled heat applied on various body regions of healthy humans: evaluation of time course and application parameters. Int J Physiol Pathophysiol Pharmacol. 2011;3:202–209. [PMC free article] [PubMed] [Google Scholar]
- 24.Nucci G, Cobelli C. Models of subcutaneous insulin kinetics. A critical review. Comput Methods Programs Biomed. 2000;62:249–257. doi: 10.1016/s0169-2607(00)00071-7. [DOI] [PubMed] [Google Scholar]
- 25.Polaschegg E. Effect of physicochemical variables of regular insulin formulations on their absorption from the subcutaneous tissue. Diabetes Res Clin Pract. 1998;40:39–44. doi: 10.1016/s0168-8227(98)00021-7. [DOI] [PubMed] [Google Scholar]
- 26.Petersen KK, Rousing ML, Jensen C, Arendt-Nielsen L, Gazerani P. Effect of local controlled heat on transdermal delivery of nicotine. Int J Physiol Pathophysiol Pharmacol. 2011;3:236–242. [PMC free article] [PubMed] [Google Scholar]
- 27.Braverman IM. The cutaneous microcirculation. J Investig Dermatol Symp Proc. 2000;5:3–9. doi: 10.1046/j.1087-0024.2000.00010.x. [DOI] [PubMed] [Google Scholar]