Abstract
In a variation of the classical Ugi reaction, an acid promoted reaction between imines and isocyanides forms both 3-aminoindoles and substituted indoxyls. A recently reported triflyl phosphoramide is shown to be critical to obtain high yields under mild conditions.
Keywords: Multicomponent Reactions, Synthetic Methods, Isocyanides, Heterocycles
1. Introduction
The nitrilium ion has been studied since the earliest days of organic chemistry, although the preparation of a stable nitrilium salt was not reported until 1955 by Klages and Meerwein.1 As a result of this longstanding interest, a variety of powerful transformations are based on nitrilium chemistry. These reactions typically feature the attack of a heteroatom- or carbon-based nucleophile at the electrophilic carbon of a nitrilium ion.2 They differ, however, in the mechanisms by which the nitrilium ion is produced. In the Beckmann rearrangement, a nitrilium species is generated by migration of a carbon-carbon bond (see ref. 3). The Houben-Hoesch cyclization4 (or Hoesch reaction5) utilizes protonated or alkylated nitriles to generate aryl ketones, and is a relative of the Friedel-Crafts reaction. The Ritter reaction relies on the addition of water to a nitrilium ion generated by the reaction between a nitrile and a carbocation as a general method for the aminoacylation of carbocations.6 A closely related variant, the Pinner reaction, involves the reaction between protonated nitriles and alcohols to generate imidate esters.7 The Bischler-Napieralski reaction is another common transformation that relies on dehydration of an amide or removal of the halide from an imidoyl halide to generate the putative nitrilium ion.8 A mild generation of nitrilium ions is also at the heart of some powerful multicomponent couplings in organic synthesis; mechanistically, the addition of an isocyanide to an oxocarbenium or iminium ion to generate a reactive nitrilium intermediate is a central step of the Passerini and Ugi reactions, respectively.9 These multicomponent reactions are of particular interest due to their wide functional group tolerance and ability to rapidly generate chemical diversity. In many cases, multiple carbon-carbon bonds, carbon-heteroatom bonds, and a stereogenic center are formed in a single operation. New variations of these nitrilium-centered reactions are of interest to the general field of chemical synthesis as they often prove useful for the rapid construction of complex, biologically active molecules.
2. Results and Discussion
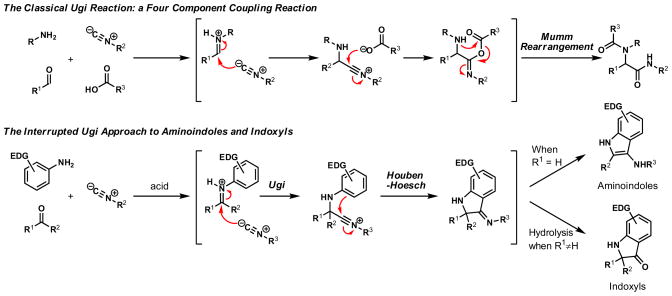
In the course of planning a synthesis of a structurally complex natural product, we were intrigued by the possibility that a nitrilium ion generated by the addition of an isocyanide to an iminium ion might be intercepted by an electron-rich arene. Such a variation on the classical Ugi reaction would result in the formation of an additional carbon-carbon bond, and, in the case of an intramolecular reaction, a new heterocyclic ring. This interrupted Ugi reaction would combine mechanistic elements of both the Ugi and Houben-Hoesch reactions. Prior studies on the Houben-Hoesch reaction have established that the reaction between a protonated nitrile and an electron-rich arene can be quite efficient. Livinghouse10 and Townsend11 have also established that electron-rich arenes react with acylated isocyanides or alkylated nitriles to form hydroquinoline derivatives and benzophenones, respectively. Other related transformations have been reported as well.5, 12–14 While these reaction manifolds have proven useful, a general strategy focused on generation of the reactive nitrilium intermediate by addition of an isocyanide to an activated imine, followed by intramolecular trapping with a pendant arene could give direct access to different structural classes of useful heterocyclic products.
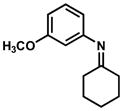
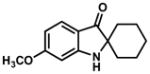
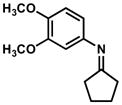
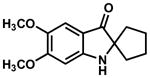
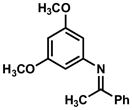
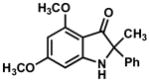
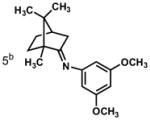
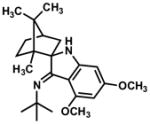
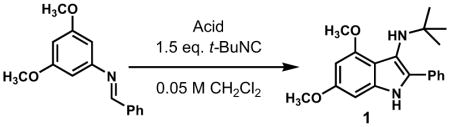
In practice, such a transformation might be accomplished by replacing the carboxylic acid component of the Ugi reaction with a Lewis acid or strong Brønsted acid and employing an aniline as the amine component. In the absence of a nucleophilic counterion in the reaction mixture, the α–amino nitrilium ion would be free to react selectively with the pendant nucleophilic π system. In the case of aldimines, the resultant cyclized imines could tautomerize to form 3-aminoindoles. The analogous ketimine-derived intermediates could lead to the production of indoxyls after hydrolysis of the initially formed bicyclic imine (Figure 1). A previous report has shown that it is feasible to generate 3-aminoindoles through this type of cyclization. Two substrates were reported and the reaction was shown to require several days with concentrated strong acid and/or at high temperature (100 °C): yields ranged from 27–68%.15 We felt that a milder procedure which also extended the reaction scope to indoxyls would be of general interest. Our first attempts to study the reaction focused on the condensation of benzaldehyde, 3,5-dimethoxyaniline, and t-butyl isocyanide (Table 1).
Figure 1.
Ugi-type reactions.
Table 1.
Screen of acids for the interrupted Ugi reaction
 | |||
|---|---|---|---|
| Entry | Acid | Equivalents | Yield % |
| 1 | None | N.A. | NR |
| 2 | HCl | 1.0 | 50 |
| 3 | Amberlyst-15 | 1.0 | NR |
| 4 | HBF4•OEt2 | 1.0 | 86 |
| 5 | Tf2NH | 1.1 | 46 |
| 6 | TfOH | 1.1 | 62 |
| 7 | TfOH | 0.3 | 42 |
| 8 | 2 | 1.1 | 96 |
| 9 | Sc(OTf)3 | 0.1 | 34 |
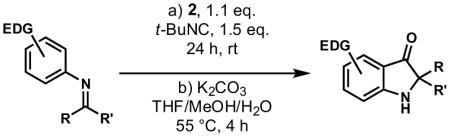
As has been observed in other studies of the Ugi reaction, we found that an imine synthesis by precondensation of the aniline and carbonyl components markedly improved the chemical yield of 3-aminoindole 1 and minimized the formation of undesired side products. A variety of Brønsted and Lewis acids were screened (Table 1). Moderate yields were observed with mineral acids such as hydrochloric acid (entry 2), although no reaction was observed with solid supported sulfonic acids such as Amberlyst-15 (entry 3). Other acids such as p-toluenesulfonic acid, camphorsulfonic acid or carboxylic acids such as trifluoroacetic or pivalic acid all provided only moderate yields of compound 1 (not shown). Some improvement was noted with stronger acids such as HBF4•OEt2 or triflic acid (entries 4, 6–7). Although Lewis acids such as scandium(III) triflate also catalyzed the reaction (entry 9), yields were generally superior with Brønsted acids. The most notable increase in yield was observed with the use of acid 2, a recently reported triflylphosphoramide.16 Acid 2 appears to provide a strong Brønsted acid with a non-nucleophilic counterion, and is surprisingly mild. Under optimized conditions, aminoindole 1 was prepared in 2 h at rt in 96% yield.
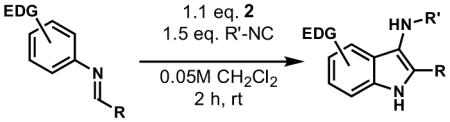
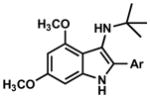
We examined the substrate scope of the reaction by studying a variety of arenes, aldehydes, and isocyanides (Table 2). In general, aldimines were prepared by condensation in toluene in the presence of molecular sieves and used without purification. The reaction was found to be tolerant of a number of substrates. Imines derived from a variety of substituted benzaldehydes (Table 2, entries 1–4) as well as ethyl glyoxalate (entries 9–17) performed well in the reaction. Branched and linear aliphatic aldimines were also shown to provide aminoindoles (entries 5, 6). Substituted alkyl and electron-rich aryl isocyanides such as p-methoxyphenyl isocyanide were also successful (entry 14–17). An attempt to use p-nitrophenyl isocyanide provided no product, presumably due to the low nucleophilicity of this compound. Although several highly electron-rich anilines provided 3-aminoindoles in general with good yield, the parent aniline (entry 9) was considerably less reactive. However, this yield could be improved with prolonged reaction time and a stronger acid.15, 17
Table 2.
Preparation of 3-aminoindoles
 | ||
|---|---|---|
| Entry | Product | Yield[%] |
| 1–4 |
|
1. Ar = Ph, 96 2. Ar = o-BrC6H4, 80 3. Ar = p-ClC6H4, 86 4. Ar = p-NO2C6H4, 81 |
| 5 |
|
81 |
| 6 |
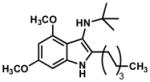
|
46 |
| 7 |
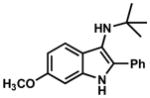
|
40 |
| 8 |
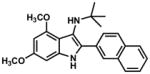
|
48 |
| 9a |
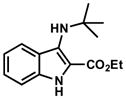
|
12 |
| 10 |
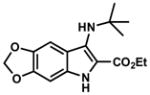
|
53 |
| 11–12 |
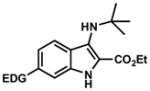
|
11. EDG = OCH3, 30 12. EDG = SCH3, 40 |
| 13 |
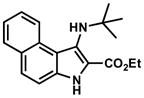
|
74 |
| 14–17 |
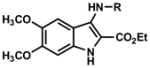
|
14. R = t-Bu, 72 15. R = p-OCH3C6H4, 25 16. R = Cy, 62 17. R = (S)-(CH3)CHC6H5, 68 |
1.1 equiv of HBF4•OEt2 was used in place of acid 2.
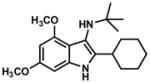
The interrupted Ugi reaction is also useful in syntheses of indoxyls (Table 3). The mild reaction conditions for these substrates are particularly notable given the formation of a fully substituted carbon and stand in contrast to the notoriously harsh conditions often encountered in the Houben-Hoesch reaction. Ketimines were generally found to require 24 hours of reaction time at room temperature under reaction conditions otherwise identical to those of aldimines. The electron-rich, sterically hindered imine intermediates initially proved resistant to acidic hydrolysis and slowly decomposed under prolonged heating in 6 N HCl. After some experimentation, it was found that the key hydrolysis step could be accomplished smoothly by treatment of the imines with 10 equiv of K2CO3 in a warm THF/MeOH/H2O mixture.18 Several substituted indoxyls were prepared in this manner (Table 3). Of particular note is the highly hindered, camphor-derived substrate (Table 3, entry 5). In this case, a product containing vicinal fully substituted carbons was easily formed, and the imine product was isolated by column chromatography.
Table 3.
Preparation of indoxyls
1.1 equiv of HBF4•OEt2 was used in place of acid 2.
Reaction time: 36 h, dr 5:1, relative stereochemistry assigned by nOe correlation.
3. Conclusions
In conclusion, we have developed a mild transformation combining mechanistic elements of the Ugi reaction and the Houben-Hoesch cyclization. Two new carbon-carbon bonds and a heterocyclic ring are formed in a single operation. The use of the recently reported, strong Brønsted acid 2 proved critical to obtaining high yields under mild conditions. In many cases, the cyclization is observed to occur at room temperature, often in as little as two hours. The acid-promoted reaction is straightforward to execute and useful in the preparation of a wide variety of 3-aminoindoles as well as several highly substituted indoxyls. It is anticipated that this reaction will find broad use in the preparation of biologically active molecules and natural products. A more complete study detailing the scope of indoxyl formation, efforts to achieve stereoselective reactions, and the application of the interrupted Ugi reaction in natural product synthesis is currently underway in our laboratory.
4. Experimental Section
4.1 General
All reactions were carried out under an atmosphere of Ar or N2 unless otherwise specified. Tetrahydrofuran (THF), toluene, and methylene chloride (CH2Cl2) were dried by passing through activated alumina columns. Commercial reagents of high purity were purchased and used without further purification, unless otherwise noted. Reactions were monitored by thin-layer chromatography (TLC) carried out on 0.25 mm Merck HPTLC silica gel plates (60 F254) using UV light as a visualizing agent and aqueous ceric sulfate/phosphomolybdic acid, ceric ammonium nitrate solution, ethanolic p-anisaldehyde solution, or potassium permanganate and heat as developing agents. E. Merck silica gel 60 (230–400 mesh) was used for flash column chromatography. FT-IR spectra were obtained on a Perkin–Elmer Paragon 500 FT–IR. NMR spectra were obtained on Bruker DRX–600, DRX–500, AMX–400, Varian Inova–500, and Inova–400 instruments and calibrated to the residual solvent peak. The multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, m = multiplet, aps = apparent singlet, apd = apparent doublet, apt = apparent triplet, brs = broad signal. Optical rotations were recorded on a Perkin-Elmer model 241 polarimeter using a 1 mL, 1 dm cell. Mass spectra were obtained using an Agilent 6210 Time of Flight-LC/MS.
4.2 General Procedure for the Synthesis of 3-aminoindoles (N-tert-butyl-4,6-dimethoxy-2-phenyl-1H-indol-3-amine, Table 2, Entry 1)
300 mg of powdered 4Å molecular sieves were added to a stirred solution of 3,5-dimethoxyaniline (50 mg, 0.33 mmol, 1 equiv) and benzaldehyde (35 mg, 0.33 mmol, 1 equiv.) in toluene (300 μL). The resulting suspension was stirred at 23 °C for 1 h, filtered through Celite, and the filtrate was concentrated to afford the crude imine which was used without further purification. tert-Butylisocyanide (41 mg, 0.49 mmol, 1.5 equiv) and 137 mg acid 2 (0.36 mmol, 1.1 equiv) in 3 mL dichloromethane (final concentration 0.05M) were added via syringe to a stirred solution of the crude imine in dichloromethane (3.6 mL, final concentration 0.05M) at 23 °C. The resulting orange solution was stirred for 2 h, and then partitioned between saturated aqueous sodium bicarbonate solution (10 mL) and dichloromethane. The organic layer was separated, and the aqueous layer was extracted with dichloromethane (2 x 10 mL). The combined organic extracts were dried over anhydrous sodium sulfate, and the dried solution was concentrated. Purification by flash column chromatography (4:1 hexanes:ethyl acetate) afforded 102 mg of aminoindole (96%) as an amorphous yellow solid.
4.2.1. N-tert-butyl-4,6-dimethoxy-2-phenyl-1H-indol-3-amine (Table 2, Entry 1)
TLC: Rf = 0.45 (20% EtOAc/Hexanes); IR (film) 3349 (br), 2967, 2931, 2901, 2838, 1624, 1603, 1514, 1494, 1465, 1453, 1431, 1388, 1360, 1350, 1295, 1265, 1219, 1201, 1150, 1130, 1072, 1043 cm−1; 1H NMR (500MHz, CDCl3) δ 7.88 (d, J = 7.3, 2H), 7.68 (brs N-H, 1H), 7.37 (apt, J = 7.73, 2H), 7.21 (t, J = 7.3, 1H), 6.33 (s, 1H), 6.09 (s, 1H), 3.83 (s, 3H), 3.75 (s, 3H), 3.25–3.45 (brs N-H, 1H), 0.91 (s, 9H); 13C NMR (125MHz, CDCl3) δ 157.4, 154.9, 135.8, 134.4, 128.3, 127.1, 126.9, 126.3, 121.2, 112.5, 91.4, 86.5, 55.8, 55.5, 54.8, 29.8; HRMS (ESI-TOF) C20H24N2O2 Calculated for [M+H]+ 325.1916, found 325.1913.
4.2.2. 2-(2-bromophenyl)-N-(tert-butyl)-4,6-dimethoxy-1H-indol-3-amine (Table 2, Entry 2)
TLC: Rf = 0.80 (50% EtOAc/Hexanes); IR (film) 3179, 2958, 2900, 2856, 1614, 1564, 1523, 1492, 1433, 1360, 1310, 1283, 1217, 1201, 1152, 1131, 1022 cm−1; 1H NMR (500MHz, CDCl3) δ 7.98 (brs N-H, 1H), 7.80 (dd, J =, 1.7, 7.7, 1H), 7.62 (dd, J = 1.1, 8.0, 1H), 7.32 (dt, J = 4.4, 12.0), 7.15 (dt, J =, 1.8, 3.7, 1H), 6.42 (s, 1H), 6.17 (s, 1H), 3.91 (s, 3H), 3.83 (s, 3H), 3.55 (brs, N-H, 1H), 0.91 (s, 9H); 13C NMR (125MHz, CDCl3) δ 157.6, 155.0, 135.3, 135.0, 133.8, 133.1, 128.7, 127.3, 125.1, 122.7, 122.2, 110.9, 91.3, 86.4, 55.6, 55.5, 54.9, 29.6 ; HRMS (ESI-TOF) C20H23BrN2O2 Calculated for [M+H]+ 403.1021, found 403.1005.
4.2.3. N-(tert-butyl)-2-(4-chlorophenyl)-4,6-dimethoxy-1H-indol-3-amine (Table 2, Entry 3)
TLC: Rf = 0.90 (50% EtOAc/Hexanes); IR (film) 3349, 2968, 2840, 1625, 1603, 1491, 1465, 1295, 1277, 1218, 1201, 1151, 1130 cm−1; 1H NMR (500MHz, CDCl3) δ 7.85 (d, J = 8.6, 2H), 7.74 (brs N-H, 1H), 7.31 (d, J = 8.6 2H), 6.39 (s, 1H), 6.16 (s, 1H), 3.89 (s, 3H), 3.82 (s, 3H), 0.98 (s, 9H); 13C NMR (125MHz, CDCl3) δ 157.6, 154.9, 136.0, 132.9, 131.6, 128.5, 128.0, 126.0, 121.7, 112.5, 91.6, 86.6, 55.9, 55.5, 54.8, 29.8; HRMS (ESI-TOF) C20H23ClN2O2 Calculated for [M+H]+ 359.1526, found 359.1529.
4.2.4. N-(tert-butyl)-4,6-dimethoxy-2-(4-nitrophenyl)-1H-indol-3-amine (Table 2, Entry 4)
TLC: Rf = 0.55 (50% EtOAc/Hexanes); IR (film) 3401, 2966, 1625, 1589, 1509, 1332, 1292, 1271, 1219, 1201, 1151, 1130, 1108 cm−1; 1H NMR (500MHz, CDCl3) δ 8.19 (d, J = 9.0, 2H), 8.13 (d, J = 9.0, 2H), 7.83 (brs, N-H, 1H), 6.40 (s, 1H), 6.16 (s, 1H), 3.91 (s, 3H), 3.84 (s, 3H), 0.98 (s, 9H); 13C NMR (125MHz, CDCl3) δ 158.7, 155.3, 145.0, 140.8, 137.1, 126.2, 125.5, 124.8, 123.9, 112.7, 92.1, 86.4, 56.6, 55.6, 54.7, 29.8; HRMS (ESI-TOF) C20H23N3O4 Calculated for [M+H]+ 370.1766, found 370.1765.
4.2.5. N-tert-butyl-2-cyclohexyl-4,6-dimethoxy-1H-indol-3-amine (Table 2, Entry 5)
TLC: Rf = 0.55 (50% EtOAc/Hexanes); IR (film) 3350 (br), 2930, 2852, 1627, 1595, 1519, 1456, 1299, 1199, 1153, 1095, 935 cm−1; 1H NMR (500MHz, CDCl3) δ 7.49 (brs, N-H, 1H), 6.37 (s, 1H) 6.14 (s, 1H), 3.87 (s, 3H), 3.80 (s, 3H), 3.25 (brs, N-H, 1H), 2.97 (m, 1H), 1.73–1.94 (m, 5H), 1.47–1.33 (m, 4H), 1.26 (m, 1H), 1.15 (s, 9H); 13C NMR (125MHz, CDCl3) δ 156.5, 154.1, 134.9, 134.8, 117.1, 111.5, 91.0, 86.7, 55.6, 54.7, 54.0, 34.4, 33.8, 29.7, 26.7, 26.2; HRMS (ESI-TOF) C20H30N2O2 Calculated for [M+H]+ 331.2385, found 331.2384.
4.2.6. N-tert-butyl-2-heptyl-4,6-dimethoxy-1H-indol-3-amine (Table 2, Entry 6)
TLC: Rf = 0.75 (50% EtOAc/Hexanes); IR (film) 3204 (br), 2956, 2927, 2856, 1627, 1515, 1456, 1387, 1360, 1302, 1201, 1154, 1129, 1051 cm−1; 1H NMR (500MHz, CDCl3) δ 7.53 (brs, N-H, 1H), 6.36 (s, 1H), 6.14 (s, 1H), 3.87 (s, 3H), 3.80 (s, 3H), 2.71 (apt, J = 8.1, 2H), 1.61 (m, 2H), 1.20–1.44 (m, 8H), 1.13 (s, 9H), 0.88 (t, J = 6.9, 3H); 13C NMR (125MHz, CDCl3) δ 156.4, 154.0, 135.0, 129.9, 118.4, 111.4, 90.9, 86.5, 55.6, 54.7, 54.0, 31.8, 29.7, 29.7, 29.5, 29.2, 26.0, 22.6, 14.1; HRMS (ESI-TOF) C21H34N2O2 Calculated for [M+H]+ 347.2698, found 347.2696.
4.2.7. N-tert-butyl-6-methoxy-2-phenyl-1H-indol-3-amine (Table 2, Entry 7)
TLC: Rf = 0.48 (20% EtOAc/Hexanes); IR (film) 3349, 2968, 1622, 1166 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.81 (dd, J = 8.3, 1.1 Hz, 3 H), 7.50 (d, J = 8.6 Hz, 1 H), 7.43 (t, J = 7.8 Hz, 2 H), 7.29 (t, J = 7.5 Hz, 1 H), 6.83 (d, J = 2.2 Hz, 1 H), 6.80 (dd, J = 8.7, 2.2 Hz, 1 H), 3.87 (s, 3 H), 2.84 (br s, 1 H), 1.05 (s, 9 H); 13C NMR (125 MHz, CDCl3) δ 156.5, 135.3, 133.9, 130.2, 128.6, 127.2, 126.9, 123.0, 120.2, 109.4, 94.2, 55.7, 55.1, 30.4; HRMS (ESI-TOF) C19H23N2O Calculated for [M+H]+ 295.1810, found 295.1811.
4.2.8. N-tert-butyl-4,6-dimethoxy-2-(naphthalen-2-yl)-1H-indol-3-amine (Table 2, Entry 8)
TLC: Rf = 0.90 (50% EtOAc/Hexanes); IR (film) 3351 (br), 2958, 2904, 2387, 1734, 1601, 1507, 1453, 1427, 1387, 1293, 1266, 1219, 1199, 1146, 1126, 1032 cm−1; 1H NMR (500MHz, CDCl3) δ 8.34 (d, J = 8.5, 1H), 8.09 (s, 1H), 7.88 (brs, N-H, 1H), 7.78–7.85 (m, 3H), 7.40–7.48 (m, 2H), 6.44 (s, 1H), 6.18 (s, 1H), 3.92 (s, 3H), 3.85 (s, 3H), 3.53 (brs, N-H, 1H), 1.00 (s, 9H); 13C 157.6, 155.0, 136.2, 133.6, 132.3, 127.7, 127.6, 127.6, 127.0, 126.3, 126.0, 125.4, 123.7, 122.0, 112.7, 91.5, 86.6, 55.9, 55.6, 54.8, 29.9; NMR (125MHz, CDCl3) δ ; HRMS (ESI-TOF) C24H26N2O2 Calculated for [M+H]+ 375.2067, found 375.2062.
4.2.9. Ethyl 3-(tert-butylamino)-1H-indole-2-carboxylate (Table 2, Entry 9)
TLC: Rf = 0.30 (25% EtOAc/Hexanes); IR (film); 3154, 2976, 1689, 1544, 1252, 1098 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.34 (br s, 1 H), 7.84 (d, J = 8.3 Hz, 1 H), 7.30 (m, 2 H), 7.08 (ddd, J = 8.1, 4.9, 3.0 Hz, 1 H), 4.42 (q, J = 7.1 Hz, 2 H), 1.44 (t, J = 7.1 Hz, 3 H), 1.35 (s, 9 H); 13C NMR (125 MHz, CDCl3) δ 162.7, 135.9, 134.0, 125.9, 123.2, 119.4, 116.5, 111.8, 60.4, 54.5, 30.7, 29.8, 14.6; HRMS (ESI-TOF) C15H21N2O2 Calculated for [M+H]+ 261.1603, found 261.1598.
4.2.10. Ethyl 7-(tert-butylamino)-5H-[1,3]dioxolo[4,5-f]indole-6-carboxylate (Table 2, Entry 10)
TLC: Rf = 0.30 (25% EtOAc/Hexanes); IR (film); 3328, 2975, 1682, 1465, 1251, 1038 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.22 (br s, 1 H), 7.15 (s, 1 H), 6.70 (s, 1 H), 5.96 (s, 2 H), 4.67 (br s, 1 H), 4.38 (q, J = 7.1 Hz, 2 H), 1.41 (t, J = 7.1 Hz, 3 H), 1.29 (s, 9 H); 13C NMR (125 MHz, CDCl3) δ 162.3, 148.2, 143.2, 132.0, 118.6, 116.5, 101.0, 100.4, 91.5, 60.2, 54.7, 30.6, 14.6; HRMS (ESI-TOF) C16H21N2O4 Calculated for [M+H]+ 305.1501, found 305.1499.
4.2.11. Ethyl 3-(tert-butylamino)-6-methoxy-1H-indole-2-carboxylate (Table 2, Entry 11)
TLC: Rf = 0.25 (20% EtOAc/Hexanes); IR (film) 3336, 2972, 1684, 1628, 1254, 1095 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.20 (br s, 1 H), 7.70 (d, J = 9.0 Hz, 1 H), 6.73 (dd, J = 9.0, 2.1 Hz, 1 H), 6.69 (d, J = 2.1 Hz, 1 H), 5.02 (br s, 1 H), 4.39 (q, J = 7.1 Hz, 2 H), 3.84 (s, 3 H), 1.42 (t, J = 7.1 Hz, 3 H), 1.34 (s, 9 H); 13C NMR (125 MHz, CDCl3) δ 162.6, 159.2, 137.2, 124.2, 118.1, 114.9, 110.7, 93.3, 60.1, 55.4, 54.3, 30.7, 29.8, 14.6; HRMS (ESI-TOF) C16H23N2O3 Calculated for [M+H]+ 291.1708, found 291.1704.
4.2.12. Ethyl 3-(tert-butylamino)-6-(methylthio)-1H-indole-2-carboxylate (Table 2, Entry 12)
TLC: Rf = 0.42 (25% EtOAc/Hexanes); IR (film); 3337, 2974, 1673, 1560, 1230, 675 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.16 (br s, 1 H), 7.73 (d, J = 8.6 Hz, 1 H), 7.14 (s, 1 H), 7.00 (d, J = 8.7 Hz, 1 H), 4.96 (br s, 1 H), 4.40 (q, J = 7.1 Hz, 2 H), 2.54 (s, 3 H), 1.43 (t, J = 7.1 Hz, 3 H), 1.34 (s, 9 H); 13C NMR (125 MHz, CDCl3) δ 162.6, 136.5, 136.4, 123.4, 121.7, 119.2, 115.8, 108.5, 60.4, 54.4, 30.6, 16.1, 14.5; HRMS (ESI-TOF) C16H23N2O2S Calculated for [M+H]+ 307.1480, found 307.1474.
4.2.13. Ethyl 1-(tert-butylamino)-3H-benzo[e]indole-2-carboxylate (Table 2, Entry 13)
TLC: Rf = 0.30 (25% EtOAc/Hexanes); IR (film) 3310, 2969, 1668, 1430, 1263, 1130 cm−1; 1H NMR (500 MHz, CDCl3) δ 9.27 (d, J = 8.3 Hz, 1 H) 9.05 (br s, 1 H), 7.85 (d, J = 8.0 Hz, 1 H), 7.66 (d, J = 8.9 Hz, 1 H), 7.56 (t, J = 7.6 Hz, 1 H), 7.43 (t, J = 7.5 Hz, 1 H), 7.40 (d, J = 8.9 Hz, 1 H), 4.46 (q, J = 7.1 Hz, 2 H), 4.17 (br s, 1 H), 1.45 (t, J = 7.1 Hz, 3 H), 1.26 (s, 9 H); 13C NMR (125 MHz, CDCl3) δ 162.4, 133.9, 132.7, 129.8, 129.5, 128.3, 127.7, 125.5, 125.0, 123.7, 119.4, 118.8, 112.9, 60.4, 56.8, 29.6, 14.5; HRMS (ESI-TOF) C19H23N2O2 Calculated for [M+H]+ 311.1759, found 311.1758.
4.2.14. Ethyl 3-(tert-butylamino)-5,6-dimethoxy-1H-indole-2-carboxylate (Table 2, Entry 14)
TLC: Rf = 0.15 (25% EtOAc/Hexanes); IR (film) 3348, 2971, 1684, 1540, 1256, 1173, 1094 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.59 (br s, 1 H), 7.16 (s, 1 H), 6.67 (s, 1 H), 4.70 (br s, 1 H), 4.36 (q, J = 7.1 Hz, 2 H), 3.87 (s, 3 H), 3.83 (s, 3 H), 1.37 (t, J = 7.1 Hz, 3 H), 1.28 (s, 9 H); 13C NMR (125 MHz, CDCl3) δ 162.4, 150.2, 144.8, 133.5, 131.2, 117.1, 116.1, 102.9, 93.3, 60.3, 56.0, 55.8, 54.8, 30.5, 14.5; HRMS (ESI-TOF) C17H25N2O4 Calculated for [M+H]+ 321.1814, found 321.1821.
4.2.15. Ethyl 5,6-dimethoxy-3-(4-methoxyphenylamino)-1H-indole-2-carboxylate (Table 2, Entry 15)
TLC: Rf = 0.23 (33% EtOAc/Hexanes); IR (film); 3351, 2934, 1670, 1510, 1245, 1174, 1093 cm−1;1H NMR (500 MHz, C6D6) δ 7.76 (br s, 1 H), 7.57 (br s, 1 H), 7.01 (d, J = 8.8 Hz, 2 H), 6.84 (s, 1 H), 6.70 (d, J = 8.8 Hz, 2 H), 6.09 (s, 1 H), 4.17 (q, J = 7.1 Hz, 2 H), 3.44 (s, 3 H), 3.29 (s, 3 H), 3.26 (s, 3 H), 1.05 (t, J = 7.1 Hz, 3 H); 13C NMR (125 MHz, CDCl3) δ 162.4, 155.0, 150.6, 144.0, 136.5, 131.7, 121.4, 114.1, 112.1, 110.5, 102.9, 93.6, 60.1, 56.0, 55.9, 55.6, 14.6; HRMS (ESI-TOF) C20H23N2O5 Calculated for [M+H]+ 371.1606, found 371.1594.
4.2.16. Ethyl 3-(cyclohexylamino)-5,6-dimethoxy-1H-indole-2-carboxylate (Table 2, Entry 16)
TLC: Rf = 0.12 (25% EtOAc/Hexanes); IR (film); 3356, 2931, 1665, 1489, 1256, 1172, 1096 cm−1;1H NMR (500 MHz, CDCl3) δ 7.74 (br s, 1 H), 7.10 (s, 1 H), 6.68 (s, 1 H), 4.35 (q, J = 7.1 Hz, 2 H), 3.91 (s, 6 H), 3.70 (br s, 1 H), 2.09 (d, J = 11.4 Hz, 2 H), 1.79 (d, J = 13.0 Hz, 2 H), 1.63 (d, J = 13.2 Hz, 1 H), 1.39 (t, J = 7.1 Hz, 3 H), 1.31 (m, 5 H); 13C NMR (125 MHz, CDCl3) δ 162.6, 150.8, 144.2, 132.5, 111.7, 103.0, 93.8, 59.6, 56.4, 55.9, 54.0, 34.3, 25.8, 24.8, 14.8; HRMS (ESI-TOF) C19H27N2O4 Calculated for [M+H]+ 347.1970, found 347.1968.
4.2.17. (S)-ethyl 5,6-dimethoxy-3-(1-phenylethylamino)-1H-indole-2-carboxylate (Table 2, Entry 17)
TLC: Rf = 0.30 (33% EtOAc/Hexanes); IR (film); 3357, 2977, 1663, 1490, 1336, 1257, 1174 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.69 (br s, 1 H), 7.45 (d, J = 8.2 Hz, 2 H), 7.31 (t, J = 7.7 Hz, 2 H), 7.21 (t, J = 7.4 Hz, 1 H), 6.80 (s, 1 H), 6.59 (s, 1 H), 5.01 (q, J = 6.5 Hz, 1 H), 4.39 (q, J = 7.1 Hz, 2 H), 3.85 (s, 3 H), 3.63 (s, 3 H), 1.63 (d, J = 6.8 Hz, 3 H), 1.41 (t, J = 7.1 Hz, 3 H); 13C NMR (125 MHz, CDCl3) δ 162.5, 150.5, 146.2, 143.8, 132.3, 128.6, 126.8, 125.6, 103.1, 93.4, 59.6, 56.1, 55.7, 55.5, 25.8, 14.7; [α]20D = 158 (c = 1.0, CHCl3); HRMS (ESI-TOF) C21H25N2O4 Calculated for [M+H]+ 369.1814, found 321.1815.
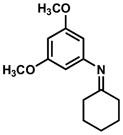
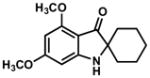
4.3 General Procedure for the Synthesis of Indoxyls (4′,6′-dimethoxyspiro[cyclohexane-1,2′-indolin]-3′-one, Table 3, Entry 1)
tert-Butylisocyanide (52 mg, 0.62 mmol, 1.5 equiv) and a solution of 175 mg acid 2 (0.46 mmol, 1.1 equiv) in 3.3 mL dichloromethane were added via syringe to a solution of imine (100mg, 0.41 mmol, 1.0 equiv) in dichloromethane (5 mL). The resulting solution was stirred at 23 °C for 24 h, then was concentrated at reduced pressure to afford an oily residue. 565 mg potassium carbonate (4.1 mmol, 10 equiv) was added to a stirred solution of the residue in tetrahydrofuran/water/methanol (1:1:1, 8 mL). A reflux condenser was attached to the flask, and the reaction mixture was heated to 55 °C for 4 h. The reaction mixture was cooled to 23 °C and the volatile solvents were evaporated under reduced pressure. The resulting aqueous solution was extracted with dichloromethane (3 x 10 mL). The combined organic extracts were dried over anhydrous sodium sulfate, and the dried solution was concentrated. Purification by flash column chromatography (3:2 hexanes:ethyl acetate) afforded 112 mg (92%) of indoxyl as a white, crystalline solid. Note: 1.1 equiv of HBF4•OEt2can be used in place of acid 2 for the preparation of indoxyls with little loss in yield. Additionally, hydrolysis conditions may decompose 2 and result in contaminating amounds of phenol if longer hydrolysis time is necessary. In this case, HBF4•OEt2 is used in place of 2.
4.3.1. 4′,6′-Dimethoxyspiro[cyclohexane-1,2′-indolin]-3′-one (Table 3, Entry 1)
TLC: Rf = 0.45 (50% EtOAc/Hexanes); IR (film) 3321 (br), 3007, 2933, 2850, 1659, 1615, 1586, 1513, 1454, 1377, 1330, 1245, 1217, 1157, 1099 cm−1; 1H NMR (500MHz, CDCl3) δ 5.89 (s, 1H), 5.78 (s, 1H), 5.09 (brs, N-H, 1H), 3.86 (s, 3H), 3.81 (s, 3H), 1.68–1.90 (m, 5H), 1.32–1.46 (m, 5H); 13C NMR (125MHz, CDCl3) δ 199.8, 169.1, 162.7, 160.1, 103.8, 90.0, 87.2, 67.5, 55.7, 55.6, 33.0, 24.7, 22.7; HRMS (ESI-TOF) C15H19NO3 Calculated for [M+H]+ 262.1433, found 262.1435.
4.3.2. 6′-Methoxyspiro[cyclohexane-1,2′-indolin]-3′-one (Table 3, Entry 2)
Note: Tetrafluoroboric acid diethyl etherate (1.1 eq.) was used. TLC: Rf = 0.40 (50% EtOAc/Hexanes); IR (film) 3342 (br), 2932, 2856, 1665, 1614, 1496, 1456, 1357, 1311, 1268, 1245, 1215, 1159, 1099, 1042, 973 cm−1; 1H NMR (500MHz, CDCl3) δ 7.52 (d, J = 8.6, 1H), 6.39 (dd, J = 8.6,2.1, 1H), 6.28 (d, J = 2.1, 1H), 5.07 (brs, N-H, 1H), 3.83 (s, 3H), 1.86 (m, 2H), 1.74 (m, 3H), 1.35–1.48 (m, 5H); 13C NMR (125MHz, CDCl3) δ 202.4, 167.5, 162.0, 126.4, 113.9, 108.6, 94.7, 67.5, 55.5, 32.9, 24.8, 22.7; HRMS (ESI-TOF) C14H17NO2 Calculated for [M+H]+ 232.1332, found 232.1330.
4.3.3. 5′,6′-Dimethoxyspiro[cyclopentane-1,2′-indolin]-3′-one (Table 3, Entry 3)
TLC: Rf = 0.30 (50% EtOAc/Hexanes); IR (film) 3312 (br), 2958, 2907, 1655, 1624, 1585, 1498, 1463, 1441, 1380, 1316, 1229, 1209, 1145, 1099, 1029, 1002 cm−1; 1H NMR (500MHz, CDCl3) δ 7.03 (s, 1H), 6.33 (s, 1H), 4.57 (brs, N-H, 1H), 3.91 (s, 3H), 3.84 (s, 3H), 2.07 (m, 2H), 1.96 (m, 2H), 1.82 (m, 2H), 1.68 (m, 2H); 13C NMR (125MHz, CDCl3) δ 203.0, 158.1, 157.4, 144.2, 112.4, 104.2, 94.7, 75.3, 56.2, 56.2, 38.1, 25.5; HRMS (ESI-TOF) C14H17NO3 Calculated for [M+H]+ 248.1281, found 248.1276.
4.3.4. 4,6-Dimethoxy-2-methyl-2-phenylindolin-3-one (Table 3, Entry 4)
TLC: Rf = 0.35 (50% EtOAc/Hexanes); IR (film) 3307 (br), 2970, 2932, 2847, 1673, 1616, 1588, 1514, 1462, 1376, 1328, 1206, 1157, 1118 cm−1; 1H NMR (500MHz, CDCl3) δ 7.48 (d, J = 7.20, 2H), 7.30 (t, J = 7.5, 2H), 7.22 (t, J = 7.3, 1H), 5.96 (s, 1H), 5.81 (s, 1H), 5.06 (brs, N-H, 1H), 3.85 (s, 3H), 3.84 (s, 3H), 1.72 (s, 3H); 13C NMR (125MHz, CDCl3) δ 197.3, 169.5, 163.0, 160.5, 140.7, 128.5, 127.4, 125.5, 102.3, 90.3, 87.0, 69.0, 55.7, 55.7, 24.5; HRMS (ESI-TOF) C17H17NO3 Calculated for [M+H]+ 284.1280, found 284.1281.
4.3.5. (±)-(E)-N-((1S,2R,4R)-4′,6′-Dimethoxy-1,7,7-trimethylspiro[bicyclo[2.2.1]heptane-2,2′-indoline]-3′-ylidene)-2-methylpropan-2-amine (Table 3, Entry 5)
TLC: Rf = 0.35 (50% EtOAc/Hexanes); IR (film) 3431 (br), 2954, 2868, 1646, 1608, 1503, 1467, 1453, 1384, 1356, 1314, 1221, 1204, 1155, 1110, 1062, 1052, 1003, 952 cm−1; 1H NMR (500MHz, CDCl3) δ 5.84 (d, J = 2.1, 1H), 5.75 (d, J = 2.1, 1H), 4.44 (brs, N-H, 1H), 3.77 (s, 3H), 3.76 (s, 3H), 2.22 (d, J = 13.0, 1H), 1.91–1.99 (m, 2H), 1.69 (t, J = 4.5, 1H), 1.44–1.55 (m, 2H), 1.35 (s, 9H), 1.11 (m, 1H), 1.06 (s, 3H), 0.84 (s, 3H), 0.57 (s, 3H); 13C NMR (125MHz, CDCl3) δ 164.4, 159.6, 158.5, 155.6, 105.0, 88.9, 87.4, 80.4, 55.6, 55.3, 54.0, 53.2, 51.7, 45.4, 42.5, 30.2, 29.4, 25.8, 20.9, 20.5, 11.2;; HRMS (ESI-TOF) C23H34N2O2 Calculated for [M+H]+ 371.2693, found 371.2688.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (GM074763), Merck Research Laboratories, Boehringer Ingelheim, a Johnson & Johnson Focused Giving Award, and Princeton University. JSS thanks the NIH for a postdoctoral fellowship (F32GM078910).
Footnotes
Dedicated with admiration to Professor Justin Dubois, recipient of the Tetrahedron Young Investigator Award.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.a) Klages F, Grill W. Liebigs Ann Chem. 1955;594:21–32. [Google Scholar]; b) Meerwein H. Angew Chem. 1955;67:374–380. [Google Scholar]; c) Meerwein H, Laasch P, Mersch R, Spille J. Chem Ber. 1956;89:209–224. [Google Scholar]
- 2.For discussions of nitrilium salt chemistry, see: Zil’berman EN. Russ Chem Rev. 1960;29(6):331–344.Kanemasa S. Science of Synthesis. 2004;(19):53–63.
- 3.a) Beckmann E. Ber Dtsch Chem Ges. 1886;19:988–993. [Google Scholar]; b) Donaruma LG, Heldt WZ. Org React. 1960;11:1–156. [Google Scholar]; c) Tatsumi T. In: Beckmann Rearrangement. Sheldon RA, Bekkum H, editors. Wiley; New York: 2001. pp. 185–204. [Google Scholar]
- 4.a) Hoesch K. Ber. 1915;48:1122–1133. [Google Scholar]; b) Houben J. Ber. 1926;59B:2878–2891. [Google Scholar]; c) Ruske W. Houben-Hoesch and Related Syntheses. In: Olah GA, editor. Friedel-Crafts and Related Reactions. Vol. 3. Interscience; New York: 1964. pp. 383–497. [Google Scholar]
- 5.For an example of a "Hoesch Reaction" relevant to this work, see: Davey AE, Schaeffer MJ, Taylor RJK. J Chem Soc Perkin Trans. 1992;1:2657–2666.
- 6.Ritter JJ, Minieri PP. J Am Chem Soc. 1948;70:4045–4048. doi: 10.1021/ja01192a022.Ritter JJ, Kalish J. J Am Chem Soc. 1948;70:4048–4050. doi: 10.1021/ja01192a023.For a review, see: Bishop R. Ritter-type Reactions. In: Trost BM, Flemming I, editors. Comp Org Synth. Vol. 6. pp. 261–300.For examples of Ritter-like reactions between nitriles and halonium ion intermediates, see Hassner A, Levy LA, Gault R. Tetrahedron Lett. 1966;(27):3119–3123.
- 7.Pinner A, Klein F. Ber. 1877;10:1889–1897.Pinner A, Klein F. Ber. 1878;11:1475–1487.For reviews, see: Zil’berman EN. Russ Chem Rev. 1962;31:615–633.Patai S, Rappaport Z, editors. The Chemistry of Amidines and Imidates. 2. Wiley; New York: 1991.
- 8.Bischler A, Napieralski B. Ber. 1893;26:1903–1908.For a Review, see: Whaley WM, Govindachari TR. Org React. 1951;6:74–150.
- 9.For comprehensive reviews on the Ugi and Passerini reactions, see: Domling A, Ugi I. Angew Chem Int Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u.Domling A. Chem Rev. 2006;106:17–89. doi: 10.1021/cr0505728.
- 10.Livingouse T. Tetrahedron. 1999;55:9947–9978.and references therein.
- 11.a) Casillas LK, Townsend CA. J Org Chem. 1999;64:4050–4059. [Google Scholar]; b) Udwary DW, Casillas LK, Townsend CA. J Am Chem Soc. 2002;124:5294–5303. doi: 10.1021/ja012185v. [DOI] [PubMed] [Google Scholar]
- 12.For other related examples of intramolecular nitrilium trapping with arenes, see: Lack O, Weber L. Chimia. 1996;50:445–447.Winkler JD, Asselin SM. Org Lett. 2006;8:3975–3977. doi: 10.1021/ol061451c.Kobayashi K, Takanohashi A, Hashimoto K, Morikawa O, Konishi H. Tetrahedron. 2006;62:10379–10382.With heteroatom nucleophiles: Groebke K, Weber L, Mehlin G. Synlett. 1998:661–663.Blackburn C. Tetrahedron Lett. 1998;39:5469–5472.Blackburn C, Guan B, Fleming P, Shiosaki K, Tsai S. Tetrahedron Lett. 1998;39:3635–3638.Bienyamé H, Bouzid K. Angew Chem Int Ed. 2005;44:6521–6525.
- 13.Harcourt DN, Hussain F, Taylor N. J Chem Soc Perkin Trans. 1986;1:1329–1338.and references therein.
- 14.For a related transformation see the Povarov reaction: Povarov LS. Russ Chem Rev. 1967;36:656.Jorgensen KA. Angew Chem. 2000;112:3702.Angew Chem Int Ed. 2000;39:3558.Kobayashi S, Akiyama R, Kitagawa H. J Comb Chem. 2001;3:196. doi: 10.1021/cc0000850.Carranco I, Díaz JL, Jiménez O, Vendrell M, Albericio F, Royo M, Lavilla R. J Comb Chem. 2005;7:33. doi: 10.1021/cc049877a.and references therein.
- 15.Deyrup JA, Vestling MM, Hagan WV, Yun HY. Tetrahedron. 1969;25:1467–1478. [Google Scholar]
- 16.Rueping M, Nachtsheim BJ, Moreth SA, Bolte M. Angew Chem Int Ed. 2008;47:593–596. doi: 10.1002/anie.200703668. [DOI] [PubMed] [Google Scholar]
- 17.There is some evidence to suggest that less electron rich arenes function by attack on a dicationic intermediate. For a discussion of dications in the Houben-Hoesch reaction, see: Sato Y, Yato M, Ohwada T, Saito S, Shudo K. J Am Chem Soc. 1995;117:3037–3043.
- 18.Modification of a procedure from reference 11a