Abstract
Increased evidence of cross-talk between NK cells and other immune cells has enhanced the possibilities of exploiting the interplay between the activation and inhibition of NK cells for immunotherapeutic purposes. The battery of receptors possessed by NK cells help them to efficiently detect aberrant and infected cells and embark on the signaling pathways necessary to eliminate them. Endogenous expansion of NK cells and their effector mechanisms are under exploration for enhancing adoptive immunotherapy prospects in combination with immunostimulatory and cell-death-sensitizing treatments against cancer, viral infections and other pathophysiological autoimmune conditions. Various modes of NK cell manipulation are being undertaken to overcome issues such as relapse and graft rejections associated with adoptive immunotherapy. While tracing the remarkable properties of NK cells and the major developments in this field, we highlight the role of immune cooperativity in the betterment of current immunotherapeutic approaches.
Keywords: adoptive therapy, autoimmunity, cancer, immunoregulation, immunotherapy, inflammation, NK cell activation, receptor signaling, viral infection
NK cells are the major cellular effectors of the innate immune system, which function alone or in synchrony with other immune cells, to abrogate a variety of targets including virally infected and transformed cells as well as those under stress or heat shock. NK cells are large granular lymphocytes, which respond spontaneously to cells under insult using their germline-encoded receptors and require no prior exposure to antigen. In fact, this ‘spontaneous’ cytotoxicity of NK cells formed the basis for their discovery in the 1970s, when investigators tried to explain the presence of cytotoxic effects of lymphocytes against tumor cells in nonimmunized experimental mice [1–3]. NK cells thus became established as a potentially useful population for adoptive immunotherapy against tumors [4]. Besides their role in tumor immunosurveillance, NK cells mediate many different biological processes ranging from the development of autoimmunity to the outcome of pregnancy and transplants or clearance of infections [5]. NK cells are not only major cytotoxic players but are also inducers of cascades of immune reactions by producing various cytokines and chemokines. Therefore, NK cells are viewed as potential immunotherapeutic modulators [6].
NK cells can act upon aberrant cells with no previous ‘priming’, but recent studies have shown that in tumor settings, in order to elicit their full cytotoxic potential, they do need help [7,8]. They are also capable of immunologic memory which was considered to be solely an adaptive immune feature [9]. Recently, NK cells were found to possess several other features of the adaptive immune response such as developmental education, clonal-like expansion, memory cell generation [10] and recall response [11,12]. This is reminiscent of adaptive characteristics of specificity and memory discovered in innate immune systems [13–15].
This article discusses current models of various strategies that NK cells may employ to mount their effector response encompassing the activating and inhibitory receptor signaling cross-talk. We also discuss NK cell subsets and their interaction with other immune cells and recapitulate the therapeutic implications consequent of the immune cross-talk between NK cells and T cells and the role played by NK cells in viral infections, autoimmunity and cancer.
Models of NK cell activation
NK cell activation is under dominant control of inhibitory receptors that bind with MHC-I molecules on surrounding tissue cells. Since MHC-I molecules are abundantly expressed on normal cells as markers of ‘self’, these cells are not affected by NK cell activity. When cells lose one or more MHC-I alleles, they are often targeted by NK cells by a process described as ‘missing-self recognition’ [16]. The engagement of inhibitory receptors on NK cells by self-MHC-I molecules decides whether an NK cell will mediate missing-self recognition and target lysis, or whether it will become hyporesponsive. NK cells express two distinct categories of inhibitory receptors: the monomeric type I glycoproteins of the immunoglobulin superfamily, for example killer cell immunoglobulin like receptors (KIRs) and the leukocyte immunoglobulin-like receptors (LIRs) and the type II glycoproteins with a C-type lectin-like scaffold, for example CD94:NK group 2, member A (NKG2A) receptors. KIRs in humans and Ly49s in mice, encoded by polygenic and polymorphic genes derived by stochastic bidirectional promoters [17,18], are MHC-I-specific inhibitory receptors [19,20]. A cluster of 17 KIR-encoding genes in humans produce over 40 haplotypes [21]. The Ly49 locus in mice also shows great variation in the number of genes and alleles [22]. Other NK cell-inhibitory receptors, such as the CD94–NKG2A heterodimeric complex binds to the nonclassical MHC-I molecules Qa-1b in mice and HLA-E in humans [23,24]. The LIRs on human NK cells recognize the nonclassical MHC-I molecule HLA-G [25].
Several models have been postulated to explain NK cell function upon MHC-I recognition by inhibitory receptors.
Licensing/arming model
This model conceptualizes that signaling from inhibitory receptors ‘licenses’ [26] or ‘arms’ [27] functional activation of NK cells, which are by default unresponsive or unlicensed. This model relies on an instructive role for inhibitory receptors. The support to this model came from the recent observation that signaling via immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing receptors can lead to phosphorylation of signaling substrates downstream of inhibitory receptors [28], implying that inhibitory receptor signaling might trigger the activation signals that are needed for stimulating NK cells.
Disarming model
The disarming model hypothesizes that all NK cells are by default in a state of responsiveness as long as they are unopposed by MHC-I-specific inhibitory receptors. Stimulation of NK cell inhibitory receptors by MHC-I molecules expressed on normal cells ‘disarms’ NK cells to render them hyporesponsive or anergic [29].
Cis-interaction model
Recent studies have shown that mouse Ly49A receptors have the ability to bind to MHC-I molecules in the same cell membrane in a cis-acting manner [30,31]. This cis interaction between MHC-I and Ly49 sequesters Ly49 receptors and prevents their relocation to the immunological synapse with the target cell [32]. Consequently, the number of unengaged Ly49 receptors on NK cells decreases, leaving NK cells more responsive [33]. However, it remains to be explored if all mouse Ly49 receptors or human KIRs can bind self MHC-I molecules in cis.
Rheostat model
NK cell responsiveness has been observed to increase or decrease according to the strength of the inhibitory signal that is received by NK cells [34–36]. The strength of the inhibitory signal correlates with the number of inhibitory receptors and their affinity with MHC-I molecules. This suggests that NK cell responsiveness operates in a quantitatively graded manner rather than in an ‘on’ or ‘off’ switch. Thus, NK cell responsiveness can be tuned up (arming-like) or tuned down (disarming-like or cis-interaction-like) as a rheostat [37].
NK cell signaling
A fine balance of activating and inhibitory receptor signaling regulates the differentiation of NK cell effector function. NK cells use a wide array of activating and inhibitory receptors, which recognize specific ligands expressed by target cells. MHC-I molecules expressed on self cells are engaged by NK cell inhibitory receptors, while the expression of stress or pathogen-induced ligands, and the downregulated expression of MHC-I on target cells, as well as ligands expressed by transformed cells are recognised by NK cell activating receptors. The precise roles of activating receptors in NK cell activation are not yet clearly understood, and the different models postulated above propose their varied requirements.
Activating receptor signaling
Activating receptors expressed by most or all NK cells are NKp46, NK1.1, NKG2D, CD16 and CD244, also known as 2B4 (which has both an activating and an inhibitory role), whereas others, such as KIRs, LY49D, Ly49H, CD226, also called DNAM-1, and NKG2C are only expressed by some NK cells. These receptors can trigger three types of signaling pathways mediated by immunoreceptor tyrosine-based activation motif (ITAM), a transmembrane adaptor polypeptide DAP10 and the receptor CD244 [19].
ITAM-mediated signaling
Activating receptors present on all mature NK cells constitutively express the transmembrane proteins FCεRI-γ, CD3-ζ and DAP12 that contain an intracellular cytoplasmic signaling motif ITAM. The pairing strategy of these protein complexes is evolutionarily conserved and common to the signaling pathways of Toll-like receptors, B- and T-cell receptor pathways and cytokine and growth factor mediated signaling pathways. Being the only known signaling motif in these proteins, mutation in ITAM causes a loss of signaling function. Different Src family tyrosine kinases expressed by NK cells phosphorylate ITAM-containing residues to bind the tyrosine kinases Syk and ZAP70. At this point, various transmembrane and cytosolic adaptors, such as the linker of activated T cells, provide multiple tyrosine phosphorylation docking sites for the SH2 domains of intracellular signaling molecules. Depending on the stage of maturation, NK cells recruit different kinases and adapters for their functioning. While the mouse NK cells employ phospholipase PLC-γ2, and the nucleotide exchange factors Vav2 and Vav3 for ITAM dependant signaling, human NK cell lines use PLC-γ1 and PLC-γ2, and human NK cells use Vav1 for the same purpose. The ITAM-mediated pathway induces actin reorganization to facilitate cell polarization and release of cytolytic granules such as granzymes and perforin, as well as the transcription of the cytokine and chemokine genes [19].
DAP10/DAP12-mediated NKG2D receptor signaling
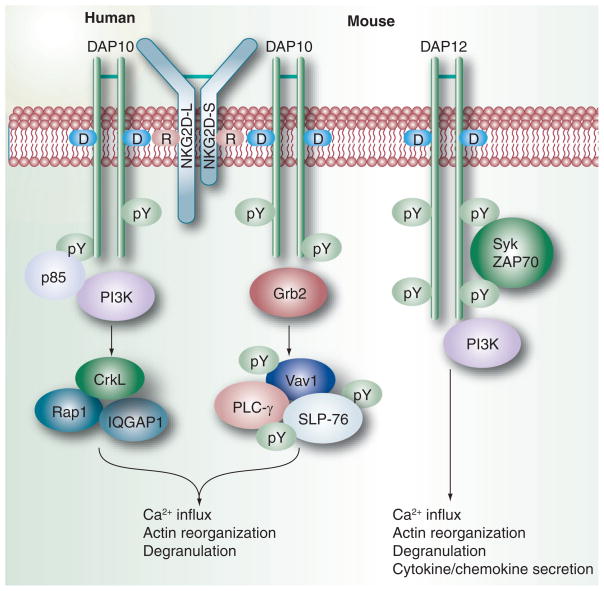
NKG2D is a type II transmembrane-anchored glycoprotein expressed as a disulfide-bonded homodimer. It was first recognized on NK cells [38], but was subsequently also found on γδT cells, activated and memory CD8+ αβT cells and macropahges [39]. It has no signaling elements in the cytoplasmic tail and follows complex activation requirements as shown in Figure 1. NKG2D is associated noncovalently with the signaling adaptor DAP10 at the cell membrane in humans, or with both DAP10 and DAP12 in mice. The genes encoding DAP10 and DAP12, HSCT and TYROBP, respectively, are arranged a few nucleotides apart in opposite transcriptional orientations [40]. An arginine residue within the transmembrane region of NKG2D associates with the aspartate residue within the transmembrane domain of the homodimeric DAP10 adaptor [40]. In mice, NKG2D exists in two isoforms, one long (NKG2D-L) and short (NKG2D-S) [41]. NKG2D-L contains 13 more amino acids in its cytoplasmic tail, which prevent its association with DAP12. NKG2D-S expression is induced after mouse NK cell activation in vitro or in vivo. A single mouse NKG2D-S may form hexameric complex with two DAP10 or DAP12 homodimers [42]. DAP10 and DAP12 do not form heterodimers [43]. Human NKG2D lacks the NKG2D-S isoform and thus can only bind with DAP10 [44].
Figure 1. NKG2D signaling.
NKG2D is a disulfide-bonded hexameric homodimer, which is associated with the adaptors DAP10 at the cell membrane in humans, or with both DAP10 and/or DAP12 in mice. In mice, NKG2D exists as two isoforms: NKG2D-L contains 13 more amino acids in its cytoplasmic tail than the NKG2D-S isoform. An arginine residue within the transmembrane region of NKG2D associates with the aspartate residue within the transmembrane domain of the homodimeric DAP10 signaling adaptor. NKG2D-S can pair with either DAP10 or DAP12, while NKG2D-L can pair only with DAP10 as the extra 13 amino acids in its tail prevent its binding with DAP12. The phosphorylation of the signal motif present in the cytoplasmic domain of DAP10, enables it to bind either the p85 subunit of PI3K or the adaptor Grb2. The binding of Grb2 induces downstream phosphorylation of Vav1 and PLC-γ2. The adaptor protein CrkL, the small GTPase Rap1 and the scaffolding protein IQGAP1 play important roles downstream of PI3K. Such signaling leads to calcium influx, actin reorganization and degranulation. DAP12 triggers immunoreceptor tyrosine-based activation motif-mediated PI3K signaling via Syk and ZAP70 protein tyrosine kinases and can activate cytokine secretion as well as cytotoxicity.
While DAP12 triggers ITAM-mediated PI3K signaling via Syk and ZAP70 protein tyrosine kinases, the short cytoplasmic domain of DAP10 contains a signaling motif sequence, YINM, which when phosphorylated is able to bind either the p85 subunit of PI3K through YXXM or the adaptor Grb2 through YXNX. As these two binding sites overlap, a single DAP10 chain will bind either p85 or Grb2, but not both. The recruitment of Grb2 induces downstream phosphorylation of Vav1, an SH2 domain-containing leukocyte protein of 76 kDa (SLP-76), and PLC-γ2. Binding with p85 results in the production of PI3K in the immune synapse between NK cells and the NKG2D ligand expressing target cells. The adaptor protein CrkL and the small GTPase Rap1 as well as the scaffolding protein IQGAP1 [45] have been implicated downstream of PI3K, to control not only integrin activation, but also cellular polarity and granule exocytosis [46]. Both p85 and Grb2 are required for optimal Ca2+ influx and degranulation following DAP10 cross-linking [47]. Any mutation in the Grb2 binding site in DAP10 does not lead to impairment of Grb2 recruitment. Thus, Grb2 maybe recruited either by direct binding to DAP10 or indirectly using the Sos1–Vav1–Grb2 complexes with phosphatidylinositol-3,4,5-trisphosphate at the immune synapse. The recruitment of p85 and Grb2 requires tyrosine phosphorylation of DAP10. A small-molecule inhibitor of Src family kinase, PP2, prevents signaling through the NKG2D–DAP10 complex, suggesting that DAP10 might be phosphorylated by a Src kinase [48]. Another possible mechanism is Jak3-mediated phosphorylation of DAP10 facilitated by the stimulated IL-15 receptor complex on NK cells following transpresentation of IL-15 by target cells or activated dendritic cell (DC) to NK cells in vivo [49–51].
There are several points of difference between the NKG2D- and the ITAM-mediated pathways: DAP10 is independent of Syk and linker of activated T-cell family tyrosine kinases; mouse DAP10 binds with Vav1 whereas ITAM couples with Vav2 and Vav3; ITAM-mediated signals can activate cytokine secretion as well as cytotoxicity, while NKG2D–DAP10 signals are only sufficient for NK cell degranulation, and not cytokine secretion [52]. A recent report has shown that NKG2D-dependent cytotoxicity is controlled by the distribution of its ligands in discrete membrane domains of target cells [53]. Moreover, activation signals mediated through NKG2D receptor can bypass signals transmitted through inhibitory receptors, possibly because NKG2D signaling is unaffected by SHP phosphatases. This might have conferred NKG2D an evolutionary advantage in immune surveillance.
CD244 signaling
The signaling lymphocyte activation molecule CD244 contains cytoplasmic immunoreceptor tyrosine-based switch motifs, which upon phosphorylation can recruit either phosphatases for inhibiting NK cell function, or the Src kinase Fyn via the adaptor SAP for activating NK cell function [54]. Engagement of CD244 can rapidly mediate leukocyte function-associated (LFA)-1 and actin-dependent NK cell adhesion to target cells [55].
Cell adhesion & intercellular communication implicated in NK cell–target interaction
Cell adhesion to target cells is essential for initiation and execution of NK cell cytotoxicity. The αLβ2 integrin LFA-1 and β1 integrins have been cited as important players in the adhesion of NK cells with their target cells [56]. LFA-1 transduces signals, which activate Vav1 phosporylation upstream of actin polymerization, cytoskeletal rearrangements and clustering of lipid rafts [57]. β1 and β2 integrins bring about the phosphorylation of protein tyrosine kinase-2, which triggers cytotoxicity through an undefined pathway [56,58]. NK cells also express αMβ2 and β1 integrins, which can function as adhesion molecules and facilitate cytotoxicity [56,59]. Another family of NK receptors called the nectins is also implicated in NK cell-mediated killing. Tumor cells express Necl-5, which is recognized by the NK cell receptors CD226 and CD96 [60–62].
Communication across intercellular contacts between NK cells and target cells is central to establishing appropriate immune effector response [63]. Signal integration from the activating and inhibitory receptors at the NK cell synapse culminates in the generation of an effector response [64]. At the interface between NK cells and target cells, proteins accumulate to form an immune synapse [65]. Recently, NK cells have been shown to form membrane nanotubes [66], which are closed-ended intercellular membranous tethers that physically link cell bodies over long distances and can vary in specific structure, process of formation and functional properties [67–72]. These can form either by actin-rich filopodial protrusions extending out from one cell to connect to a distant cell or when cells are in close contact and subsequently depart [67].
Nanotubes can traffic vesicles [69,73] or transmit calcium-mediated signals [74] and potentiate NK cells to interact functionally with target cells over long distances to aid the lysis of distant target cells either directly or by moving target cells along the nanotube path into close contact for lysis by a conventional immune synapse. Target cells that remain connected to an NK cell by a nanotube are frequently lysed, whereas absence of a nanotube reduces target cell lysis. The frequency of nanotube formation increases following NK cell activation and is dependent on the number of receptor/ligand interactions and the local microenvironment, which includes the cytokine milieu. NK cell nanotubes contain a submicron junction where proteins accumulate, including the signaling adaptor DAP10 that associates with the NK cell activating receptor NKG2D and its cognate ligand [66].
Redundancies are found between adaptor and intermediate signaling molecules downstream of various NK cell activating receptors. Moreover, it appears that following target recognition and NK cell immune synapse, NK cell production of cytokines and cytotoxicity are controlled by different signaling pathways. This suggests a functional heterogeneity in the NK cell population, supported by the notion that NK cells with potentially distinct functional properties occupy different niches in the body [75].
Inhibitory receptor signaling
All NK inhibitory receptors possess a common ITIM in their cytoplasmic region, but their extracellular domains vary so as to attract a diverse array of ligands. Upon recognition of a ligand by inhibitory receptors, the ITIM tyrosine residue is phosphorylated by an Src family kinase, leading to the recruitment of mainly SHP-1 and also SHP-2 and SHIP-1 [19]. The classical view is that these phosphatases recruited by the inhibitory receptors at the NK–target cell interface dephosphorylate the Vav factors, leading to a block in the downstream propagation of activating receptor signals [76]. A second, simultaneous consequence of inhibitory signaling downstream of the ITIM motifs is the phosphorylation of the adaptor protein Crk by the tyrosine protein kinase Abl-1 (also known as c-Abl) that disrupts an activation complex comprising the E3 ubiquitin ligase Cbl, Crk and the guanine exchange factor C3G [28,77]. A third, recently proposed pathway of inhibition downstream of ITIM signaling is the inhibition of phosphorylated Vav by Cbl, which counteracts activating receptor signals [78].
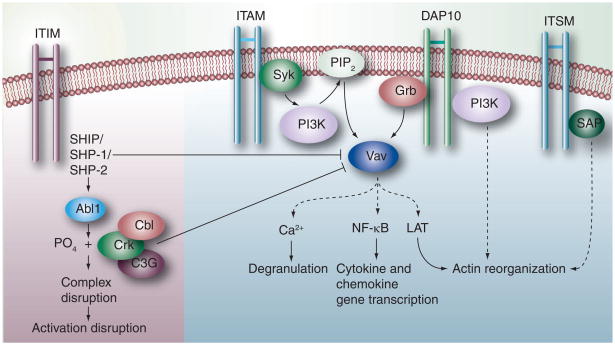
Thus, NK cell signaling from inhibitory receptors and the balance between activation and inhibition is a complex phenomenon requiring several signals acting synergistically. The dynamic fluorescence spectroscopic analysis of the mobility of surface receptors in membrane nanodomains show that the engagement of inhibitory receptors regulate the ultrafine cell-membrane organization of activating receptors [79]. Moreover, Vav phosphorylation acts as a switch-like regulator of NK cell activation and cytotoxicity [80]. This has been illustrated in Figure 2. The net outcome of these signals is likely to be dependent on tissue location, differentiation stage of NK cells and the micro-environmental factors provided by the tissue and other immune cells.
Figure 2. NK signaling cross-talk.
Upon engagement of a ligand by the inhibitory receptor, the tyrosine residue of inhibitory motif ITIM is phosphorylated so as to recruit the lipid phosphatase SHIP or the tyrosine phosphatase SHP-1 or SHP-2. These dephosphorylate Vav of the activation pathway and block the subsequent signaling for activation. Alternatively, phosphorylation of the adaptor protein Crk by Abl1 disrupts the activation complex. Cbl may also inhibit phosphorylation of Vav leading to a blockade of the activation pathways. The ITAM-containing residues are phosphorylated by the Src family protein tyrosine kinases to bind Syk and ZAP tyrosine kinases. At this point, intracellular signaling molecules such as PLC-γ and the nucleotide exchange factors of the Vav family are recruited by NK cells depending on their stage of maturation. A cascade of reactions follows which induces degranulation using Ca2+ ions, NF-κB for transcription of cytokine and chemokine genes or LAT-mediated actin reorganization and degranulation. The recruitment of Grb to the DAP10 signaling motif triggers the downstream phosphorylation of Vav1, ultimately leading to actin reorganization and degranulation through a series of reactions. The ITSM of the CD244 receptor molecules is recognized by SAP (SH2D1A), which is a cytoplasmic SH2 domain-containing adaptor protein. Tyrosine phosphorylation of the ITSM by SAP leads to the activation of NK cells via a series of reactions.
ITAM: Immunoreceptor tyrosine-based activation motif; ITIM: Immunoreceptor tyrosine-based inhibitory motif; ITSM: Immunoreceptor tyrosine-based switch motif; LAT: Linker of activated T cell.
NK cell maturation & subsets
NK cells share a common ancestor with the T and B lymphocytes of the adaptive immune system, but unlike the T and B cells, the precise location for the development of NK cells and the factors that control their maturation are not clearly defined. The earliest committed NK cell precursors that express high levels of the IL-15/IL-2 receptor β-chain (CD122) can be isolated from fetal thymus or adult bone marrow [81]. The general consensus is that NK cells are educated by self-MHC-I and develop in the bone marrow [82]. Peripheral NK cells from MHC-I-deficient mice or patients are apparently not mature as they are hyporesponsive to stimulation. Moreover, experimental ablation of the bone marrow affects the development of NK cells and athymic or thymectomized mice and humans have normal numbers of NK cells [83]. However, peripheral NK cells from MHC-I-deficient mice gain responsiveness upon their transfer into an MHC-sufficient mouse [84,85]. IL-15-dependent induction of Blimp1 gene in NK cells regulates NK cell maturation, homeostasis and proliferation [86]. The transcription factors, interferon regulatory factor 4 and B-cell lymphoma 6, which are crucial for the regulation of Blimp1 in B and T cells, are dispensable for Blimp1 expression of NK cells, highlighting the distinct nature of the NK cell gene regulation. Following the NK cell lineage commitment, NK cells undergo a gradual maturation process that leads to the expression of NK cell receptors and acquisition of effector functions. In mice, four sequential maturation stages are described based on the surface expression of CD11b and CD27 markers: CD27−CD11b−, CD27+CD11b−, CD27+CD11b+ and CD27−CD11b+ [87]. CD11b− NK cells are considered immature. In humans, NK cells undergo a maturation process associated with a gradual loss of CD94:NKG2A heterodimers [88], CD62L [89] and CCR7 [90] and a concomitant acquisition of KIRs and CD57 [88]. These markers along with the adhesion molecule CD56 define at least three distinct subsets of NK cells with different homing and effector functions: CD56brightCD94/NKG2A+CD62L+CCR7+, CD56dimCD57−CD62L +/− CCR7− and CD56dimCD57+/−CD62L−7−. CD56bright NK cells express a more rudimentary repertoire of inhibitory NK cell receptors, with a capability to secrete cytokines, than cytotoxic CD56dim NK cells. While immature NK cells predominate in bone marrow and lymph nodes (LNs), mature NK cells populate the blood, liver, spleen and lung [91,92]. Levels of NK cells in the body vary with hormonal changes such as during the menstrual cycle or treatment with epinephrine [93,94]. At steady state levels, NK cells comprise up to 5% of LN mononuclear cells in humans and 0.5% in mice. This is due, in part, to a difference in the expression of homing receptor CCR7 that regulate the recruitment of NK cells to LNs. Mouse NK cells do not express CCR7.
NK cell immune cross-talk
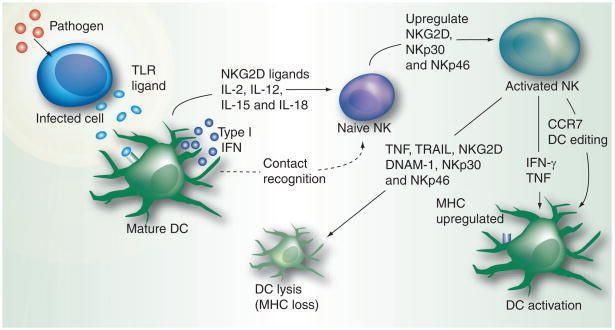
NK cells interact with a number of other cells of the immune system, most notably, DCs, macrophages and T cells [8,95–99]. NK cell and DC interaction first came to light in a study where it was demonstrated that NK cells eliminated DCs loaded with antigens to facilitate the contraction phase of the adaptive T-cell response [100]. Later on, it was found that NK cells selectively kill immature DCs through the TNF-related apoptosis-inducing ligand-mediated pathway, most likely owing to their reduced expression of MHC-I molecules, while mature DCs are unaffected [98]. However, in NK–DC coculture experiments, immature DCs have been shown to attain maturity, secrete TNF and IL-12 as well as upregulate the expression of costimulatory ligands, such as CD86 [101]. The outcome of the DC–NK interaction (killing versus maturation of DC) may depend on NK:DC ratios. At low NK:DC ratios, DC responses are amplified in a TNF-α/contact-dependent manner, whereas at high ratios, inhibition of DC functions is the dominant feature [102]. Thus, NK cells possess DC-editing capability. Moreover, DC-derived signals via cytokines and cell contact are important for activation of NK cells [103]. IL-12 produced by DCs plays a role in the secretion of IFN-γ by NK cells [98]. IL-15 receptors on DCs participate in the trans-presentation of IL-15 found to be necessary for the production of IFN-γ and cytotoxic activity of NK cells [104]. In case of bacterial infections, IL-2 secreted by DCs regulates the production of IFN-γ by NK cells. Also, DCs which mature in the presence of IL-4 lose their ability to activate NK cells; alternatively, they recruit killer-cell activating-receptor-associated protein-dependent receptors to activate NK cells [98]. NK cells were shown to require multiple contacts with DCs to upregulate CD69, secrete granzyme B but no IFN-γ and achieve full activation [104]. DCs have also been found to activate NK cell activating receptors NKp30, NKp46 and NKG2D [105–107]. Injection of plasmacytoid DCs in the peritoneal cavity of mice evoked a strong recruitment of NK cells [108]. In another study, adenovirus transduction and lipopolysaccharide/IFN-γ-induced DCs led to enhanced NK cell activation through the activity of transmembrane TNF and trans-IL-15 mediated by direct cellular contact [109]. DC–NK bidirectional cross-talk is illustrated in Figure 3.
Figure 3. NK–dendritic cell cross-talk.
The TLR ligands secreted by an infected cell are captured by the DCs, the professional antigen-presenting cells. This stimulates the DCs to produce type I interferon through the IRF-3 and IRF-7-mediated JAK–STAT pathway. IL-2, IL-15 and other cytokines presented by the DC to naive NK cells, together with type I interferons, activate the naive NK cells and stimulate their activation and proliferation. NK cells, in turn, regulate the functioning of DCs by eliminating immature DCs with low MHC expression through various effector pathways. Mature DCs with an upregulated expression of MHC are not affected by such pathways; instead, they undergo further activation.
DC: Dendritic cell; DNAM: DNAX accessory molecule; TLR: Toll-like receptor; TRAIL: TNF-related apoptosis-inducing ligand.
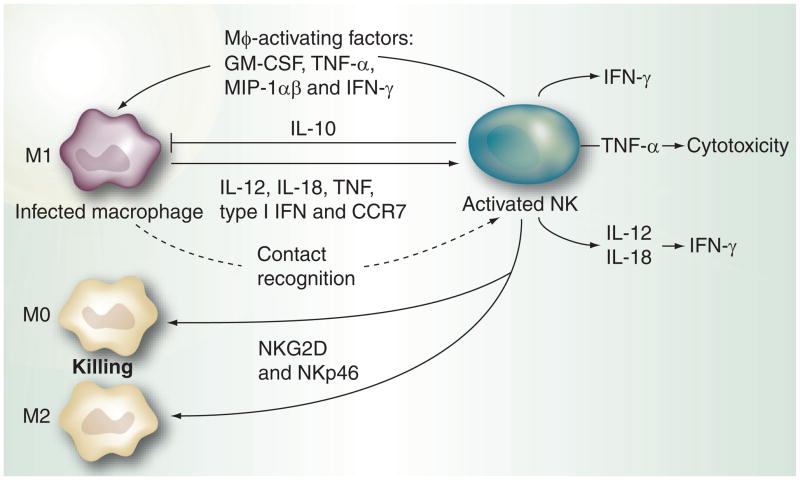
Cross-regulation between NK cells and macrophages has also received much attention recently [110–113]. Macrophages display a correlated sequence of events between the upregulation of MHC-I chain related molecule A (MICA) expression on their surface and the secretion of proinflammatory cytokines such as IL-12, IL-18, TNF, type I IFN and CCR7 for NK cell stimulation [99]. M0 and M2 macrophages become polarized towards M1 phenotype soon after capturing microbial products. This polarization leads to manifold increase in NK cell activation. Once activated, NK cells kill the M0 and M2 macrophages owing to low level of HLA expression, while M1 remains resistant to NK attack [95]. The role of IL-12 and IL-18 cytokines has been emphasized in this cross-talk by showing that their combined blocking led to inhibited expression of CD69 and cytotoxicity in NK cells cocultured with Salmonella-infected macrophages [112]. Activated macrophages and microglial cells, the resident macrophages of the brain, are lysed by activated NK cells via NKG2D and NKp46-mediated recognition [114,115]. In contrast to DCs and microglia, macrophages are resistant to NK cell cytotoxicity, unless they become activated and express NKG2D ligands [114]. This reinforces the fact that the stimulatory signals resulting from NKG2D engagement overcome the inhibitory signal provided by MHC-I ligands during recognition of activated macrophages by NK cells. Moreover, in certain inflammatory situations, NK cells secrete IL-10 that dampens macrophage activation [116]. Macrophage–NK interaction is illustrated in Figure 4. NK cell cross-talk with DCs and macrophages suggests that activated NK cells play a regulatory role by selectively editing antigen-presenting cells (APCs) during the course of immune responses. By lysing the M0 and M2 macrophages and immature DCs, NK cells allow fully activated APCs to present antigens to responding T cells in a controlled fashion as needed.
Figure 4. NK–macrophage cross-talk.
Following microbial infection macrophages secrete proinflammatory cytokines IL-12, IL-18, TNF-α, type I interferon and CCR7, which activate NK cells. Polarization of M0 and M2 macrophages to M1 leads to enhanced NK activation. Activated NK cells eliminate M0 and M2 macrophages as they have low levels of MHC expression on their surface. M1s escape this attack owing to upregulated MHC on their surface, but in certain situations can be inhibited by NK cell-secreted IL-10. However, in most cases macrophage activation factors secreted by activated NK cells lead to further activation of macrophages.
In addition to regulation of myeloid cells by NK cells, data on the cooperativity of NK cells with T cells are emerging that point towards a bidirectional cross-talk between these two cell types. During experimental Leishmania major infection, NK cells are recruited to LNs where they function in close contact with DCs to provide the IFN-γ required for the induction of Th1 polarization [117]. Similarly, in allogeneic immune responses, NK cells secrete huge amounts of IFN-γ that acts on naive T cells directly to mediate Th1 polarization [118,119]. NK cells expressing OX40 ligand and CD86 upon ligation of their activating receptor CD16 can induce IFN-γ production and proliferation of autologous T cells [120]. NK cells also contribute to the resolution of adaptive immune responses via deletion of activated T cells. Following activation, T cells upregulate NKG2D ligands and become susceptible to NK cell-mediated lysis [121]. By this mechanism, NK cells can also regulate the frequency of T regulatory cells [122]. Moreover, in murine cytomegalovirus (CMV) infection, NK cells can influence the quality and quantity of virus-specific CD4+ and CD8+ T-cell responses by limiting the exposure of T cells to infected DCs [123]. It has also been shown that NK cells can negatively regulate the activity of antigen-stimulated T cells via a mechanism requiring the cooperation between DNAM-1 and NKG2D [124]. However, DNAM-1 has also been shown to activate CD8+ T cells and NK cells against a range of target cells that evade recognition by other activating or accessory molecules [125]. Recently, in a reciprocal interaction, activated T cells have been found to provide necessary ‘help’ to NK cells in the abrogation of an experimentally induced mastocytoma in a mouse model. Such a collaborative action has been effective in checking the development of tumor escape variants before metastasis [8,97]. NK and T cells both contributed to the development of an effective antitumor response and it was observed that NK cells needed an activation signal from tumor antigen-specific CD8+ T cells. The exact mechanism for this cooperativity remains undeciphered. In case of parasitic infections by Plasmodium, IFN-γ response from NK cell was found to depend on IL-2 secretion of CD4+ T cells in an MHC-II-dependent manner [126]. Similarly, vaccine-induced cellular immune responses to rabies virus were found to involve cross-talk between T cells and NK cells [127]. Potential molecules involved in NK cell–T cell interaction are summarized in Figure 5. The studies discussed here highlight the provision of balancing the activation of innate effector mechanisms, in addition to T-cell effectors, that may be therapeutically helpful in an immunosuppressive tumor microenvironment and other pathological conditions as discussed recently [128].
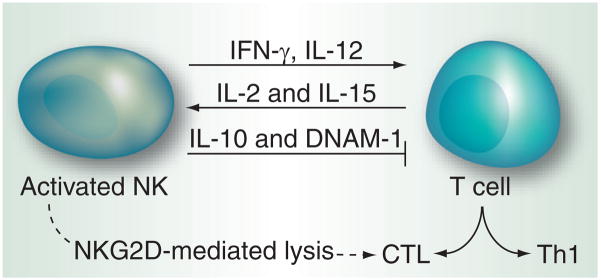
Figure 5. NK–T cell cross-talk.
T cells secrete IL-2 and IL-15, whose roles in NK activation are well established. IFN-γ and IL-12 secreted by NK cells help in T cell activation and their proliferation into CTL and Th. IL-10 and DNAM-1 secreted by NK cells inhibit T-cell response. NK cells can also contribute to the resolution of T-cell response via the deletion of CTL.
CTL: Cytolytic T lymphocytes; DNAM: DNAX accessory molecule; Th: Helper T cells.
NK cells & inflammation
Besides the cytotoxic functions of NK cells in cancer and infections, NK cells also contribute to chronic inflammation and induce pathology in certain conditions. This is particularly the case during pulmonary infections of respiratory syncytial virus that causes bronchiolitis, especially in infants. NK cell numbers increased on the second day of the disease, but upon NK depletion, the disease was prevented [129]. NK cells are a potent and early source of cytokines, such as IFN-γ, Th2-associated cytokines, such as IL-5 and IL-13, and the regulatory cytokine IL-10 [130]. The activation status of NK cells may have dual implications for chronic inflammatory diseases, such as asthma and chronic obstructive pulmonary disease, which are exacerbated by respiratory infection. Their role in these diseases is not fully understood. Their ability to produce Th2 cytokines may promote lung inflammation, whereas their production of IFN-γ, and other actions, may reduce lung pathology. Although many potential interactions of NK cells with DCs, macrophages and T cells have been discussed earlier, their location, timing and importance during different phases of an ongoing infection or inflammatory response are still largely unknown, as is the role of different NK cell subsets. Recently, it was shown that NK cell granzyme M augments the inflammatory cascade downstream of lipopolysaccharide–TLR4 signaling, highlighting a role for APC–NK cell cross-talk as a potential regulator of inflammation [131]. The downregulation of NK cell response after a pathogen has been cleared or to prevent pathology during inflammation is an area that could provide insights into the mechanisms underlying NK cell-dependent inflammatory processes.
Role in viral immunity
NK cells are essential for controlling viral infections in mammals. Depletion of NK cells in a mouse infected with mouse CMV (MCMV) or a defect in their production of antiviral factors such as IFN-γ renders the host more susceptible to infection [132]. Conversely, adoptive NK-cell therapy in such mice makes them resistant to infection. Mouse NK cells use multiple mechanisms to act against MCMV. While in C57BL/6 mice, the MCMC-encoded cell surface molecule m157 is directly recognized by the NK cell activating receptor Ly49H, in other inbred strains of mice, additional activating receptors such as Ly49P, Ly49D2 and Ly49L recognize the MCMV-encoded molecule m04 in the presence of MHC-I alleles [132]. Thus, NK cell recognition of MCMV-infected cells is dependent on the MHC-I haplotype of the strain. Various cytokines such as the type I IFN and IL-12 secreted by DCs and chemokines such as CCL3, CXCL10 and CXCL9 regulate the movement and activity of NK cells. IκBζ, a nuclear IκB-like protein encoded by Nfkbiz, is expressed in NK cells following stimulation by IL-18 or IL-12 cytokines. Its expression has been shown to be essential for the production of IFN-γ and cytotoxic functions of NK cells. Mice deficient in Nfkbiz expression are highly vulnerable to MCMV infection [133]. In the case of human CMV, granzyme along with the perforin analog streptolysin-O inhibits viral replication in fibroblasts. Granzyme could also specifically cleave phosphoprotein 71, an HCMV tegument protein most essential for viral replication [134].
Certain viral infections become successfully established in the host by evading the host immune response or modifying the secretory or cytolytic mechanisms of the host NK cells. Downregulation of the NK cell activating receptor NKG2D is one mechanism of immune evasion by viral infections [135] as is the case with tumor cell evasion discussed later. For example, human CMV establishes itself in immunocompromised patients owing to successful evasion of NKG2D receptor. A recombinant MCMV expressing the high-affinity NKG2D ligand RAE-1γ showed virus attenuation, lower viral loads and efficiently controlled infection in immunodeficient or immunosuppressed mice [136]. Immunization with MCMV-RAE-1γ also protected neonates from MCMV disease and the RAE-1γ transgene did not exhibit sequence variation following infection. In rat CMV infections, RCTL – a viral gene product – acts as a decoy for rat Clr-b ligand, which is inhibitory towards NK cell receptor NKR-P1B.
Persistent hepatitis C virus (HCV) infections establish themselves by modification of IFN signaling and cytokine production of host NK cells along with interference in immune effector cell functions and continual genetic variation by the virus. NK cells can be inhibited in vitro by recombinant HCV glycoprotein E2 via cross-linking of CD81, a cellular coreceptor for the virus [137]. Susceptibility to viral infections has been correlated with KIR/HLA haplotypes [138,139]. In HCV, KIR2DL3 and HLA-C1 homozygocity is beneficial in eradication of infection [140,141]. Homozygocity of KIR2DL3 and HLA-C1 alleles has been correlated to lower NK cell inhibition than other pairs of KIR ligand combinations [142]. In chronic hepatitis B patients, NK cells, which are enriched in the liver, upregulate the expression of the death ligand TRAIL leading to elimination of hepatocytes bearing the TRAIL receptors. Simultaneously, however, with liver inflammation upon infection, IFN-γ production by NK cells is suppressed by IL-10 and TGF-β as shown by blocking studies [143].
NK cell–DC cross-talk plays an important role in HIV infections. In early-stage HIV infections, DCs capture the virus particles through specific receptors and transport them to the lymphocytes. The role of NK cells is to keep the number of such infected DCs under control by killing them via apoptosis through a TNF-related death receptor-4 pathway. However, when infected with HIV, the DCs become resistant to apoptosis by NK cells owing to the expression of two antiapoptotic molecules, c-FLIP and CIAP2, as shown by specific siRNA silencing studies that restored NK-mediated apoptosis [144]. The activating receptor KIR3DS1 and its putative ligand HLABw4-I80 play key roles in preventing HIV infection [145–147]. Recently, differential mRNA regulation of HLA-C polymorphism has beeen associated with HIV control [148]. Attempts have been made to augment NK activity in HIV patients using KIR mAb 1-7F9 [149].
NK cells rapidly mount an antiviral response in the case of Chikungunya viral infections. This is accompanied with high levels of IFN-α and circulating IL-12 for a prolonged period in chronic infection with Chikungunya virus [150]. In human hantavirus infections, a significant portion of the expanding NK population expressed the NK activating receptor NKG2C and the inhibitory receptor KIR. The NK cell population not only responded to target cell stimulation, but also persisted in the circulation at elevated levels for a prolonged period after the infection [151].
Role in autoimmunity
The cytokine production profile of NK cells matches closely with their developmental stage, with immature NK cells producing type 2 cytokines and mature NK cells producing type 1 cytokines. Consequently, NK cells can act as a two-edged weapon in autoimmune reactions. Animal models of autoimmunity provide evidence for both disease-promoting and disease-preventing effects of NK cells. In experimental autoimmune encephalomyelitis, the pathogenicity is determined by NK–T cell cross-talk. NK cells have been shown to be pathogenic by shaping Th1 adaptive immune response and by activating DCs infiltrating the CNS [152]. Indeed, mice which have defective NK cell functions, such as IL-18−/− or T-bet−/− mice, are resistant to encephalomyelitis induction [153,154]. However, humans with multiple sclerosis and systemic lupus erythematosus have decreased NK cell number and activity compared with healthy individuals [155]. By contrast, patients in remission from multiple sclerosis manifest high frequencies of blood NK cells that secrete IL-5 and IL-15 as observed in a Phase II clinical trial with a humanized monoclonal antibody (mAb) against the IL-2Rα chain [156]. In hemophagocytic lymphohistiocytosis, a condition characterized by impaired perforin/granzyme system, and also in systemic juvenile rheumatoid arthritis, a decreased NK cell activity has been observed [157,158]. Indicative of a disease-promoting role, synovial fluid NK cells that are CD56bright, however, express a high level of activation markers and produce IFN-γ upon cytokine stimulation [159,160]. Likewise, patients with psoriasis and psoriatic arthritis show a higher frequency of activating KIR genes (KIR2DS1/KIR2DS2) [161]. Recently, a distinct subset of NKp46+ NK cells, which have the capacity to produce large amounts of IL-22, have been identified in the skin that contribute to skin pathologies, such as atopic dermatitis and psoriasis [162]. Isolated lesional NK cells from patients with atopic dermatitis and psoriasis harbor highly activated NK cells as indicated by a high spontaneous release of IL-4, IL-5, IL-13 and IFN-γ [163,164]. Similarly, in alopecia areata, an inflammatory autoimmune disease causing hair loss, an upregulation of MICA in follicular lesions enhances the susceptibility of these hair follicles for an attack by NKG2D+ NK cells [165]. Also, in pregnancy, which is a controlled state of inflammation during early implantation and later systemically, the predominant immune interactions in the decidua are between the placental trophoblast and maternal NK cells. Dysfunctional NK cell activation in such a condition can result in the maternal syndrome of preeclampsia [166]. Thus, both genetic and immunological observations suggest an involvement of NK cells in autoimmune conditions. Increased understanding of NK cell actions and immune cross-talk in such conditions will provide insights for new therapeutic approaches towards alleviating or preventing such diseases.
Role in tumor immunity
Experimental evidence has shown that NK cells are active players in the immunosurveillance against tumors. Mice deficient in NK cell activating receptor NKG2D are more susceptible to spontaneous cancer than wild-type mice [167]. NK cells have been found capable of eradicating established hematological malignancies such as acute myeloid leukemia or pediatric acute lymphocytic leukemia [168]. In an experimentally induced mouse mastocytoma model, NK cells have been found critical for tumor abrogation. In addition, in this model activated T cells were found necessary to provide some activation ‘help’ to NK cells. Such a collaborative T cell–NK cell immune cross-talk has been effective in checking the development of tumor escape variants before metastasis [8,97].
The missing self recognition is one simplistic mechanism by which NK cells target tumor cells deficient in MHC-I expression [169]. In case of allogeneic NK cells transplanted from a donor, a KIR ligand mismatch helps the donor NK cells to avoid inhibition owing to lack of syngeneic inhibitory MHC-I ligands in the recipient. Allotransplanted NK cells thus get activated and target tumor cells [170]. In addition, NK cells may target NKG2D and NKp30 ligands that are induced during carcinogenesis [171]. NK cells possess a vast machinery to mount a cytotoxic response, such as exocytosis of lytic granules containing perforin and granzymes, and the engagement of cell-death receptors by TNF superfamily ligands. Different NK cell-based strategies for tumor elimination outlined in Figure 6 have, therefore, evolved with a major focus on activation of endogenous NK cells, hematopoietic stem cell therapy, allogeneic NK cell transfers and pharmacological and genetic modulation of NK cells [6].
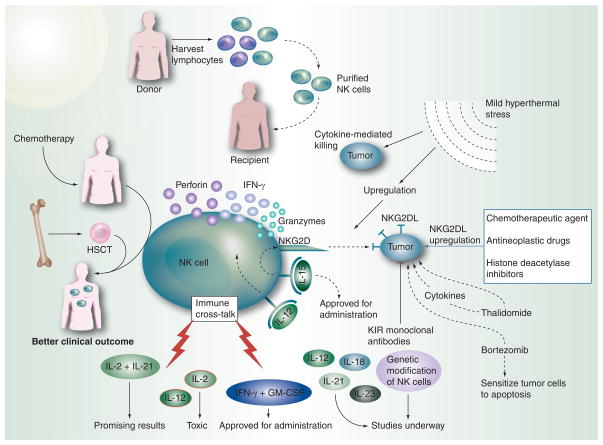
Figure 6. NK cell-based strategies for cancer therapy.
Allogeneic NK cell transfers are considered advantageous for cancer therapy owing to the better graft-versus-tumor effects and lower graft-versus-host disease effects encountered in patients undergoing such transplants. The lymphocytes harvested from the donor are purified to obtain NK cells while removing the T cells that usually bring about the graft-versus-host disease. Purified NK cells, when transplanted into the recipient, provide long-term remission. Hematopoietic stem cells (HSC) are preferred over bone marrow stem cells as they can bring the cells of the immune system to prechemotherapy levels. Such a strategy provides a good clinical outcome, especially with haploidentical HSC transfers. The rate of graft-versus-tumor, however, is better with allogeneic NK cell transfers as compared with HSC transfers. External administration of NK cell-stimulatory cytokines enhances endogenous NK cell activation. While IFN-γ and GM-CSF have been approved for administration in cancer patients, studies are underway for IL-12, IL-18, IL-21 and IL-23, as well as for genetically modified NK cells. The combined effects of IL-2 and IL-21 have shown promising results by improving immune cross-talk, but the individual administration of these cytokines as well as IL-12 has been shown to produce toxic effects. Various drugs that increase tumor cell expression of ligands for NK cell-activating receptors are currently under investigation currently. The different modes by which these act mostly involve upregulation of NKG2D ligands on tumor cells or the induction of immunostimulatory cytokines. Some drugs such as thalidomide have direct antitumor effects, while others such as bortezomib, a proteasome inhibitor, sensitize tumors to apoptosis and upregulate TRAIL receptors, as well as increasing the expression of NKG2D ligands on tumor cells. Clinical trial with a blocking killer cell immunoglobulin like receptors monoclonal antibody, 1-7F9, which recognizes KIR2DL1, 2 and 3 and thus blocks the inhibitory signaling by almost all MHC class I alleles, is under development. Mild hyperthermal stress brings about increased apoptosis of tumor cells and leads to an increase in NKG2D-mediated cytotoxic killing by NK cells. Heat treatment of NK cells close to the physiological range brings about cellular changes that lead to NK cell activation.
HSCT: Hematopoietic stem cell transplantation.
NK-stimulatory cytokines
Activation of endogenous NK cells can be enhanced by the administration of several cytokines and colony-stimulating factors. IFN-α and GM-CSF have been approved for administration in cancer patients [172–173]. IL-2 was approved by the US FDA to treat metastatic renal cell cancer and malignant melanoma [174]. IL-2, however, is associated with capillary leak syndrome, activation-induced cell death, and expansion of immunosuppressive regulatory T cells [175]. Studies are underway to assess the effects of IL-12 and IL-21 in cancer patients. Although IL-2 and IL-12 have shown to produce toxic effects individually, the combined effects of IL-2 and IL-21 have shown promising results. IL-15 along with type-I interferons also plays an important role in the survival and activation of NK cells [7,176]. IL-15 uses a heterotrimeric receptor that includes the IL-15Rα and IL-2/IL-15Rβ subunits shared with IL-2 and the common γ chain shared with IL-2, IL-4, IL-7, IL-9 and IL-21 cytokines [177]. IL-15 together with IL-12 stimulates the production of IFN-γ by human NK cells [178]. IL-15 receptor signaling has been shown to be coupled with the activation of NK cell activating receptor NKG2D [49] and in the induction of its signaling adaptor DAP10 [179]. It has also been shown that activating signals dominate inhibitory signals in IL-15 activated NK cells [180]. A recent pharmacokinetic and safety study supports the administration of IL-15 in patients with metastatic malignancies [181]. Roles of IL-18 and IL-23 have also been implicated in NK cell priming [182–183], and need to be tested for therapeutic intervention.
Hematopoietic stem cell transplantation
Following chemotherapy, cancer patients have been introduced to donor stem cell transplantation. Hematopoietic stem cells derived from peripheral blood are the cells of choice for this transplantation (HSCT) as they bring the immune cells to prechemotherapy levels and can induce greater numbers of NK cells as compared with bone marrow stem cells [184,185]. Such a regimen has been reported to provide prolonged disease-free state in several tumor types with reduced chances of relapse, and this can be achieved in a shorter treatment period [184,186–191]. The HSCT exerts a graft-versus-tumor (GVT) effect against tumor cells in the host. A class of NK cells that lack inhibitory receptors for self MHC-I ligands have also been reported to mediate GVT effects following HSCT [192]. NK cells that are partially functionally disabled, owing to their expression of self MHC-incompatible KIRs, do exist in individuals and mice [27,193,194]. Additional studies are required to gauge the therapeutic importance of these cells.
Haploidentical HSCT, where one MHC allele is similar between donor and recipient whereas the other is mismatched, has been found to be effective against tumor cells in the recipient [195]. A number of retrospective HSCT studies have led to differing clinical outcomes [196]. Recently, following a haploidentical HSCT consisting of high numbers of purified donor CD34− cells with very few mature cells, which were injected after an intensive immunosuppressive regimen (to remove recipient hematopoetic cells), alloreactive NK cells were found ex vivo in an activated state, with improved clinical outcomes [197]. However, immunosuppressive treatment-related morbidities caused by infections are high following such haploidentical transplant procedures. Surprisingly, even in completely MHC-identical transplants, functionally alloreactive NK cells have been reported with an improved clinical outcome [198,199]. Mechanisms underlying such observations are still not clear. The cytokine milieu, the strength of inhibitory signaling and the presence of different activating receptor genes, as recently observed in a selection of donors differing in KIR genotype [200], may be the underlying basis for the development of alloreactive NK cells in such identical transplants.
Allogeneic NK cell transfers
To overcome the relapse associated with HSCT, allogeneic NK cell transfers have been explored as they provide better GVT in some relapse patients. The major risk with these NK cell transfers is that of graft-versus-host disease (GVHD), where the host cells are not recognized by the grafted NK cells as ‘self’ and are thus rejected. To reduce this effect, transfer of purified donor NK cells, depleted of T cells, has been performed. Such transfers have shown to generate long-term remission in patients with leukemia relapse [6,201]. Fourfold expansion of transferred CD3−CD56+NK cells in an adult patient with advanced acute myeloid leukemia has been observed with impressive tumor killing in vivo [202]. Based on these promising results, additional studies are needed to optimize the scheduling of NK cell or HSCT infusions. Also, protocols for the large-scale in vitro expansion of GMP-grade purified NK cells are needed. Some initial NK cell purification protocols have demonstrated safety in Phase I clinical trials [203,204]. Efforts are underway to further refine such protocols [205,206]. Another key issue is the fate of NK cells after transfer. Protocols usually involve daily injections of IL-2 to sustain NK levels and activity [201]. IL-2, however, has toxic side effects, including expansion of immunosuppressive T regulatory cells [175], which results in poor clinical outcome [203,207]. Protocols that improve long-term NK cell memory are also desirable. Improved NK cell activation and memory was observed with IL-12 and IL-18, along with a low dose of IL-15 [208]. Similar cocktails of cytokines need to be tested along with NK cell transfer protocols. Moreover, since NK cells represent only approximately 5–10% of circulating lymphocytes [116] and the purification and enrichment of clinical grade NK cells is cumbersome, alternative sources of NK cells should be considered. NK-92 clonal cells, established from a patient with large granular lymphoma, do not express KIRs and produce toxic effects towards a diverse range of tumor cell lines, human leukemia as well as mice melanomas. When cultured under GMP, NK-92 infusion in patients has safely generated antitumor effects in a few cases [209,210]. In view of recent data showing collaborative effects of T cells and NK cells in mounting an effector response against tumors [8,97], it is worth investigating the combination of T cells and NK cells in adoptive transfer protocols.
Pharmacological modulation of NK cells
Certain drugs can increase tumor cell expression of ligands for NK cell activating receptors. Chemotherapeutic agents, such as. 5-fluorouracil, Ara-C and cisplatin, radiation as well as antineoplastic drugs such as 5-aza-2′-deoxycytidine, trichostatin A, bryostatin-1 and all-trans-retinoic acid can increase the expression of NKG2D ligands on tumor cells [211,212]. Clinical trial with a blocking KIR mAb, 1-7F9, which recognizes KIR2DL1, 2 and 3 and thus blocks the inhibitory signaling by almost all MHC-I-C alleles, is under development [213,214]. More recently, a proteasome inhibitor bortezomib, which is an approved therapeutic drug against multiple myeloma and mantle cell lymphoma, has shown NK stimulatory effects by increasing the expression of NKG2D ligands on tumors [206,215–218]. This is therapeutically important as downregulation of NKG2D ligand expression facilitates the escape of MHC-I-negative tumor cells from NKG2D-mediated killing by NK cells, as shown in melanoma cells [219]. Molecular targeting by bortezomib apparently acts as a two-edged sword. It can sensitize tumors to apoptosis by increasing caspase-8 activity [220,221] as well as potentiate NK cell-mediated killing by upregulating NKG2D ligands and TRAIL receptors on tumor cells. Another drug, thalidomide, besides having direct antitumor effects, has also been shown to upregulate NK cell activity through induction of immunostimulatory cytokines and NK cell stimulatory ligands on tumor cells [222,223]. Drugs that act by histone deacetylase inhibition have also been found to upregulate the expression of NKG2D ligands on tumor target cells [218,224,225]. Some of these pharmacological drugs may also have potential immunosuppressive effects as is suspected for bortezomib [226]. Therefore, in vivo dose concentrations and treatment schedules of these drugs should be carefully optimized before they can be used in vivo to enhance the tumor-targeting ability of NK cells without negatively affecting immune cell cross-talk.
Hyperthermal therapy
Hyperthermia is defined as a simple addition of excess heat, sufficient to increase body temperature both locally or systemically [227]. Even though the benefits of using heat treatments for cancer date as far back in history as the writings of Hippocrates, serious evaluation of these methods began only a few decades ago. It is now known that elevated temperatures enhance the effect of standard cancer therapies such as radiation and chemotherapy [228]. Current hyperthermia protocols are focused on delivering excess heat directly within the tumor cells so as to modulate their DNA repair pathway, maximize the damage to tumor cells, enhance immune effector functions and avoid heating normal tissues around the site of tumor [229]. Treatments with temperatures over 42°C sensitize tumor cells to apoptosis, enhance drug uptake by tumor cells and reduce or reverse resistance to drugs. Mild thermal therapy activates NK cell activity and induces vascular perfusion. However, localized heating above 42°C often remains unachieved largely owing to vascular drainage. Therefore, efforts are now on to stimulate different branches of the immune system using a mild hyperthermia approach [227]. Mild hyperthermal stress has been shown to upregulate NKG2D clustering and MICA expression, both of which are essential for tumor target recognition and NK cell cytotoxicity [229]. It has also been demonstrated using various tumor models that thermal stress increases the migration of immune cells such as NK cells, monocytes and macrophages to the site of the tumor [227]. Fever-range thermal stress has been shown to promote lymphocyte trafficking across high endothelial venules via an IL-6 trans-signaling mechanism and through induction of intravascular ICAM-1 [230,231]. In addition, there is increased apoptosis within the tumor bed [228,232] and cytotoxic killing by NK cells [233]. Treatment of human NK cells with 39.5°C hyperthermia has been shown to play a role in the localization of NKG2D to the lipid rafts within NK cell plasma membrane, which play an important role in cell signaling leading to NK cell activation [227]. Studies suggest that the activation of NK cells following hyperthermal treatments at temperatures close to the physiological range and no higher, points towards a physiologically relevant and evolutionarily conserved temperature gradient to which the NK cells have become responsive [233].
Genetic modification of NK cells
Genetic modification of NK cells could endow them with cytotoxicity against otherwise NK-resistant target cells by overcoming NK cell inhibitory signals. We discuss below various chimeric receptors used to activate NK cells, as well as studies modifying NK cells with cytokine transgenes.
Chimeric NKG2D receptor
Keeping in mind the fact that tumor cells express ligands for the NK cell activating receptor NKG2D, which are not expressed by normal body cells, a chimeric NKG2D (chNKG2D) receptor was designed by fusing the NKG2D receptor with the cytoplasmic domain of the CD3ζ chain and then expressing them on T cells [234]. T cells do not express DAP10 while NK cells express both DAP10 and DAP12. Both these adaptor proteins play an important role in cell activation. DAP12 becomes essential in this context as it contains an ITAM that provides primary signals for NK cell activation, while DAP10 can only transduce costimulatory signals for cell activation. The interaction of such a fused chimeric receptor with its ligands expressed on tumor cells resulted in the production of cytokines and chemokines that induced an antitumor effector response, and recruited macrophages to the site of tumor in an MHC-independent manner. In the case of ovarian cancer, substantially beneficial results have been obtained with chNKG2D receptors [235]. MHC polymorphism plays an important role in developing tumor escape variants as tumor cells downregulate the expression of MHC molecules or mimic MHC-I expression using polymorphic molecules. Since chNKG2D is independent of MHC expression, it reduces the possibility of developing escape variants. Similar results were also obtained in the case of myeloma patients where wild-type NKG2D could not recognize myeloma cell lines, while chNKG2D recognized and lysed myeloma cells. The same study also reports the secretion of IFN-γ, GM-CSF and TNF-α during coculture with myeloma cells [236]. In addition, mice completely rejected ovarian tumor and developed immunological memory, which afforded protection against subsequent challenge with same ovarian tumor cells. It was also observed that T cells expressing chNKG2D receptor induced the activation of NK cells against tumors, while reducing Foxp3+ regulatory T cells in a perforin-dependent manner and enhancing the production of IFN-γ, CCL-3, CCL-5 and GM-CSF at the site of the tumor. Recently, a new bifunctional fusion protein, single-chain variable fragment (scFv)–NKG2D, which binds tumor cells through NKG2D and recruits and stimulates T cells through an anti-CD3 single-chain fragment has shown antitumor effects in murine lymphoma and B16F10 tumor models [237].
Chimeric CD19 receptor
CD19 is a receptor widely expressed among B-cell malignancies and delivers different primary and costimulatory signals. CD56+ NK cells were transduced with chimeric receptors directed against CD19. Expression of anti-CD19 receptors linked to CD3ζ chain overcame intrinsic NK cell inhibitory signals and augmented NK cell-mediated killing of autologous leukemic cells [238]. The cytotoxic killing was further improved by adding 4-1BB costimulatory molecule to the chimeric anti-CD19-CD3ζ receptor, suggesting that 4-1BB-mediated signals enhanced NK cell cytotoxicity.
Chimeric erbB2-CD28 receptor
Primary NK cells in mice have been modified to express a chimeric scFv receptor specific for the human erbB2 tumor-associated antigen. The chimeric receptor was composed of the extracellular scFv of erbB2 antibody linked to the transmembrane and cytoplasmic CD28 and TCR-ζ signaling domains (scFv–CD28-ζ). NK cells genetically modified with such a chimera could mediate enhanced killing of an erbB2+ MHC-I+ lymphoma in a perforin-dependent manner [239]. Adoptive transfer of such NK cells also enhanced the survival of mice bearing established erbB2+ lymphoma.
Cytokine transgenic NK cells
Human NK cell line NK-92 stably transduced with the IL-2 gene secretes greater amounts of IFN-γ and TNF-α, in addition to IL-2, and shows increased cytotoxicity against tumor targets [240]. These NK-92 cells also show improved therapeutic potential in tumor-bearing mice. Similarly, IL-15 gene-modified human NK cell line showed increased proliferation at low doses of IL–2, which was associated with the upregulation of antiapoptosis genes Bcl-2, Bcl-xl and Mcl-1 as well as the downregulation of apoptosis genes Bim and Noxa. Moreover, these IL-15-transduced NK cell line cells showed enhanced anti-tumor cytotoxicity against hepatocarcinoma cells, partly through increasing expression of IFN-γ, perforin and FasL [241].
Roadblocks in NK cell therapy
Allogeneic transplantations of NK cells from a donor are usually associated with an extensive chemotherapeutic regimen that suppresses the recipient’s immune system in order to increase the chances of graft survival. Moreover, the KIR ligand mismatch can lead to graft rejection. Efforts are aimed at reducing GVHD while increasing GVT, but a fail-safe mechanism for this approach is yet to be developed. In the case of exogenously expanded NK cells, there is also a possibility that a specific subset of NK cells may expand selectively as compared with the entire cell population. This subset may be an altered phenotype or a lineage different from the one that was originally desired. Such an expansion may lead to aberrant NK cell growth and transplantation anomalies. While selecting a donor for allogeneic transplant, the availability of an approporiate KIR-mismatched donor remains a challenge. Siblings or relatives of the patient may not possess allotypes compatible with the patient and may result in graft rejection or GVHD. These issues point towards the efficacy of a naive or endogenous NK cell expansion as the most suitable method for NK-mediated immunotherapy, but positive results from such studies remain elusive.
Furthermore, certain tumors such as myeloid leukemia are more susceptible to NK cells while others such as lymphoid leukemia pose a greater difficulty probably owing to selective ligand expression on some tumors and insufficient expression on others. Targeting large, solid tumor masses with NK cells is another issue. NK cell migration to the site of tumor and their infiltration inside the tumor mass is perhaps more suitable in the case of smaller tumors. Tumors often resort to deceptive forces such as downregulation of NK cell activating receptors, mimicking inhibitory receptors, or secreting immunosuppressive cytokines. Moreover, mesenchymal stem cells in the tumor microenvironment can alter the phenotype of NK cells and suppress their proliferation, cytokine secretion and cytotoxicity against HLA-expressing targets [242]. These factors coupled with a lack of complete understanding of NK homing and infiltration mechanisms has hampered efforts to specifically direct NK cells to tumor sites for enhanced and effective antitumor activity.
Another practical roadblock is the complexity of current NK cell culture protocols and their compliance with regulatory standards. Successful NK cell therapy protocols in clinical trials require a large-scale production of NK cells with a capability to sustain them for a long term in vivo without losing their viability and functionality. Available NK cell culture protocols have so far failed to meet this standard. Another significant challenge in this field is to identify markers that can distinguish naive cells from activated NK cells. So far, the activity of NK cells is gauged by functional assays such as in vivo target rejection, cell–cell interaction assays, stimulation of activating receptors and changes in the inhibitory receptor repertoire. All these assays need to be performed in parallel to make any conclusions on NK cell activity. Refined quantitative markers of NK cell activation that are independent from NK cell function and can be measured in vitro are desirable.
Conclusion
Despite major strides to understand interactive immune functioning, a consolidated immunotherapeutic approach to cancer and other immunopathologies remains elusive. Signaling through the different activating and inhibitory receptors on NK cells regulates the threshold for NK cell activation, which is necessary to bring about abrogation of target cells. A fine balance between the different receptor signaling mechanisms and intercellular contacts form the basis of how NK cells ‘see’ their target. The net outcome of these signals is dependent on tissue location, NK cell differentiation and the microenvironmental factors. Immune crosstalk leads to enhanced activation and proliferation of NK cells. Emerging data suggest that the provision of balancing the activation of NK cell effector mechanisms, in addition to T-cell effectors, may be therapeutically helpful in an immunosuppressive tumor microenvironment and other pathological conditions. Many newer approaches such as the allogeneic NK cell transfers, the extraneous administration of cytokines as well as the use of drugs for pharmacological manipulation of NK cells or genetically modified NK cells are finding their way into clinical trials. Special emphasis is being laid on designing combinatorial cell transfer regimens along with immunostimulatory and tumor cell-death-sensitizing treatments.
Future perspective
Numerous developments in the field of immunotherapy, which point towards a combinatorial regimen involving NK cells as a major component, look promising. Protocols relying on endogenous expansion of NK cells in vivo with the use of appropriate cytokines such as a cocktail including IL-12 and IL-15 are emerging. Efforts should also focus on combining immunotherapy with tumor-sensitizing approaches that can increase the susceptibility of tumors to cell death. Increasing the expression of NK cell activating ligands and death receptors on target cells is one potential headway. Several drugs are being explored to increase the sensitivity of NK cells to tumor cells [243]. A combination of different therapeutics, such as initial priming of the tumor with a sensitizing drug before administering conventional chemotherapy or ionizing radiation therapy, leads to considerably amplified antitumor effects [6,244]. Manipulation of NK cell receptors such as by the use of chimeric NKG2D receptors on NK cell surface [245] also holds promise for the future. Humanized mAbs to block the inhibitory receptors on NK cells are worth pursuing in view of the recent findings. NK cell transfer protocols with optimized treatment conditions represent attractive possibilities to be translated in the clinic.
The emerging role of immune cross-talk in the activation and proliferation of NK cells needs to be delved in deeper so as to fully exploit the vast potential of NK cells in cancer and other pathologies.
Keeping in view the promising aspects of immune cooperativity in effector functions and the combinatorial trend of recent therapeutic approaches, it seems inevitable that an interactive immunotherapeutic cell transfer regimen would emerge that would benefit from the existing immunostimulatory and tumor cell-death-sensitizing treatments. Activation of the innate and adaptive components of the immune system simultaneously with molecular targeting and sensitization of target cells can potentially lead to successful and effective therapeutic strategies in cancer and other pathological conditions.
Executive summary.
Immunosurveillance mechanisms of NK cells rely on a fine balance of NK cell activating and inhibitory receptor signaling. NK cell activation is under dominant control of inhibitory receptors that bind with MHC-I molecules. Several models have been postulated to explain how NK cells function upon MHC-I recognition, namely, licensing/arming, disarming, cis-interaction and rheostat.
Activating receptors expressed by most or all NK cells are NKp46, NK1.1, NKG2D, CD16 and CD244 (which has both an activating and an inhibitory role), whereas others, such as KIRs, LY49D, Ly49H, CD226 and NKG2C are only expressed by some NK cells. The receptors trigger signaling pathways mediated by a immunoreceptor tyrosine-based activation motif or a transmembrane adaptor polypeptide DAP10 or DAP12 or the receptor CD244.
NK cell inhibitory receptors primarily consist of the signaling motif called immunoreceptor tyrosine-based inhibitory motif, which inhibits activating receptor signaling by dephosphorylating the Vav factors in the activation pathway, by phosphorylation of Crk leading to disruption of the activation complex, or by inhibition of phosphorylation of Vav by Cbl which counteracts activation signals.
NK cells interact with different immune cells like dendritic cells, macrophages and T cells, leading to mutual regulation and enhanced production of cytokines and effector molecules.
For tumor immunotherapy purposes, endogenous activation of NK cells by using appropriate cytokine cocktails, allogeneic NK cell transfer for increasing graft-versus-tumor and reducing graft-versus-host disease, donor hematopoietic stem cell transplantation for greater NK cell proliferation and drug modulation are the approaches under exploration.
NK cells help control viral infections by upregulating the expression of NKG2D, IFN signaling and cytokine and chemokine secretion, which bring about cytolysis of the virally infected cells.
In certain conditions, NK cells contribute to pathology and chronic inflammation by their secretion of proinflammatory cytokines or crosstalk with other immune cells. Results thus far also indicate a disease-promoting role of NK cells in autoimmune disease.
A combinatorial therapeutic regimen comprising of conventional, immunostimulatory and cell-death-sensitizing treatments holds the most promise to overcome the drawbacks associated with NK cell therapy and to provide a successful stimulant to the current clinical approaches.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
A Shanker and A Malhotra are supported by funds from the U54 CA091408 grant from the National Cancer Institute to the Meharry Medical College–Vanderbilt-Ingram Cancer Center partnership in eliminating cancer disparities. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1▪.Greenberg AH, Hudson L, Shen L, Roitt IM. Antibody-dependent cell-mediated cytotoxicity due to a ‘null’ lymphoid cell. Nat New Biol. 242(117):111–113. doi: 10.1038/newbio242111a0. Together with references [2] and [3], was the first paper to demonstrate the existence of NK cells capable of spontaneous cytotoxicity. [DOI] [PubMed] [Google Scholar]
- 2▪.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 16(2):216–229. doi: 10.1002/ijc.2910160204. Together with references [1] and [3], was the first paper to demonstrate the existence of NK cells capable of spontaneous cytotoxicity. [DOI] [PubMed] [Google Scholar]
- 3▪.Kiessling R, Klein E, Wigzell H. ‘Natural’ killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 5(2):112–117. doi: 10.1002/eji.1830050208. Together with references [1] and [2], was the first paper to demonstrate the existence of NK cells capable of spontaneous cytotoxicity. [DOI] [PubMed] [Google Scholar]
- 4.Topalian SL, Rosenberg SA. Therapy of cancer using the adoptive transfer of activated killer cells and interleukin-2. Acta Haematol. 1987;78(Suppl 1):75–76. doi: 10.1159/000205907. [DOI] [PubMed] [Google Scholar]
- 5▪▪.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 9(5):503–510. doi: 10.1038/ni1582. Extensive review that discusses the various functions of NK cells. [DOI] [PubMed] [Google Scholar]
- 6.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7(5):329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 7.Boudreau JE, Stephenson KB, Wang F, et al. IL-15 and type I interferon are required for activation of tumoricidal NK cells by virus-infected dendritic cells. Cancer Res. 2011;71(7):2497–2506. doi: 10.1158/0008-5472.CAN-10-3025. [DOI] [PubMed] [Google Scholar]
- 8▪▪.Shanker A, Verdeil G, Buferne M, et al. CD8 T cell help for innate antitumor immunity. J Immunol. 179(10):6651–6662. doi: 10.4049/jimmunol.179.10.6651. Showed for the first time that activated antigen-specific CD8 T cells have the potential to activate dormant NK cell function in a tumor model. [DOI] [PubMed] [Google Scholar]
- 9▪.O’leary JG, Goodarzi M, Drayton DL, Von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 7(5):507–516. doi: 10.1038/ni1332. Together with references [10] and [12], presented the first evidence for adaptive immune features in NK cells. [DOI] [PubMed] [Google Scholar]
- 10▪.Paust S, Gill HS, Wang BZ, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 11(12):1127–1135. doi: 10.1038/ni.1953. Together with references [9] and [12], presented the first evidence for adaptive immune features in NK cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun JC, Lanier LL. Versatility in NK cell memory. Immunol Cell Biol. 2011;89(3):327–329. doi: 10.1038/icb.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 457(7229):557–561. doi: 10.1038/nature07665. Together with references [9] and [10], presented the first evidence for adaptive immune features in NK cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz J. Specific memory within innate immune systems. Trends Immunol. 2005;26(4):186–192. doi: 10.1016/j.it.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Roth O, Sadd BM, Schmid-Hempel P, Kurtz J. Strain-specific priming of resistance in the red flour beetleTribolium castaneum. Proc Biol Sci. 2009;276(1654):145–151. doi: 10.1098/rspb.2008.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth O, Kurtz J. Phagocytosis mediates specificity in the immune defence of an invertebrate, the woodlouse Porcellio scaber (Crustacea: Isopoda) Dev Comp Immunol. 2009;33(11):1151–1155. doi: 10.1016/j.dci.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 17▪.Saleh A, Davies GE, Pascal V, et al. Identification of probabilistic transcriptional switches in the Ly49 gene cluster: a eukaryotic mechanism for selective gene activation. Immunity. 21(1):55–66. doi: 10.1016/j.immuni.2004.06.005. References [17] and [18] were the first to demonstrate the existence of stochastic bidirectional promoter for Ly49 and KIR gene clusters. [DOI] [PubMed] [Google Scholar]
- 18▪.Davies GE, Locke SM, Wright PW, et al. Identification of bidirectional promoters in the human KIR genes. Genes Immun. 8(3):245–253. doi: 10.1038/sj.gene.6364381. References [17] and [18] were the first to demonstrate the existence of stochastic bidirectional promoter for Ly49 and KIR gene clusters. [DOI] [PubMed] [Google Scholar]
- 19.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358(6381):66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 21.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5(3):201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 22.Carlyle JR, Mesci A, Fine JH, et al. Evolution of the Ly49 and Nkrp1 recognition systems. Semin Immunol. 2008;20(6):321–330. doi: 10.1016/j.smim.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Braud VM, Allan DS, O’Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391(6669):795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 24.Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b) J Exp Med. 1998;188(10):1841–1848. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guma M, Angulo A, Lopez-Botet M. NK cell receptors involved in the response to human cytomegalovirus infection. Curr Top Microbiol Immunol. 2006;298:207–223. doi: 10.1007/3-540-27743-9_11. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 27.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6(7):520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 28▪.Peterson ME, Long EO. Inhibitory receptor signaling via tyrosine phosphorylation of the adaptor Crk. Immunity. 29(4):578–588. doi: 10.1016/j.immuni.2008.07.014. Study identified the potential pathways of interaction between the NK cell inhibitory receptor signaling and activating receptor signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasser S, Raulet DH. Activation and self-tolerance of natural killer cells. Immunol Rev. 2006;214:130–142. doi: 10.1111/j.1600-065X.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 30▪.Chalifour A, Scarpellino L, Back J, et al. A role for cis interaction between the inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 30(3):337–347. doi: 10.1016/j.immuni.2008.12.019. Study showed that NK cells can be activated by cis-interactions between Ly49 receptors and MHC-I molecules on NK cell membranes. [DOI] [PubMed] [Google Scholar]
- 31.Back J, Malchiodi EL, Cho S, et al. Distinct conformations of Ly49 natural killer cell receptors mediate MHC class I recognition in trans and cis. Immunity. 2009;31(4):598–608. doi: 10.1016/j.immuni.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson KE, Williams GS, Davis DM, Hoglund P. Quantifying the reduction in accessibility of the inhibitory NK cell receptor Ly49A caused by binding MHC class I proteins in cis. Eur J Immunol. 2007;37(2):516–527. doi: 10.1002/eji.200636693. [DOI] [PubMed] [Google Scholar]
- 33.Jonsson AH, Yang L, Kim S, Taffner SM, Yokoyama WM. Effects of MHC class I alleles on licensing of Ly49A+ NK cells. J Immunol. 2010;184(7):3424–3432. doi: 10.4049/jimmunol.0904057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪.Johansson S, Johansson M, Rosmaraki E, et al. Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules. J Exp Med. 201(7):1145–1155. doi: 10.1084/jem.20050167. Demonstrated for the first time that NK cell activation is a quantitative process dependent on different MHC-I alleles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182(8):4572–4580. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 113(11):2434–2441. doi: 10.1182/blood-2008-05-156836. Demonstrated that the strength of inhibitory signal by MHC-I ligands through Ly49 receptors determines the threshold for NK cell activation. [DOI] [PubMed] [Google Scholar]
- 37.Brodin P, Karre K, Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30(4):143–149. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17(1):19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 39.Ogasawara K, Lanier Ll. NKG2D in NK and T cell-mediated immunity. J Clin Immunol. 2005;25(6):534–540. doi: 10.1007/s10875-005-8786-4. [DOI] [PubMed] [Google Scholar]
- 40.Wu J, Song Y, Bakker Ab, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285(5428):730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 41.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3(12):1150–1155. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 42.Garrity D, Call ME, Feng J, Wucherpfennig KW. The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proc Natl Acad Sci USA. 2005;102(21):7641–7646. doi: 10.1073/pnas.0502439102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J, Cherwinski H, Spies T, Phillips JH, Lanier LL. DAP10 and DAP12 form distinct, but functionally cooperative, receptor complexes in natural killer cells. J Exp Med. 2000;192(7):1059–1068. doi: 10.1084/jem.192.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosen DB, Araki M, Hamerman JA, Chen T, Yamamura T, Lanier LL. A structural basis for the association of DAP12 with mouse, but not human, NKG2D. J Immunol. 2004;173(4):2470–2478. doi: 10.4049/jimmunol.173.4.2470. [DOI] [PubMed] [Google Scholar]
- 45.Awasthi A, Samarakoon A, Chu H, et al. Rap1b facilitates NK cell functions via IQGAP1-mediated signalosomes. J Exp Med. 2010;207(9):1923–1938. doi: 10.1084/jem.20100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segovis CM, Schoon RA, Dick CJ, Nacusi LP, Leibson PJ, Billadeau DD. PI3K links NKG2D signaling to a CrkL pathway involved in natural killer cell adhesion, polarity, and granule secretion. J Immunol. 2009;182(11):6933–6942. doi: 10.4049/jimmunol.0803840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Upshaw JL, Arneson LN, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nat Immunol. 2006;7(5):524–532. doi: 10.1038/ni1325. [DOI] [PubMed] [Google Scholar]
- 48.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol. 2003;4(6):557–564. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 49▪.Horng T, Bezbradica JS, Medzhitov R. NKG2D signaling is coupled to the interleukin 15 receptor signaling pathway. Nat Immunol. 8(12):1345–1352. doi: 10.1038/ni1524. References [49] and [50] showed that NKG2D signaling is coupled to IL-15 heterologous receptor signaling. [DOI] [PubMed] [Google Scholar]
- 50▪.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 26(4):503–517. doi: 10.1016/j.immuni.2007.03.006. References [49] and [50] showed that NKG2D signaling is coupled to IL-15 heterologous receptor signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huntington ND, Legrand N, Alves NL, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206(1):25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306(5701):1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 53.Martinez E, Brzostowski JA, Long EO, Gross CC. Cutting edge: NKG2D-dependent cytotoxicity is controlled by ligand distribution in the target cell membrane. J Immunol. 2011;186(10):5538–5542. doi: 10.4049/jimmunol.1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bryceson YT, Chiang SC, Darmanin S, et al. Molecular mechanisms of natural killer cell activation. J Innate Immun. 2011;3(3):216–226. doi: 10.1159/000325265. [DOI] [PubMed] [Google Scholar]
- 55.Hoffmann SC, Cohnen A, Ludwig T, Watzl C. 2B4 Engagement mediates rapid LFA-1 and actin-dependent NK cell adhesion to tumor cells as measured by single cell force spectroscopy. J Immunol. 2011;186(5):2757–2764. doi: 10.4049/jimmunol.1002867. [DOI] [PubMed] [Google Scholar]
- 56.Tassi I, Klesney-Tait J, Colonna M. Dissecting natural killer cell activation pathways through analysis of genetic mutations in human and mouse. Immunol Rev. 2006;214:92–105. doi: 10.1111/j.1600-065X.2006.00463.x. [DOI] [PubMed] [Google Scholar]
- 57.Riteau B, Barber DF, Long EO. Vav1 phosphorylation is induced by β2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J Exp Med. 2003;198(3):469–474. doi: 10.1084/jem.20021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gismondi A, Bisogno L, Mainiero F, et al. Proline-rich tyrosine kinase-2 activation by β 1 integrin fibronectin receptor cross-linking and association with paxillin in human natural killer cells. J Immunol. 1997;159(10):4729–4736. [PubMed] [Google Scholar]
- 59.Palmieri G, Serra A, De Maria R, et al. Cross-linking of β 4 β 1 and β 5 β 1 fibronectin receptors enhances natural killer cell cytotoxic activity. J Immunol. 1995;155(11):5314–5322. [PubMed] [Google Scholar]
- 60.Bottino C, Castriconi R, Pende D, et al. Identification of PVR (CD155) and nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198(4):557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tahara-Hanaoka S, Miyamoto A, Hara A, Honda S, Shibuya K, Shibuya A. Identification and characterization of murine DNAM-1 (CD226) and its poliovirus receptor family ligands. Biochem Biophys Res Commun. 2005;329(3):996–1000. doi: 10.1016/j.bbrc.2005.02.067. [DOI] [PubMed] [Google Scholar]
- 62.Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155) J Immunol. 2004;172(7):3994–3998. doi: 10.4049/jimmunol.172.7.3994. [DOI] [PubMed] [Google Scholar]
- 63.Davis DM. Mechanisms and functions for the duration of intercellular contacts made by lymphocytes. Nat Rev Immunol. 2009;9(8):543–555. doi: 10.1038/nri2602. [DOI] [PubMed] [Google Scholar]
- 64.Culley FJ, Johnson M, Evans JH, et al. Natural killer cell signal integration balances synapse symmetry and migration. PLoS Biol. 2009;7(7):e1000159. doi: 10.1371/journal.pbio.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8(9):713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66▪▪.Chauveau A, Aucher A, Eissmann P, Vivier E, Davis DM. Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proc Natl Acad Sci USA. 107(12):5545–5550. doi: 10.1073/pnas.0910074107. Demonstrates the importance of nanotubes in NK cell-mediated target cell recognition and cytotoxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9(6):431–436. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- 68.Sowinski S, Jolly C, Berninghausen O, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10(2):211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 69.Onfelt B, Nedvetzki S, Benninger RK, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177(12):8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 70.Onfelt B, Purbhoo MA, Nedvetzki S, Sowinski S, Davis DM. Long-distance calls between cells connected by tunneling nanotubules. Sci STKE. 2005;(313):pe55. doi: 10.1126/stke.3132005pe55. [DOI] [PubMed] [Google Scholar]
- 71.Onfelt B, Davis DM. Can membrane nanotubes facilitate communication between immune cells? Biochem Soc Trans. 2004;32(Pt 5):676–678. doi: 10.1042/BST0320676. [DOI] [PubMed] [Google Scholar]
- 72.Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: membrane nanotubes connect immune cells. J Immunol. 2004;173(3):1511–1513. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- 73.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303(5660):1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 74.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23(3):309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 75.Di Santo JP. Natural killer cells: diversity in search of a niche. Nat Immunol. 2008;9(5):473–475. doi: 10.1038/ni.f.201. [DOI] [PubMed] [Google Scholar]
- 76▪.Stebbins CC, Watzl C, Billadeau DD, Leibson PJ, Burshtyn DN, Long EO. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol. 23(17):6291–6299. doi: 10.1128/MCB.23.17.6291-6299.2003. Showed for the first time that NK cell inhibitory receptor signaling can inhibit activating signals by dephosphorylating Vav1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yokoyama WM. Inhibitory receptors signal activation. Immunity. 2008;29(4):515–517. doi: 10.1016/j.immuni.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 78.Kim HS, Das A, Gross CC, Bryceson YT, Long EO. Synergistic signals for natural cytotoxicity are required to overcome inhibition by c-Cbl ubiquitin ligase. Immunity. 2010;32(2):175–186. doi: 10.1016/j.immuni.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guia S, Jaeger BN, Piatek S, et al. Confinement of activating receptors at the plasma membrane controls natural killer cell tolerance. Sci Signal. 2011;4(167):ra21. doi: 10.1126/scisignal.2001608. [DOI] [PubMed] [Google Scholar]
- 80.Mesecke S, Urlaub D, Busch H, Eils R, Watzl C. Integration of activating and inhibitory receptor signaling by regulated phosphorylation of vav1 in immune cells. Sci Signal. 2011;4(175):ra36. doi: 10.1126/scisignal.2001325. [DOI] [PubMed] [Google Scholar]
- 81.Vosshenrich CA, Ranson T, Samson SI, et al. Roles for common cytokine receptor γ-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174(3):1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 82.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fehniger TA, Cooper MA, Nuovo GJ, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101(8):3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 84▪.Joncker NT, Shifrin N, Delebecque F, Raulet DH. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 207(10):2065–2072. doi: 10.1084/jem.20100570. References [84] and [85] show the importance of MHC-I environment in NK cell activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85▪.Elliott JM, Wahle JA, Yokoyama WM. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J Exp Med. 207(10):2073–2079. doi: 10.1084/jem.20100986. References [84] and [85] show the importance of MHC-I environment in NK cell activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kallies A, Carotta S, Huntington ND, et al. A role for Blimp1 in the transcriptional network controlling natural killer cell maturation. Blood. 2011;117(6):1869–1879. doi: 10.1182/blood-2010-08-303123. [DOI] [PubMed] [Google Scholar]
- 87.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113(22):5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 88.Bjorkstrom NK, Riese P, Heuts F, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116(19):3853–3864. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 89.Juelke K, Killig M, Luetke-Eversloh M, et al. CD62L expression identifies a unique subset of polyfunctional CD56dim NK cells. Blood. 2010;116(8):1299–1307. doi: 10.1182/blood-2009-11-253286. [DOI] [PubMed] [Google Scholar]
- 90.Marcenaro E, Cantoni C, Pesce S, et al. Uptake of CCR7 and acquisition of migratory properties by human KIR+ NK cells interacting with monocyte-derived DC or EBV cell lines: regulation by KIR/HLA-class I interaction. Blood. 2009;114(19):4108–4116. doi: 10.1182/blood-2009-05-222265. [DOI] [PubMed] [Google Scholar]
- 91.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176(3):1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 92.Ferlazzo G, Thomas D, Lin SL, et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172(3):1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 93.Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2010;184(1):503–511. doi: 10.4049/jimmunol.0902189. [DOI] [PubMed] [Google Scholar]
- 94.Lee S, Kim J, Jang B, et al. Fluctuation of peripheral blood T, B, and NK cells during a menstrual cycle of normal healthy women. J Immunol. 2010;185(1):756–762. doi: 10.4049/jimmunol.0904192. [DOI] [PubMed] [Google Scholar]
- 95.Bellora F, Castriconi R, Dondero A, et al. The interaction of human natural killer cells with either unpolarized or polarized macrophages results in different functional outcomes. Proc Natl Acad Sci USA. 2010;107(50):21659–21664. doi: 10.1073/pnas.1007654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shanker A. Adaptive control of innate immunity. Immunol Lett. 2010;131(2):107–112. doi: 10.1016/j.imlet.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shanker A, Buferne M, Schmitt-Verhulst AM. Cooperative action of CD8 T lymphocytes and natural killer cells controls tumour growth under conditions of restricted T-cell receptor diversity. Immunology. 2010;129(1):41–54. doi: 10.1111/j.1365-2567.2009.03150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5(2):112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 99.Eissmann P, Evans JH, Mehrabi M, Rose EL, Nedvetzki S, Davis DM. Multiple mechanisms downstream of TLR-4 stimulation allow expression of NKG2D ligands to facilitate macrophage/NK cell crosstalk. J Immunol. 2010;184(12):6901–6909. doi: 10.4049/jimmunol.0903985. [DOI] [PubMed] [Google Scholar]
- 100.Shah PD, Gilbertson SM, Rowley DA. Dendritic cells that have interacted with antigen are targets for natural killer cells. J Exp Med. 1985;162(2):625–636. doi: 10.1084/jem.162.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5(10):996–1002. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- 102.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195(3):335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti tumor immune responses in vivo. Nat Med. 1999;5(4):405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 104.Long EO. Ready for prime time: NK cell priming by dendritic cells. Immunity. 2007;26(4):385–387. doi: 10.1016/j.immuni.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 105.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195(3):343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poggi A, Prevosto C, Zancolli M, Canevali P, Musso A, Zocchi MR. NKG2D and natural cytotoxicity receptors are involved in natural killer cell interaction with self-antigen presenting cells and stromal cells. Ann NY Acad Sci. 2007;1109:47–57. doi: 10.1196/annals.1398.007. [DOI] [PubMed] [Google Scholar]
- 107.Wai LE, Garcia JA, Martinez OM, Krams SM. Distinct roles for the NK cell-activating receptors in mediating interactions with dendritic cells and tumor cells. J Immunol. 2011;186(1):222–229. doi: 10.4049/jimmunol.1002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Persson CM, Chambers BJ. Plasmacytoid dendritic cell-induced migration and activation of NK cells in vivo. Eur J Immunol. 2010;40(8):2155–2164. doi: 10.1002/eji.200940098. [DOI] [PubMed] [Google Scholar]
- 109.Vujanovic L, Szymkowski DE, Alber S, Watkins SC, Vujanovic NL, Butterfield LH. Virally infected and matured human dendritic cells activate natural killer cells via cooperative activity of plasma membrane-bound TNF and IL-15. Blood. 2010;116(4):575–583. doi: 10.1182/blood-2009-08-240325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kloss M, Decker P, Baltz KM, et al. Interaction of monocytes with NK cells upon Toll-like receptor-induced expression of the NKG2D ligand MICA. J Immunol. 2008;181(10):6711–6719. doi: 10.4049/jimmunol.181.10.6711. [DOI] [PubMed] [Google Scholar]
- 111.Newman KC, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol. 2007;7(4):279–291. doi: 10.1038/nri2057. [DOI] [PubMed] [Google Scholar]
- 112.Lapaque N, Walzer T, Meresse S, Vivier E, Trowsdale J. Interactions between human NK cells and macrophages in response to Salmonella infection. J Immunol. 2009;182(7):4339–4348. doi: 10.4049/jimmunol.0803329. [DOI] [PubMed] [Google Scholar]
- 113.Basu S, Eriksson M, Pioli PA, et al. Human uterine NK cells interact with uterine macrophages via NKG2D upon stimulation with PAMPs. Am J Reprod Immunol. 2009;61(1):52–61. doi: 10.1111/j.1600-0897.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nedvetzki S, Sowinski S, Eagle RA, et al. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109(9):3776–3785. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 115.Lunemann A, Lunemann JD, Roberts S, et al. Human NK cells kill resting but not activated microglia via NKG2D- and NKp46-mediated recognition. J Immunol. 2008;181(9):6170–6177. doi: 10.4049/jimmunol.181.9.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bajenoff M, Breart B, Huang AY, et al. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203(3):619–631. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nat Immunol. 2005;6(6):600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 119.Morandi B, Bougras G, Muller WA, Ferlazzo G, Munz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-γ secretion. Eur J Immunol. 2006;36(9):2394–2400. doi: 10.1002/eji.200636290. [DOI] [PubMed] [Google Scholar]
- 120.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40–OX40 ligand interactions. J Immunol. 2004;173(6):3716–3724. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 121.Cerboni C, Zingoni A, Cippitelli M, Piccoli M, Frati L, Santoni A. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK- cell lysis. Blood. 2007;110(2):606–615. doi: 10.1182/blood-2006-10-052720. [DOI] [PubMed] [Google Scholar]
- 122.Roy S, Barnes PF, Garg A, Wu S, Cosman D, Vankayalapati R. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J Immunol. 2008;180(3):1729–1736. doi: 10.4049/jimmunol.180.3.1729. [DOI] [PubMed] [Google Scholar]
- 123.Andrews DM, Estcourt MJ, Andoniou CE, et al. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med. 2010;207(6):1333–1343. doi: 10.1084/jem.20091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ardolino M, Zingoni A, Cerboni C, et al. DNAM-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK–T cell interaction. Blood. 2011;117(18):4778–4786. doi: 10.1182/blood-2010-08-300954. [DOI] [PubMed] [Google Scholar]
- 125.Gilfillan S, Chan CJ, Cella M, et al. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med. 2008;205(13):2965–2973. doi: 10.1084/jem.20081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol. 2010;184(11):6043–6052. doi: 10.4049/jimmunol.1000106. [DOI] [PubMed] [Google Scholar]
- 127.Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol. 2010;185(5):2808–2818. doi: 10.4049/jimmunol.1000844. [DOI] [PubMed] [Google Scholar]
- 128.Shanker A, Marincola FM. Cooperativity of adaptive and innate immunity: implications for cancer therapy. Cancer Immunol Immunother. 2011;60(8):1061–1074. doi: 10.1007/s00262-011-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harker JA, Godlee A, Wahlsten JL, et al. Interleukin 18 coexpression during respiratory syncytial virus infection results in enhanced disease mediated by natural killer cells. J Virol. 2010;84(8):4073–4082. doi: 10.1128/JVI.02014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology. 2009;128(2):151–163. doi: 10.1111/j.1365-2567.2009.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anthony DA, Andrews DM, Chow M, et al. A role for granzyme M in TLR4-driven inflammation and endotoxicosis. J Immunol. 2010;185(3):1794–1803. doi: 10.4049/jimmunol.1000430. [DOI] [PubMed] [Google Scholar]
- 132.Colonna M, Jonjic S, Watzl C. Natural killer cells: fighting viruses and much more. Nat Immunol. 2011;12(2):107–110. doi: 10.1038/ni0211-107. [DOI] [PubMed] [Google Scholar]
- 133.Miyake T, Satoh T, Kato H, et al. IκBζ is essential for natural killer cell activation in response to IL-12 and IL-18. Proc Natl Acad Sci USA. 2010;107(41):17680–17685. doi: 10.1073/pnas.1012977107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Van Domselaar R, Philippen LE, Quadir R, Wiertz EJ, Kummer JA, Bovenschen N. Noncytotoxic inhibition of cytomegalovirus replication through NK cell protease granzyme M-mediated cleavage of viral phosphoprotein 71. J Immunol. 2010;185(12):7605–7613. doi: 10.4049/jimmunol.1001503. [DOI] [PubMed] [Google Scholar]
- 135.Muntasell A, Magri G, Pende D, Angulo A, Lopez-Botet M. Inhibition of NKG2D expression in NK cells by cytokines secreted in response to human cytomegalovirus infection. Blood. 2010;115(25):5170–5179. doi: 10.1182/blood-2009-11-256479. [DOI] [PubMed] [Google Scholar]
- 136.Slavuljica I, Busche A, Babic M, et al. Recombinant mouse cytomegalovirus expressing a ligand for the NKG2D receptor is attenuated and has improved vaccine properties. J Clin Invest. 2010;120(12):4532–4545. doi: 10.1172/JCI43961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Crotta S, Brazzoli M, Piccioli D, Valiante NM, Wack A. Hepatitis C virions subvert natural killer cell activation to generate a cytokine environment permissive for infection. J Hepatol. 2010;52(2):183–190. doi: 10.1016/j.jhep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 138.Carrington M, Martin MP, Van Bergen J. KIR-HLA intercourse in HIV disease. Trends Microbiol. 2008;16(12):620–627. doi: 10.1016/j.tim.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim AY, Kuntzen T, Timm J, et al. Spontaneous control of HCV is associated with expression of HLA-B 57 and preservation of targeted epitopes. Gastroenterology. 2011;140(2):686–696. doi: 10.1053/j.gastro.2010.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Khakoo SI, Thio CL, Martin MP, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305(5685):872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 141.Vidal-Castineira JR, Lopez-Vazquez A, Diaz-Pena R, et al. Effect of killer immunoglobulin-like receptors in the response to combined treatment in patients with chronic hepatitis C virus infection. J Virol. 2010;84(1):475–481. doi: 10.1128/JVI.01285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ahlenstiel G, Martin MP, Gao X, Carrington M, Rehermann B. Distinct KIR/HLA compound genotypes affect the kinetics of human antiviral natural killer cell responses. J Clin Invest. 2008;118(3):1017–1026. doi: 10.1172/JCI32400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peppa D, Micco L, Javaid A, et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog. 2010;6(12):e1001227. doi: 10.1371/journal.ppat.1001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Melki MT, Saidi H, Dufour A, Olivo-Marin JC, Gougeon ML. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk – a pivotal role of HMGB1. PLoS Pathog. 2010;6(4):e1000862. doi: 10.1371/journal.ppat.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alter G, Martin MP, Teigen N, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204(12):3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alter G, Rihn S, Walter K, et al. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J Virol. 2009;83(13):6798–6805. doi: 10.1128/JVI.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alter G, Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J Intern Med. 2009;265(1):29–42. doi: 10.1111/j.1365-2796.2008.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kulkarni S, Savan R, Qi Y, et al. Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature. 2011;472(7344):495–498. doi: 10.1038/nature09914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Johansson SE, Hejdeman B, Hinkula J, et al. NK cell activation by KIR-binding antibody 1–7F9 and response to HIV-infected autologous cells in viremic and controller HIV-infected patients. Clin Immunol. 2010;134(2):158–168. doi: 10.1016/j.clim.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 150.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184(10):5914–5927. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 151.Bjorkstrom NK, Lindgren T, Stoltz M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208(1):13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Winkler-Pickett R, Young HA, Cherry JM, et al. In vivo regulation of experimental autoimmune encephalomyelitis by NK cells: alteration of primary adaptive responses. J Immunol. 2008;180(7):4495–4506. doi: 10.4049/jimmunol.180.7.4495. [DOI] [PubMed] [Google Scholar]
- 153.Shi FD, Takeda K, Akira S, Sarvetnick N, Ljunggren HG. IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-γ by NK cells. J Immunol. 2000;165(6):3099–3104. doi: 10.4049/jimmunol.165.6.3099. [DOI] [PubMed] [Google Scholar]
- 154.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200(1):79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hamann I, Unterwalder N, Cardona AE, et al. Analyses of phenotypic and functional characteristics of CX3CR1-expressing natural killer cells. Immunology. 2011;133(1):62–73. doi: 10.1111/j.1365-2567.2011.03409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bielekova B, Becker BL. Monoclonal antibodies in MS: mechanisms of action. Neurology. 2010;74(Suppl 1):S31–S40. doi: 10.1212/WNL.0b013e3181c97ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Verbsky JW, Grossman WJ. Hemophagocytic lymphohistiocytosis: diagnosis, pathophysiology, treatment, and future perspectives. Ann Med. 2006;38(1):20–31. doi: 10.1080/07853890500465189. [DOI] [PubMed] [Google Scholar]
- 158.Villanueva J, Lee S, Giannini EH, et al. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res Ther. 2005;7(1):R30–R37. doi: 10.1186/ar1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Soderstrom K, Stein E, Colmenero P, et al. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc Natl Acad Sci USA. 2010;107(29):13028–13033. doi: 10.1073/pnas.1000546107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.De Matos CT, Berg L, Michaelsson J, Fellander-Tsai L, Karre K, Soderstrom K. Activating and inhibitory receptors on synovial fluid natural killer cells of arthritis patients: role of CD94/NKG2A in control of cytokine secretion. Immunology. 2007;122(2):291–301. doi: 10.1111/j.1365-2567.2007.02638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nelson GW, Martin MP, Gladman D, Wade J, Trowsdale J, Carrington M. Cutting edge: heterozygote advantage in autoimmune disease: hierarchy of protection/susceptibility conferred by HLA and killer Ig-like receptor combinations in psoriatic arthritis. J Immunol. 2004;173(7):4273–4276. doi: 10.4049/jimmunol.173.7.4273. [DOI] [PubMed] [Google Scholar]
- 162.Luci C, Reynders A, Ivanov Ii, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10(1):75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 163.Aktas E, Akdis M, Bilgic S, et al. Different natural killer (NK) receptor expression and immunoglobulin E (IgE) regulation by NK1 and NK2 cells. Clin Exp Immunol. 2005;140(2):301–309. doi: 10.1111/j.1365-2249.2005.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ottaviani C, Nasorri F, Bedini C, De Pita O, Girolomoni G, Cavani A. CD56brightCD16(−) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006;36(1):118–128. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- 165.Ito T, Ito N, Saatoff M, et al. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermatol. 2008;128(5):1196–1206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- 166.Sargent IL, Borzychowski AM, Redman CW. NK cells and pre eclampsia. J Reprod Immunol. 2007;76(1–2):40–44. doi: 10.1016/j.jri.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 167.Guerra N, Tan YX, Joncker NT, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9(5):486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 169.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 170.Pende D, Marcenaro S, Falco M, et al. Anti leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113(13):3119–3129. doi: 10.1182/blood-2008-06-164103. [DOI] [PubMed] [Google Scholar]
- 171.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wadler S, Schwartz EL. Antineoplastic activity of the combination of interferon and cytotoxic agents against experimental and human malignancies: a review. Cancer Res. 1990;50(12):3473–3486. [PubMed] [Google Scholar]
- 173.Metcalf D. The colony-stimulating factors and cancer. Nat Rev Cancer. 2010;10(6):425–434. doi: 10.1038/nrc2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271(12):907–913. [PubMed] [Google Scholar]
- 175.Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol. 2005;6(11):1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- 176.Kobayashi H, Dubois S, Sato N, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105(2):721–727. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 177.Bodnar A, Nizsaloczki E, Mocsar G, et al. A biophysical approach to IL-2 and IL-15 receptor function: localization, conformation and interactions. Immunol Lett. 2008;116(2):117–125. doi: 10.1016/j.imlet.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 178.Carson WE, Ross ME, Baiocchi RA, et al. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-γ by natural killer cells in vitro. J Clin Invest. 1995;96(6):2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Meresse B, Chen Z, Ciszewski C, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21(3):357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 180.Zhang H, Cui Y, Voong N, et al. Activating signals dominate inhibitory signals in CD137L/IL-15 activated natural killer cells. J Immunother. 2011;34(2):187–195. doi: 10.1097/CJI.0b013e31820d2a21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Waldmann TA, Lugli E, Roederer M, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117(18):4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chaix J, Tessmer MS, Hoebe K, et al. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181(3):1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Guia S, Cognet C, De Beaucoudrey L, et al. A role for interleukin-12/23 in the maturation of human natural killer and CD56+ T cells in vivo. Blood. 2008;111(10):5008–5016. doi: 10.1182/blood-2007-11-122259. [DOI] [PubMed] [Google Scholar]
- 184.Sagar J, Chaib B, Sales K, Winslet M, Seifalian A. Role of stem cells in cancer therapy and cancer stem cells: a review. Cancer Cell Int. 2007;7:9. doi: 10.1186/1475-2867-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Talmadge JE, Reed E, Ino K, et al. Rapid immunologic reconstitution following transplantation with mobilized peripheral blood stem cells as compared with bone marrow. Bone Marrow Transplant. 1997;19(2):161–172. doi: 10.1038/sj.bmt.1700626. [DOI] [PubMed] [Google Scholar]
- 186.Fermand JP, Katsahian S, Divine M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol. 2005;23(36):9227–9233. doi: 10.1200/JCO.2005.03.0551. [DOI] [PubMed] [Google Scholar]
- 187.Nitz UA, Mohrmann S, Fischer J, et al. Comparison of rapidly cycled tandem high-dose chemotherapy plus peripheral-blood stem-cell support versus dose-dense conventional chemotherapy for adjuvant treatment of high-risk breast cancer: results of a multicentre Phase III trial. Lancet. 2005;366(9501):1935–1944. doi: 10.1016/S0140-6736(05)67784-7. [DOI] [PubMed] [Google Scholar]
- 188.Balduzzi A, Valsecchi MG, Uderzo C, et al. Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet. 2005;366(9486):635–642. doi: 10.1016/S0140-6736(05)66998-X. [DOI] [PubMed] [Google Scholar]
- 189.Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6(9):649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 190.Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105(7):2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 191.Valteau-Couanet D, Faucher C, Auperin A, et al. Cost effectiveness of day 5 G-CSF (Lenograstim) administration after PBSC transplantation: results of a SFGM-TC randomised trial. Bone Marrow Transplant. 2005;36(6):547–552. doi: 10.1038/sj.bmt.1705097. [DOI] [PubMed] [Google Scholar]
- 192.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105(11):4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Anfossi N, Andre P, Guia S, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 194.Cooley S, Xiao F, Pitt M, et al. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110(2):578–586. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110(1):433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Witt CS. The influence of NK alloreactivity on matched unrelated donor and HLA identical sibling haematopoietic stem cell transplantation. Curr Opin Immunol. 2009;21(5):531–537. doi: 10.1016/j.coi.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 197.Moretta L, Locatelli F, Pende D, Mingari MC, Moretta A. Natural killer alloeffector responses in haploidentical hemopoietic stem cell transplantation to treat high-risk leukemias. Tissue Antigens. 2010;75(2):103–109. doi: 10.1111/j.1399-0039.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- 198.Sobecks RM, Ball EJ, Maciejewski JP, et al. Survival of AML patients receiving HLA-matched sibling donor allogeneic Bone Marrow Transplantation correlates with HLA-Cw ligand groups for killer immunoglobulin-like receptors. Bone Marrow Transplant. 2007;39(7):417–424. doi: 10.1038/sj.bmt.1705609. [DOI] [PubMed] [Google Scholar]
- 199.Yu J, Venstrom JM, Liu XR, et al. Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non self HLA exhibit effector function after T cell-depleted allogeneic hematopoietic cell transplantation. Blood. 2009;113(16):3875–3884. doi: 10.1182/blood-2008-09-177055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(14):2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201▪.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. Provided first demonstration of therapeutic benefits of haploidential NK cell transfers. [DOI] [PubMed] [Google Scholar]
- 202.Nguyen S, Beziat V, Norol F, et al. Infusion of allogeneic natural killer cells in a patient with acute myeloid leukemia in relapse after haploidentical hematopoietic stem cell transplantation. Transfusion. 2011;51(8):1769–1778. doi: 10.1111/j.1537-2995.2010.03058.x. [DOI] [PubMed] [Google Scholar]
- 203.Barkholt L, Alici E, Conrad R, et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a phase I clinical study. Immunotherapy. 2009;1(5):753–764. doi: 10.2217/imt.09.47. [DOI] [PubMed] [Google Scholar]
- 204.Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69(9):4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Berg M, Childs R. Ex-vivo expansion of NK cells: what is the priority – high yield or high purity? Cytotherapy. 2010;12(8):969–970. doi: 10.3109/14653249.2010.536216. [DOI] [PubMed] [Google Scholar]
- 206.Berg M, Lundqvist A, Mccoy P, Jr, et al. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. 2009;11(3):341–355. doi: 10.1080/14653240902807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Geller MA, Cooley S, Judson PL, et al. A Phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13(1):98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106(6):1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tam YK, Martinson JA, Doligosa K, Klingemann HG. Ex vivo expansion of the highly cytotoxic human natural killer-92 cell-line under current good manufacturing practice conditions for clinical adoptive cellular immunotherapy. Cytotherapy. 2003;5(3):259–272. doi: 10.1080/14653240310001523. [DOI] [PubMed] [Google Scholar]
- 210.Klingemann HG. Natural killer cell-based immunotherapeutic strategies. Cytotherapy. 2005;7(1):16–22. doi: 10.1080/14653240510018000. [DOI] [PubMed] [Google Scholar]
- 211.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rohner A, Langenkamp U, Siegler U, Kalberer CP, Wodnar-Filipowicz A. Differentiation-promoting drugs up-regulate NKG2D ligand expression and enhance the susceptibility of acute myeloid leukemia cells to natural killer cell-mediated lysis. Leuk Res. 2007;31(10):1393–1402. doi: 10.1016/j.leukres.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 213.Vahlne G, Lindholm K, Meier A, et al. In vivo tumor cell rejection induced by NK cell inhibitory receptor blockade: maintained tolerance to normal cells even in the presence of IL-2. Eur J Immunol. 2010;40(3):813–823. doi: 10.1002/eji.200939755. [DOI] [PubMed] [Google Scholar]
- 214.Romagne F, Andre P, Spee P, et al. Preclinical characterization of 1–7F9, a novel human anti KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114(13):2667–2677. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215▪.Ames E, Hallett WH, Murphy WJ. Sensitization of human breast cancer cells to natural killer cell-mediated cytotoxicity by proteasome inhibition. Clin Exp Immunol. 155(3):504–513. doi: 10.1111/j.1365-2249.2008.03818.x. Along with references [217] and [218], showed that bortezomib could be combined with NK cell transfers in vivo for therapeutic benefits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Butler JE, Moore MB, Presnell SR, Chan HW, Chalupny NJ, Lutz CT. Proteasome regulation of ULBP1 transcription. J Immunol. 2009;182(10):6600–6609. doi: 10.4049/jimmunol.0801214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hallett WH, Ames E, Motarjemi M, et al. Sensitization of tumor cells to NK cell-mediated killing by proteasome inhibition. J Immunol. 180(1):163–170. doi: 10.4049/jimmunol.180.1.163. Along with references [215] and [218], showed that bortezomib could be combined with NK cell transfers in vivo for therapeutic benefits. [DOI] [PubMed] [Google Scholar]
- 218.Lundqvist A, Yokoyama H, Smith A, Berg M, Childs R. Bortezomib treatment and regulatory T-cell depletion enhance the antitumor effects of adoptively infused NK cells. Blood. 113(24):6120–6127. doi: 10.1182/blood-2008-11-190421. Along with references [215] and [217], showed that bortezomib could be combined with NK cell transfers in vivo for therapeutic benefits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schwinn N, Vokhminova D, Sucker A, et al. Interferon-γ down-regulates NKG2D ligand expression and impairs the NKG2D-mediated cytolysis of MHC class I-deficient melanoma by natural killer cells. Int J Cancer. 2009;124(7):1594–1604. doi: 10.1002/ijc.24098. [DOI] [PubMed] [Google Scholar]
- 220.Shanker A, Brooks AD, Tristan CA, et al. Treating metastatic solid tumors with bortezomib and a tumor necrosis factor-related apoptosis-inducing ligand receptor agonist antibody. J Natl Cancer Inst. 2008;100(9):649–662. doi: 10.1093/jnci/djn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Brooks AD, Jacobsen KM, Li W, Shanker A, Sayers TJ. Bortezomib sensitizes human renal cell carcinomas to TRAIL apoptosis through increased activation of caspase-8 in the death-inducing signaling complex. Mol Cancer Res. 2010;8(5):729–738. doi: 10.1158/1541-7786.MCR-10-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wu P, Davies FE, Horton C, et al. The combination of cyclophosphomide, thalidomide and dexamethasone is an effective alternative to cyclophosphamide – vincristine – doxorubicin – methylprednisolone as induction chemotherapy prior to autologous transplantation for multiple myeloma: a case-matched analysis. Leuk Lymphoma. 2006;47(11):2335–2338. doi: 10.1080/10428190600821955. [DOI] [PubMed] [Google Scholar]
- 223.Davies FE, Raje N, Hideshima T, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98(1):210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 224.Diermayr S, Himmelreich H, Durovic B, et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood. 2008;111(3):1428–1436. doi: 10.1182/blood-2007-07-101311. [DOI] [PubMed] [Google Scholar]
- 225.Vanoosten RL, Moore JM, Karacay B, Griffith TS. Histone deacetylase inhibitors modulate renal cell carcinoma sensitivity to TRAIL/Apo-2L-induced apoptosis by enhancing TRAIL-R2 expression. Cancer Biol Ther. 2005;4(10):1104–1112. doi: 10.4161/cbt.4.10.2022. [DOI] [PubMed] [Google Scholar]
- 226.Markasz L, Stuber G, Vanherberghen B, et al. Effect of frequently used chemotherapeutic drugs on the cytotoxic activity of human natural killer cells. Mol Cancer Ther. 2007;6(2):644–654. doi: 10.1158/1535-7163.MCT-06-0358. [DOI] [PubMed] [Google Scholar]
- 227.Peer AJ, Grimm MJ, Zynda ER, Repasky EA. Diverse immune mechanisms may contribute to the survival benefit seen in cancer patients receiving hyperthermia. Immunol Res. 2010;46(1–3):137–154. doi: 10.1007/s12026-009-8115-8. [DOI] [PubMed] [Google Scholar]
- 228.Skitzki Jj, Repasky EA, Evans SS. Hyperthermia as an immunotherapy strategy for cancer. Curr Opin Investig Drugs. 2009;10(6):550–558. [PMC free article] [PubMed] [Google Scholar]
- 229.Lee CT, Mace T, Repasky EA. Hypoxia-driven immunosuppression: a new reason to use thermal therapy in the treatment of cancer? Int J Hyperthermia. 2010;26(3):232–246. doi: 10.3109/02656731003601745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chen Q, Appenheimer MM, Muhitch JB, et al. Thermal facilitation of lymphocyte trafficking involves temporal induction of intravascular ICAM-1. Microcirculation. 2009;16(2):143–158. doi: 10.1080/10739680802353850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chen Q, Fisher DT, Clancy KA, et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol. 2006;7(12):1299–1308. doi: 10.1038/ni1406. [DOI] [PubMed] [Google Scholar]
- 232.Burd R, Dziedzic TS, Xu Y, Caligiuri MA, Subjeck JR, Repasky EA. Tumor cell apoptosis, lymphocyte recruitment and tumor vascular changes are induced by low temperature, long duration (fever-like) whole body hyperthermia. J Cell Physiol. 1998;177(1):137–147. doi: 10.1002/(SICI)1097-4652(199810)177:1<137::AID-JCP15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 233.Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E, Repasky EA. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukoc Biol. 2007;82(5):1322–1331. doi: 10.1189/jlb.1106699. [DOI] [PubMed] [Google Scholar]
- 234.Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106(5):1544–1551. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Barber A, Zhang T, Sentman CL. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J Immunol. 2008;180(1):72–78. doi: 10.4049/jimmunol.180.1.72. [DOI] [PubMed] [Google Scholar]
- 236.Barber A, Rynda A, Sentman CL. Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol. 2009;183(11):6939–6947. doi: 10.4049/jimmunol.0902000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang T, Sentman CL. Cancer immunotherapy using a bispecific NK receptor fusion protein that engages both T cells and tumor cells. Cancer Res. 2011;71(6):2066–2076. doi: 10.1158/0008-5472.CAN-10-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106(1):376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pegram HJ, Jackson JT, Smyth MJ, Kershaw MH, Darcy PK. Adoptive transfer of gene-modified primary NK cells can specifically inhibit tumor progression in vivo. J Immunol. 2008;181(5):3449–3455. doi: 10.4049/jimmunol.181.5.3449. [DOI] [PubMed] [Google Scholar]
- 240.Nagashima S, Mailliard R, Kashii Y, et al. Stable transduction of the interleukin-2 gene into human natural killer cell lines and their phenotypic and functional characterization in vitro and in vivo. Blood. 1998;91(10):3850–3861. [PubMed] [Google Scholar]
- 241.Jiang W, Zhang J, Tian Z. Functional characterization of interleukin-15 gene transduction into the human natural killer cell line NKL. Cytotherapy. 2008;10(3):265–274. doi: 10.1080/14653240801965156. [DOI] [PubMed] [Google Scholar]
- 242.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 243.Shanker A, Sayers T. Sensitizing tumor cells to immune-mediated cytotoxicity. Adv Exp Med Biol. 2007;601:163–171. doi: 10.1007/978-0-387-72005-0_17. [DOI] [PubMed] [Google Scholar]
- 244▪▪.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 118(6):1991–2001. doi: 10.1172/JCI35180. Extensive review that discusses combinatorial approaches for enhancing immunotherapeutic benefits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Klingemann H, Boissel L. Targeted cellular therapy with natural killer cells. Horm Metab Res. 2008;40(2):122–125. doi: 10.1055/s-2007-1004576. [DOI] [PubMed] [Google Scholar]