Caspase-5 mRNA synthesis, protein expression, and catalytic activation were highly regulated in response to various proinflammatory stimuli, ATP, and ER stress inducers. Mutual activation of caspase-5 and -1 suggests caspase-5 may work predominantly in concert with caspase-1 in modulating hRPE inflammatory responses.
Abstract
Purpose.
To investigate the expression, activation, and functional involvement of caspase-5 in human retinal pigment epithelial (hRPE) cells.
Methods.
Expression and activation of caspase-5 in primary cultured hRPE cells, telomerase-immortalized hTERT-RPE1 cells (hTERT-RPE1), or both, were measured after stimulation with proinflammatory agents IL-1β, TNF-α, lipopolysaccharide (LPS), interferon-γ, monocyte coculture, adenosine triphosphate (ATP), or endoplasmic reticulum (ER) stress inducers. Immunomodulating agents dexamethasone (Dex), IL-10, and triamcinolone acetonide (TA) were used to antagonize proinflammatory stimulation. Cell death ELISA and TUNEL staining assays were used to assess apoptosis.
Results.
Caspase-5 mRNA expression and protein activation were induced by LPS and monocyte-hRPE coculture. Caspase-5 activation appeared as early as 2 hours after challenge by LPS and consistently increased to 24 hours. Meanwhile, caspase-1 expression and protein activation were induced by LPS. Activation of caspase-5 was blocked or reduced by Dex, IL-10, and TA. Activation of caspase-5 and -1 was also enhanced by ATP and ER stress inducers. Expression and activation of caspase-5 were inhibited by a caspase-1–specific inhibitor. Caspase-5 knockdown reduced caspase-1 protein expression and activation and inhibited TNF-α–induced IL-8 and MCP-1. In contrast to caspase-4, the contribution of caspase-5 to stress-induced apoptosis was moderate.
Conclusions.
Caspase-5 mRNA synthesis, protein expression, and catalytic activation were highly regulated in response to various proinflammatory stimuli, ATP, and ER stress inducers. Mutual activation between caspase-5 and -1 suggests caspase-5 may work predominantly in concert with caspase-1 in modulating hRPE inflammatory responses.
Caspase-5 was originally cloned from human THP-1 cells and placenta tissue and was named ICErelIII and TY, respectively.1,2 Caspase-5 has high amino acid sequence homology with caspase-1 (51%) and caspase-4 (74%). As with caspase-1 and -4, the overexpression of caspase-5 in HeLa and COS cells induces apoptotic cell death. In contrast to the ubiquitous distribution of caspase-4 in tissues, caspase-5 is expressed only in a restricted manner with its detection in placenta, lung, liver, spleen, small intestine, colon, and peripheral blood lymphocytes.1,3 There are six splice variants of caspase-5, assigned as variants a to f, that encode 447 (f), 434 (a), 376 (b), 292 (c), 108 (e), and 24 (d) amino acids.4 These splice variants result from alternative splicing and with different start sites for translation.
Caspase-5 belongs to a family of cytosolic, aspartate-specific, cysteine proteases involved in apoptosis, inflammation, proliferation, and differentiation.5–8 At least 17 members of the caspase family have been identified, of which 13 are found in humans.9 Chromosomal mapping reveals that the human caspase-5 gene is colocalized within a cluster of functionally related genes (caspase-1, -4, and -12) and caspase-1 pseudogenes (ICEBERG, COP, and INCA) in chromosome 11q22–23.10,11 Phylogenetic analysis assigns caspase-5, together with caspase-1, -4, and -12, to an inflammatory caspase subfamily (group 1 caspases).8 The functional roles of group 1 caspases are represented by the well-characterized caspase-1, which plays a crucial role in processing the proinflammatory cytokines IL-1β, IL-18, and IL-33.5,8,12 The chromosomal colocalization of caspase-5 with inflammatory caspases implies that these caspases evolved from a common ancestor through multiplication of a mouse caspase-11-like gene and that they may share common functions in innate immunity and inflammation.13
As is the case with murine caspase-11, human caspase-5 is a poorly characterized member of the human caspase-1 subfamily.5 Like murine caspase-11, caspase-5 has been known to be regulated by the proinflammatory agents lipopolysaccharide (LPS) and interferon (IFN)-γ, suggesting that caspase-5 may play roles in inflammation and innate immune responses.3 In addition, experiments with immunoprecipitation demonstrate that caspase-5 and -1 are coimmunoprecipitated with several other proteins, forming a protein complex called inflammasome NALP1. Evidence shows that caspase-5 also participates in IL-1β processing, because pro-IL-1β processing occurs most efficiently when both caspase-1 and caspase-5 are coactivated in a cell-free system.12
Human RPE is clinically involved in many ocular inflammatory diseases. RPE cells and infiltrating leukocytes produce inflammatory cytokines that are essential mediators of the innate immune response within the ocular microenvironment. Because hRPE cells are believed to actively participate in propagating many retinal diseases with inflammatory components by secreting cytokines, caspase-mediated inflammatory and apoptotic pathways may be important mechanisms by which hRPE cells function in these diseases. The functional roles of caspase-5 in ocular inflammatory diseases are essentially unknown. Thus far, there has been only a single report on caspase-5 existence in eye tissues.14 In this report, the authors examined the protein profile in the mixtures of spent media from hRPE cultures that were isolated from age-related macular degeneration (AMD) patients. Caspase-5 protein was the one of proteins detected in the samples. Our previous studies have demonstrated that in most human populations, caspase-12 is truncated and catalytically deficient but still regulated by proinflammatory stimuli.15 Caspase-4 is involved in both proinflammatory and endoplasmic reticulum (ER) stress-induced apoptotic hRPE responses.16 We now examine the functional roles of caspase-5 in hRPE cells. In this study, we provide evidence that caspase-5 functionally differs from caspase-4 by only moderate involvement in ER stress-induced apoptosis. Caspase-5 is involved in proinflammatory agent–induced IL-8 and MCP-1. Caspase-5 appears to interplay with caspase-1 in inflammatory responses in hRPE cells.
Materials and Methods
Materials
Recombinant human IL-1β, TNF-α, IFN-γ, and IL-10 were purchased from R&D Systems (Minneapolis, MN). Dexamethasone, puromycin, polybrene, and tunicamycin were purchased from Sigma-Aldrich (St. Louis, MO). The caspase-1 inhibitor Z-YVAD-FMK and the caspase-5 and -1 inhibitor Z-WEHD-fmk and caspase-5 colorimetric assay kit were from Clontech (Mountain View, CA) and BioVision (Mountain View, CA), respectively. Rabbit polyclonal antibody against caspase-1 was from Abcam (Cambridge, MA). Goat active polyclonal antibody against caspase-5 was from BioVision. Plasmid (EndoFree Maxi Kit), homogenization (QIAshredder), and mini (RNeasy) kits were purchased from Qiagen (Valencia, CA). Reverse transcription system and transfection buffer (OPTI-MEM) were obtained from Invitrogen (Carlsbad, CA). Transfection reagent (Fugene 6) was purchased from Roche Molecular Biochemicals (Indianapolis, IN). All other reagents were obtained from Sigma-Aldrich and Fisher Scientific (Pittsburgh, PA). Mission nontarget shRNA control vector and mission shRNA bacterial glycerol stock specific for caspase-5 were purchased from Sigma-Aldrich.
Cell Isolation and Culture
The hRPE cells were isolated within 24 hours of death from the donor eyes, as previously described, in accordance with the Declaration of Helsinki.17–19 In brief, the sensory retina tissue was separated gently from the hRPE monolayer, and the hRPE cells were removed from Bruch's membrane with papain (5 U/mL). hRPE cells were cultured in Dulbecco's modified Eagle's/Ham's F12 nutrient mixture medium (DMEM/Ham's F12), containing 15% fetal bovine serum, penicillin G (100 U/mL), streptomycin sulfate (100 μg/mL), and amphotericin B (0.25 μg/mL) in culture plates (Falcon Primaria; BD Biosciences, Franklin Lakes, NJ) to inhibit fibroblast growth. The hRPE monolayers exhibited uniform immunohistochemical staining for cytokeratin 8/18, fibronectin, laminin, and type IV collagen in the chicken-wire distribution characteristic of these epithelial cells. Cells were subcultured, grown to confluence, and exposed to the same medium but contained reduced serum (5%) for further experiments. The hTERT-RPE1 cell line is a human retinal pigment epithelial cell line that stably expresses the human telomerase reverse transcriptase (hTERT) subunit, which does not induce changes associated with a transformed phenotype.
Monocyte Isolation and hRPE-Monocyte Coculture
Human monocytes were freshly isolated from the peripheral blood of healthy volunteers, as described previously, in accordance with the Declaration of Helsinki.19 In brief, peripheral blood was drawn into a heparinized syringe and 1:1 diluted in 0.9% saline. Mononuclear cells were separated by density gradient centrifugation. The cells were washed and then layered onto density-gradient monocytes (1.068 g/mL; Fico[b]-Lite[b], Atlanta Biologicals, Norcross, GA) for the enrichment of monocytes. The isolated cells were then washed, cytospun onto a glass slide, stained (Diff-Quick; Baxter Healthcare, Deerfield, IL), and differentially counted. The purity of the cell was >97%. For hRPE cells, monocyte coculture and enriched monocyte populations (3 × 105) were overlaid onto near-confluent hRPE cultures (2 × 105) for 6 hours. After coculture, the monocytes were removed as previously described,19 and hRPE cells were subjected to further analyses.
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction
Total cellular RNA was isolated from hRPE cells (QIAshredder and RNeasy mini kit; Qiagen) according to the manufacturer's protocol. cDNA synthesis was set up according to the protocol for a reverse transcription system. Briefly, 5 μg RNA was added to the reaction mixture with reverse transcriptase (Superscript III, 200 U/μL; Invitrogen) and 1 μL oligo d(T)20 (0.5 μg/μL) in a total volume of 20 μL. The linear range of β-actin PCR reaction was predetermined by using a series of cycles (15–35 cycles). The midlinear portion of the response curve was selected as the condition for semiquantitative PCR. The primer and conditions for caspase-5 PCR were as described by Lin et al.3 and were confirmed by first examining three cycles (15, 25, and 35), and then cycle 32 was selected. The reaction was initiated by the addition of 0.15 μL Taq DNA polymerase (5 U/μL) to a final volume of 20 μL. Resultant cDNAs were amplified though 32 and 20 cycles for caspase-5 and β-actin, respectively. Primer sequences for human caspase-5 genes were 5′-TAGACTCTTTGCGAAAGAATCGCGTGGCTCAT-3′ (forward) and 5′-CACCTCTGCAGGCCTGGACAATG ATGAC-3′ (reverse). To ensure that an equal amount of template was used in each amplification reaction, human β-actin sense (5′-GTGGGGCGCCCCAGGCACCA-3′) and antisense (5′-GCTCGGCCGTGGTGGTGAAGC-3′) primers were used in parallel. The following conditions were used in RT-PCR reaction for caspase-5 and β-actin: denaturation at 95°C for 45 seconds (caspase-5) or 1 minute (β-actin), annealing at 65°C for 1 minute (caspase-5) or at 62°C (β-actin) for 1 minute, and extension at 72°C for 1 minute (caspase-5) or 2 minutes (β-actin) for 32 (caspase-4) or 20 (β-actin) cycles. RT-PCR products were analyzed by electrophoresis on a 2% agarose gel and stained with ethidium bromide.
ELISA
The levels of immunoreactive IL-8 and MCP-1 in the hRPE supernatants were determined by modification of a double-ligand ELISA method, as previously described.20 Briefly, diluted supernatants from hRPE or HTERT-RPE 1 (50 μL) were added and incubated for 1 hour. The plates were then subjected to sequential incubations with biotinylated rabbit anti–IL-8 or anti–MCP-1 and streptavidin-peroxidase conjugate. Chromogen substrate (OPD) was added, the plates were incubated to desired extinction, and the reaction was terminated with 3M H2SO4. Absorbance for each well at 490 nm was read. Standards included half-log dilution of corresponding chemokines at concentrations from 1 pg to 100 ng/well. This ELISA method consistently detected chemokine concentrations greater than 10 pg/mL in a linear fashion.
Western Blot Analysis
Cellular extracts from hRPE cells were processed for Western blot analysis according to the manufacturer's procedure (Sigma-Aldrich). Then, 20 to 50 μg protein/sample was analyzed by SDS-PAGE. Protein was electrotransferred to nitrocellulose membrane, blocked with a solution of TBS containing 5% nonfat milk and 0.1% Tween-20 (TBST) for 1 hour, and probed with primary antibodies overnight, followed by washing three times with TBST. Next, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature and were washed three additional times with TBST. The membrane was then visualized using an enhanced chemiluminescence technique.
Caspase-5 Activity Assay
A commercially available caspase-5 assay kit (BioVision; Qiagen) was used for analysis. In accordance with the manufacturer's protocol, 200 μg protein was used for each assay. The activity of caspase-5 was determined by the colorimetric method based on spectrophotometric detection of the chromophore p-nitroaniline (pNA) after cleavage from the labeled substrate WEHD-pNA. Absorbance was read at 405 nm.
Knockdown of Caspase-5 in hTERT-RPE1 Cells
Because of the low transfection efficiency in primary hRPE cells, the hTERT-RPE1 cell line was selected as a replacement for primary cultures. This cell line is a human RPE cell line that stably expresses human telomerase reverse transcriptase (hTERT) and is a useful tool for biochemical and physiological studies (Clontech, Mountain View, CA). For lentivirus packaging of shRNA expression vector specific for caspase-5, human embryonic kidney (HEK) 293T cells were grown in DMEM. The hTERT-RPE1 cells were grown in DMEM/F-12 medium. Both media were supplemented with 10% heat-inactivated fetal bovine serum, penicillin, and streptomycin at 37°C in a humidified atmosphere with 5% CO2.
Mission shRNA is represented by5 multiple vector constructs targeting different regions of the gene sequence. Each shRNA is cloned into the pLKO.1 lentiviral vector, and each construct is sequence verified to ensure a match to the target gene. The following mission shRNA was selected in this study: #3554, CCGGCACCATAGAACG AGCAACCTTCTCGAGAAGGTTGCTCGTTCTATGGTGTTTTT; #3555, CCGGCA CTTCTCAATATGGACCAAACTCGAGTTTGGTCCATATTGAGAAGTGTTTTT; #3556, CCGGTCATTACGGAACTCATCACATCTCGAGATGTGATGAGTTCCGTAATGATTTTT
First, high-copy of transfection-grade plasmid DNA was made using a plasmid maxi kit (EndoFree; Qiagen). Transient transfection was performed in HEK293T or hTERT-RPE1 cells by using transfection reagent (Fugene 6; Roche) according to the manufacturer's protocol. Western blot analysis was performed to assess the expression of shRNA clones and to test the efficiency of caspase-5 knockdown in the HEK293 cell line.
For long-term caspase-5 knockdown, we used a two-component viral packaging system (Lentiviral expression vector/PVSV) and selected shRNA clones in HEK293 packaging cells. The packaging was prepared with PVSV, PMD26 Lenti, and caspase-5 shRNA or a scrambled shRNA, and then the supernatants with shRNA Lentiviral particles were collected and transfected to hTERT-RPE1 cells at 30% to 40% confluence with polybrene (10 μg/mL). hTERT-RPE1 cells were also transiently transfected with shRNA clones and transfection reagent (Fugene 6; Roche) according to the manufacturer's protocol as described.
Apoptosis Assay
Cell death detection ELISA was performed according to the manufacturer's protocol. Briefly, hRPE cells were seeded and grown in 96-well plates until the cells were 90% confluent. After treating the cells with or without inducers or inhibitors for 24, 48, or 72 hours, apoptosis was quantified by using a cell death detection ELISA kit. Cultures were lysed with the lysis buffer, and then the cytoplasmic fraction was transferred into the wells coated by streptavidin for further analysis. Next, the immune-reagent was added to each well, which contained anti–histone-biotin and anti–DNA-peroxidase. The immune-reagents bonded to or reacted with the histone and DNA portion of the mononucleosomes and oligonucleosomes released from cells undergoing apoptosis. After removal of the unbound components with wash, the substrate 2, 2′-azino-di-[3-ethylbenzthiazoline sulfonate (ABTS) was added to determine peroxidase activity based on the absorbance difference between A405nm and A490nM in an ELISA reader.
TUNEL Staining
The cells were stained with TdT-mediated dUTP nick-end labeling (TUNEL) according to the manufacturer's protocol. Briefly, hRPE cells were fixed and incubated with TUNEL mixtures for 1 hour at 37°C. The incorporated fluorescein was detected by sheep anti–fluorescein antibody conjugated with horseradish peroxidase using the substrate diaminobenzidine. hRPE cells were distinguished by subsequent labeling with anti–vimentin antibody, alkaline phosphatase-labeled polymer, and permanent red substrate (EnVision G/2 system; Dako, Carpinteria, CA). The stained cells were analyzed by light microscopy. Apoptotic cells in the cultures were quantified by counting the number of TUNEL-positive cells in five random microscopic fields.
Statistical Analysis
Each experiment was confirmed by testing samples from three independent hRPE cell lines and monocyte donors; thus, results were representative of three independent experiments. For ELISA and functional assays, the results shown are representative of three independent experiments with similar results, with each data point performed in triplicate. All data were analyzed using statistics software (StatView; SAS Institute Inc., Cary, NC). Data were first evaluated by ANOVA F-test and were followed by post hoc analyses as follows: for Western blot and PCR, analyses were performed using the Tukey-Kramer method comparing various treatments with control or stimulated without inhibitor; for caspase-5, ELISA, and apoptosis assays, Student's two-tailed t-test was used to compare two preselected conditions in which one experimental condition (stimulation) was compared with another (unstimulated or stimulated inhibitor), either control or stimulated, without inhibitor; for caspase knockdown studies, the Student's two-tailed t-test was used for comparisons of two conditions. P < 0.05 was considered statistically significant. For ELISA and functional assays, the values represented mean ± SEM. For Western blot analysis and RT-PCR, a representative experiment was selected for illustration.
Results
Expression and Activation of Caspase-5 and -1 in Response to Proinflammatory Stimulation
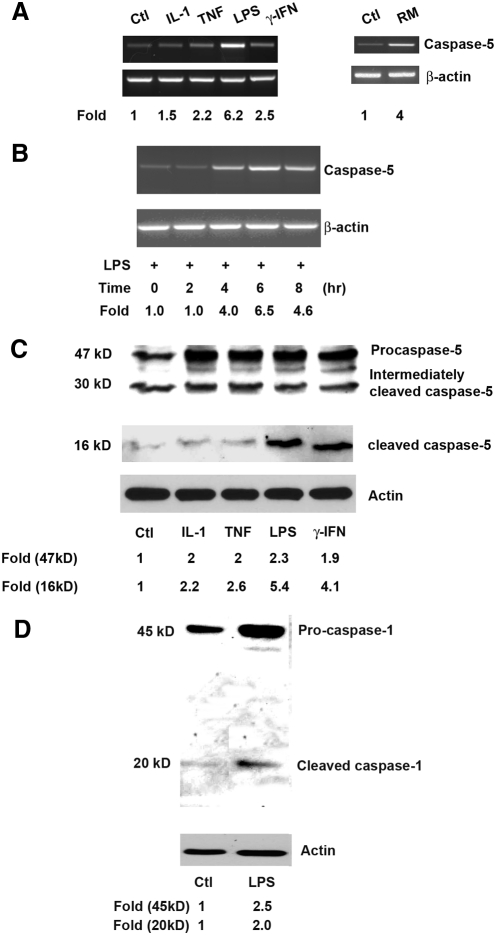
Various microbial agents such as LPS are known to induce the activation of caspase-1.5 To determine the involvement of caspase-5 and -1 in proinflammatory responses by hRPE cells, in addition to the two previously reported caspase-5 inducers (namely LPS and IFN-γ), a group of known proinflammatory agents or conditions was selected for this study, including IL-1β, TNF-α, and RPE-monocyte coculture. The hRPE cells were incubated with IL-1β (2 ng/mL), TNF-α (20 ng/mL), LPS (1000 ng/mL), IFN-γ (500 U/mL), and monocytes to maximally induce proinflammatory responses.17,19,21,22 After treating hRPE cells with above agents for 0, 2, 4, 6 or 8 hours, the total cellular mRNA was isolated and subjected to RT-PCR analysis. This primer pair is able to detect all three larger caspase-5 variants (a, b, and f), yielding a single PCR product of 505 bp on agarose gels. These three variants are the only caspase-5 variants that code for both the large prodomain and the catalytic domain.4 To compare caspase-5 mRNA levels, expression of the housekeeping gene β-actin was used to monitor gel loading. As shown in Figure 1A, treatment of hRPE cells with IL-1β, TNF-α, LPS, IFN-γ, and RPE-monocyte coculture increased caspase-5 mRNA synthesis by 1.5-, 2.2-, 6.2-, 2.5-, and 4.0-fold, respectively. Stimulation of caspase-5 mRNA synthesis by IL-1β, TNF-α, LPS, and RPE-monocyte coculture from independent experiments was statistically significant, with average fold increases of 2.7 ± 0.3 (P < 0.01), 2.2 ± 0.2 (P < 0.01), 4.5 ± 1.0 (P < 0.05), and 3.8 ± 0.8 (P < 0.05), respectively. However, stimulation by IFN-γ did not produce a statistically significant increase (2.0 ± 0.5; P > 0.05). The LPS-induced caspase-5 mRNA expression was time dependent and appeared as early as 4 hours after stimulation (Fig. 1B). Expression of caspase-5 mRNA peaked at 6 hours with a 6.4 ± 0.1 fold increase (P < 0.001), compared with 0 hours, and declined by 8 hours after the induction.
Figure 1.
Stimulation of caspase-5 mRNA synthesis (A, B) and caspase-5 and -1 protein cleavage (C, D) in hRPE cells. The hRPE cells were cultured either without (untreated; Ctl) or with IL-1β (IL-1, 2 ng/mL), TNF-α (TNF, 20 ng/mL), LPS (1000 ng/mL), IFN-γ (500 U/mL), or overlaid monocytes (RM) and were incubated for 6 hours (A), 0, 2, 4, 6, and 8 hours (B), or 24 hours (C, D). The data shown represent results from a typical experiment. (A, B) The steady state caspase-5 mRNA levels determined by RT-PCR. (C, D) Western blot analysis of caspase-5 protein. The fold changes were calculated by normalization against β-actin and comparison with untreated control (A) or with treated control at 0 hours (B) of mRNA levels in RT-PCR, or by normalization against actin and making a comparison with untreated control levels of caspase-5 in Western blot analysis (C, D). The bands at approximately 30 kDa (C) are presumably either nonspecific bands or intermediately cleaved caspase-5 protein.
To determine caspase-5 and caspase-1 activation, whole cell lysates from the hRPE cells treated under the same conditions as described for 24 hours were subjected to Western blot analysis. Activation of pro-caspase-5 protein was evident by the appearance of cleaved caspase-5 products (Fig. 1C). Although IL-1β, TNF-α, LPS and IFN-γ increased caspase-5 protein production by 2.0-, 2.0-, 2.3-, and 1.9-fold, activation of the caspase-5 protein was also enhanced by 2.2-, 2.6-, 5.4-, and 4.1-fold, respectively. The fold increases induced by IL-1β, TNF-α, LPS, and IFN-γ from independent experiments were 2.3 ± 0.1 (P < 0.001), 2.6 ± 0.4 (P < 0.05), 2.2 ± 0.2 (P < 0.01), and 1.8 ± 0.1 (P < 0.001) for caspase-5 protein production and 2.3 ± 0.0 (P < 0.001), 2.1 ± 0.0 (P < 0.05), 3.9 ± 0.7 (P < 0.01), and 3.1 ± 0.4 (P < 0.01) for caspase-5 protein activation, respectively. Similar to the results for caspase-5, LPS increased caspase-1 protein production and activation by 2.5- and 2.0-fold, respectively (Fig. 1D); the average fold increases from independent experiments were 2.4 ± 0.2 (P < 0.01) and 1.9 ± 0.1 (P < 0.01), respectively.
To further determine the effects of proinflammatory and anti-inflammatory agents on caspase-5 protein function, caspase-5 catalytic activity was assessed using a caspase-5 assay kit. Human RPE cells were stimulated by LPS (1000 ng/mL) for 0, 1, 2, 7, and 24 hours with or without caspase-1 inhibitor Z-YVAD-fmk (2 μM). By comparing the results from treated samples with untreated control or 0-hour treatment, the fold increases in caspase-5 activity were calculated (Fig. 2). The time course of induction showed that the stimulated activation began as early as 2 hours after challenge with LPS and consistently increased from 7 to 24 hours by 2.0- to 2.5-fold. The caspase-5 activity after 24-hour stimulation by LPS increased by 2.0-fold and was significantly reduced with the caspase-1 specific inhibitor by 36%.
Figure 2.
Induction of caspase-5 catalytic activity by LPS. The hRPE cells were stimulated by LPS (1000 ng/mL, A, B) for 0, 1, 2, 7 (A), and 24 (A, B) hours, with or without caspase -1 inhibitor Z-YVAD-fm (2 μM) or Dex (10−6 M). Two hundred micrograms of protein from the hRPE cell lysate was analyzed in each assay using a caspase-5 assay kit. The data are representative of three independent experiments with similar results, as shown by fold increases in caspase-5 activity. Values represent mean ± SEM. *P < 0.05; ***P < 0.001.
Expression and Activation of Caspase-5 and Caspase-1 in Response to ATP and ER Stress
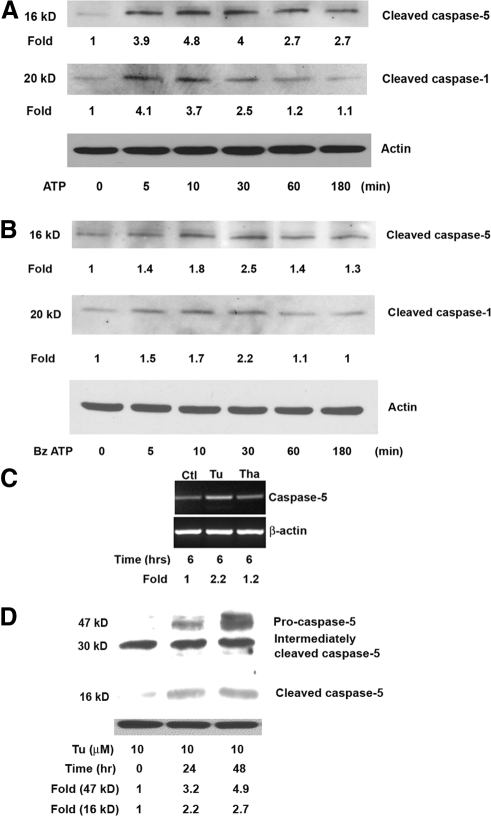
Extracellular ATP is an inflammatory mediator that is released by stressed or infected eukaryotic cells.23 It has been shown that LPS priming can increase the efficiency of ATP-mediated caspase-1 activation.24 To determine whether stimulation with ATP may also result in the activation of caspase-5 and caspase-1 in hRPE cells, hRPE cells were primed by LPS (1000 ng/mL) for 4 hours and then cultured with either ATP at 3 mM or BzATP, an agonist of nucleotide receptor P2X7, at 300 μM for various times. Treating the cells with ATP for 5, 10, 30, 60, and 180 minutes increased caspase-5 activation by 3.9-, 4.8-, 4.0-, 2.7-, and 2.7-fold and caspase-1 activation for 5, 10, and 30 minutes by 4.1-, 3.7-, and 2.5-fold, respectively (Fig. 3A); no difference in caspase-1 activation for 60 and 180 minutes was tested. Statistical analysis of results from three independent experiments showed significant, transient increases of caspase-5 protein activation by 3.6 ± 0.6 (P < 0.01), 4.4 ± 0.4 (P < 0.05), 4.2 ± 0.2 (P < 0.01), 2.4 ± 0.3 (P > 0.05), and 2.4 ± 0.3 (P > 0.05) fold after treating cells with ATP for 5, 10, 30, 60, and 180 minutes, respectively. The transient increases in caspase-1 protein activation were 4.2 ± 0.1 (P < 0.001), 4.0 ± 0.3 (P < 0.001), and 2.4 ± 0.4 (P > 0.05) fold after ATP treatment for 5, 10, and 30 minutes, respectively. Similar results were observed for BzATP. BzATP treatment for 30 minutes caused a 2.5-fold increase in caspase-5 activation and a 2.2-fold increase in caspase-1 activation (Fig. 3B). Statistical analysis confirmed that the increase in caspase-5 and -1 protein activation at 30 minutes after BzATP stimulation was significant; the average fold changes at 30 minutes from independent experiments were 2.4 ± 0.2 (P < 0.01) for activation of caspase-5 protein and 2.4 ± 0.2 (P < 0.05) for activation of caspase-1 protein. The induction peak was reached in 5 to 30 minutes after ATP treatment and approximately 30 minutes for BzATP. The levels of activation by ATP were higher than those by BzATP. Activation of caspase-5 or caspase-1 in response to ATP or BzATP was not observed without LPS treatment in hRPE cells (data not shown).
Figure 3.
Stimulation of human RPE caspase-5 mRNA synthesis (C) and protein maturation (A, B, D) by ATP and ER stress. hRPE cells were pretreated with LPS (1000 ng/mL, A, B) for 4 hours and cultured either with ATP (3 mM) or BzATP (300 μM) for 0, 5, 10, 30, 60, and 100 minutes, tunicamycin (10 μM), or thapsigargin (25 ng/mL) for 6 (C), 0, 24, and 48 hours (D). The data shown represent results from a typical experiment. (C) Steady state caspase-5 mRNA, as determined by RT-PCR. The fold changes were calculated by normalization against β-actin and comparison with control cells without the treatment. (A, B, D) Western blot analysis of caspase-5, caspase-1, and actin proteins. (D) The bands at approximately 30 kDa are presumably either nonspecific bands or intermediately cleaved caspase-5 protein.
Sequence comparison among inflammatory caspases reveals that both caspase-4 and caspase-5 probably originated from duplication of a caspase-11 ancestor gene.13 Our previous study has shown that ER stress causes the activation of caspase-4.16 We thus predicted that ER stress might also enhance the activation of caspase-5. Tunicamycin and thapsigargin are two well-known ER stress inducers that act, respectively, by blocking N-glycosylation of newly synthesized proteins and inhibiting Ca2+-ATPase, which is responsible for maintaining Ca2+ homeostasis in the ER. Similar to the results described in our caspase-4 study,16 tunicamycin (3 μM) increased caspase-5 mRNA expression by 2.2-fold, and thapsigargin (25 ng/mL) did not cause significant change (Fig. 3C). Independent experiments showed that tunicamycin induced a 2.5 ± 0.4-fold (P < 0.05) average increase in caspase-5 mRNA synthesis. Tunicamycin at 10 μM induced caspase-5 protein production and cleavage as early as 24 hours after stimulation, and activation persisted up to 48 hours (Fig. 3D). At 24 and 48 hours after stimulation, caspase-5 protein production was enhanced by 3.2- and 4.9-fold, and caspase-5 activation was moderately by 2.2- and 2.7-fold, respectively. Analysis of three independent experiments showed the average fold increases in caspase-5 protein production at 24 and 48 hours after tunicamycin stimulation to be 3.8 ± 0.6 (P < 0.05) and 5.9 ± 1.0 (P < 0.05); increases in activation of caspase-5 protein were 2.2 ± 0.1 (P < 0.05) and 2.6 ± 0.1 (P < 0.05), respectively.
Inhibition of Induced Activation of Caspase-5 by Dex, IL-10, and Triamcinolone Acetonide
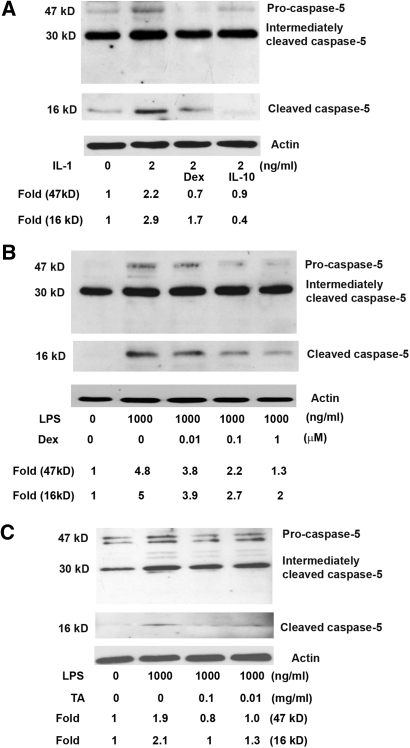
Given that Dex and triamcinolone acetonide (TA) have been widely used in various ocular conditions, we wanted to know whether proinflammatory agent-induced hRPE caspase-5 expression and activation may be counteracted by these two drugs and anti-inflammatory IL-10. As expected, treatment with Dex (10−6 M) and IL-10 (100 U/mL) totally blocked IL-1β–induced caspase-5 production and reduced the induced cleavage of pro-caspase-5 by 63% and 100%, respectively (Fig. 4A). Inhibitions by Dex and IL-10 were statistically significant. The average fold changes from three independent experiments with IL-1β, IL-1β + Dex, and IL-1β + IL-10 were 2.3 ± 0.5, 0.9 ± 0.2 (P < 0.05), and 1.2 ± 0.3 (P < 0.05) in caspase-5 protein production and 2.7 ± 0.3, 1.5 ± 0.2 (P < 0.05), and 0.7 ± 0.1 (P < 0.05) in caspase-5 activation, respectively. Inhibition by Dex on LPS-induced caspase-5 expression and activity was dose dependent at the concentrations ranging from 10−8 to 10− M (Fig. 4B). Dex (10−6 M) treatment reduced LPS-induced caspase-5 production and caspase-5 activation by 71% and 64%, respectively. From three independent experiments, the average fold change for caspase-5 protein production was reduced from 4.2 ± 0.7 by LPS alone to 1.2 ± 0.2 (P < 0.05) by LPS+Dex (10−6 M), whereas caspase-5 activation was reduced from 4.5 ± 0.5 to 1.6 ± 0.5 (P < 0.05). Inhibition by Dex was also confirmed using a caspase-5 functional assay. In the presence of Dex, LPS-induced caspase-5 enzyme activity was reduced by 32% (Fig. 2).
Figure 4.
The effect of Dex, IL-10, and TA on caspase-5 protein production and maturation by IL-1β (A) and LPS (B, C) in hRPE cells. hRPE cells were pretreated with Dex (10−8 or 10−7 to 10−6 M), IL-10 (100 U/mL), or TA (0.1 or 0.01 mg/mL) for 30 minutes and then coincubated with IL-1β (2 ng/mL) or LPS (1000 ng/mL) for an additional 24 hours. Proteins from the whole hRPE cell lysates were detected by anti–caspase-5 antibody specific for pro-caspase-5 and cleaved caspase-5. Fold changes of the cleaved caspase-5 were calculated by relative density between treated and untreated samples, as determined by densitometry after normalization with actin protein.
TA is a synthetic crystalline corticosteroid with potent anti-inflammatory properties. This drug has recently been used as an effective therapeutic agent in various retinal vascular and inflammatory diseases. We therefore investigated the effect of TA on caspase-5 expression and activity. TA significantly reduced the production and activation of caspase-5 by 55% ± 4% (P < 0.05) and 52% ± 1% (P < 0.05) at a TA concentration of 0.1 mg/mL and by 32% ± 9% (P > 0.05) and 35% ± 1% (P > 0.05) at 0.01 mg/mL, respectively.
Mutual Inhibition of Gene Expression between Caspase-5 and -1
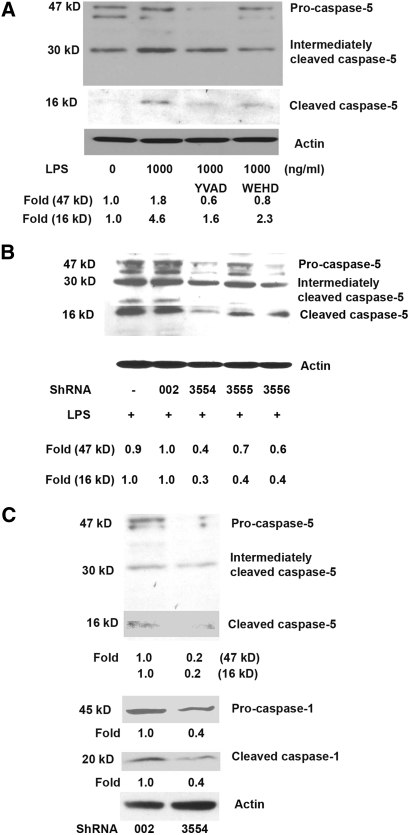
Previous reports have shown that caspase-1 and -5 coexist in one type of inflammasome, NALP1, suggesting their close association in cellular inflammatory responses. To examine the effect of caspase-1 on caspase-5 gene expression and protein activation, the caspase-1 inhibitor Z-YVAD-fmk and the caspase-1 and -5 inhibitor Z-WEHD-fmk were used. Treatment with Z-YVAD-fmk (2 μM) and Z-WEHD-fmk (2 μM) reduced LPS-induced production of caspase-5 by 74% and 64% and LPS-induced cleavage of pro-caspase-5 by 83% and 64%, respectively (Fig. 5A). The average fold reductions from three independent experiments with LPS, LPS+caspase-1 inhibitor Z-YVAD-fmk, and LPS+caspase-1 and -5 inhibitor Z-WEHD-fmk were 2.2 ± 0.2, 0.9 ± 0.3 (P < 0.05), and 0.8 ± 0.0 (P < 0.05) for caspase-5 protein production, and 4.2 ± 0.6, 1.8 ± 0.2 (P < 0.05), and 1.7 ± 0.3 (P < 0.05) for caspase-5 activation, respectively.
Figure 5.
Blockade of caspase-5 activation by caspase-1 inhibitors (A) and caspase-5 and -1 activation by caspase-5 knockdown (B, C). (A) hRPE cells were pretreated with caspase-1 inhibitor Z-YVAD-fmk or caspase-1 and -5 inhibitor Z-WEHD-fmk for 30 minutes and then coincubated with or without LPS (1000 ng/mL) for an additional 24 hours. Proteins from the whole hRPE cell lysates were subjected to Western blot analysis using anti–caspase-5 antibody. (B, C) Whole cell lysates from stably knockdown hTERT-RPE 1 cells with caspase-5 shRNA and control shRNA were detected by antibodies specific for pro-caspase-5 or cleaved caspase-5 or -1. Fold changes of cleaved caspase-5 and -1 were calculated by relative density between scrambled shRNA002 and caspase-5–specific shRNA3554 using densitometry after normalization to actin protein.
Conversely, to show the role of caspase-5 on caspase-1 expression, we used shRNA-mediated suppression of caspase-5 in hTERT-RPE1 cells. We selected three caspase-5–specific shRNA constructs—shRNA3554, shRNA3555, and shRNA3556—to transfect hTERT-RPE1 cells. Scrambled shRNA (shRNA002) was used as the control. Because shRNA3554 showed the highest blockade of caspase-5 expression, we determined that using it with shRNA002 as the control was best for further studies.
To determine the effects of caspase-5 knockdown, we first investigated caspase-5 expression and maturation in shRNA transiently transfected hTERT-RPE 1 cells. In hTERT-RPE 1 cells, transient transfection with shRNA3554, shRNA3555, and shRNA3556 reduced the expression of caspase-5 by 60%, 30%, and 40% and caspase-5 activation by 70%, 60%, and 60%, respectively (Fig. 5B). The average fold inhibitions from three independent experiments by shRNA002 (scrambled shRNA control)—caspase-5 knockdown shRNA3554, shRNA3555, and shRNA3556—were from 0.98 ± 0.0 to 0.4 ± 0.0 (P < 0.001), 0.7 ± 0.1 (P < 0.05), and 0.7 ± 0.1 (P < 0.05) for caspase-5 protein production and from 0.9 ± 0.1 to 0.3 ± 0.1 (P < 0.05), 0.4 ± 0.0 (P < 0.05), and 0.5 ± 0.1 (P > 0.05) for caspase-5 protein activation, respectively.
Interestingly, the stable knockdown of caspase-5 by shRNA3554 also eliminated endogenous caspase-1 expression (Fig. 5C). Compared with scrambled shRNA control, the average fold reductions by LPS for caspase-5 and caspase-1 protein production in shRNA3554 cells were 0.3 ± 0.1 (P < 0.05) and 0.4 ± 0.1 (P < 0.01), respectively, and reductions for activation of caspase-5 and caspase-1 protein were 0.3 ± 0.1 (P < 0.05) and 0.5 ± 0.1 (P < 0.01), respectively.
Functional Role of Caspase-5 and -1 on hRPE IL-8 and MCP-1 Secretion
To determine the functional role of caspase-5 and -1 in hRPE chemokine secretion, human RPE cells were challenged with IL-1β (2 ng/mL), TNF-α (20 ng/mL), or LPS (1000 ng/mL) in the presence or absence of caspase-1 inhibitor Z-YVAD-fmk (2 μM), pan-caspase inhibitor Z-VAD-fmk, or caspase-5 and -1 inhibitor Z-WEHD-fmk (2 μM). The media were harvested after 24 hours of stimulation. As shown in Figure 6A, Z-WEHD-fmk reduced IL-1β, TNF-α, and LPS-induced IL-8 secretion by 37%, 22%, and 28%, respectively. Similar results were observed in MCP-1 secretion (Fig. 6B). In this case, Z-WEHD-fmk reduced IL-1β, TNF-α, and LPS-induced MCP-1 secretion by 36%, 38%, and 32%, respectively.
Figure 6.
Inhibition of human RPE IL-8 and MCP-1 production by caspase inhibitors (A, B, E, F) and caspase-5 shRNA knockdown (C, D). hRPE cells (A, B, E, F) were pretreated with caspase-5 and -1 inhibitor Z-WEHD-fmk (2 μM), caspase-1 inhibitor Z-YVAD-fmk (2 μM), or pan-caspase inhibitor Z-VAD-fmk (50 μM) and were costimulated with IL-1β (2 ng/mL), TNF-α (20 ng/mL), or LPS (1000 ng/mL) for 24 hours. (C, D) hTERT-RPE 1 cells were transfected with scrambled shRNA002 or caspase-5–specific shRNA3554. Secretion of IL-8 and MCP-1 was determined by ELISA. Values represent mean ± SEM (n = 3). Statistical analysis was carried out for all values with inhibitor versus the corresponding control without inhibitor (A, B, E, F) or for all values with scrambled shRNA versus caspase-5–specific shRNA. Data are representative of three independent experiments with similar results. Ctrl, untreated; IL-1, IL-1β; TNF, TNF-α.
To further differentiate the contribution of caspase-5 from that of caspase-1 to hRPE chemokine induction, the secretion of IL-8 and MCP-1 was examined in hTERT-RPE-1 cells by shRNA knockdown of caspase-5 (Figs. 6C, 6D). Compared with scrambled shRNA (shRNA002), TNF-α–stimulated IL-8 secretion and MCP-1 secretion were reduced by 56% and 35%, respectively.
To determine caspase-1 contribution to the induced chemokine secretion, we then stimulated hRPE cells with TNF-α (20 ng/mL) or LPS (1000 ng/mL) and in the presence or absence of caspase-1 inhibitor Z-YVAD-fmk or pan-caspases inhibitor Z-VAD-fmk. HRPE IL-8 and MCP-1 induction by TNF-α was only weakly inhibited by Z-YVAD-fmk, whereas that by Z-VAD-fmk was reduced by 37% and 26%, respectively (Figs. 6E, 6F). In contrast to the weak effect of Z-YVAD-fmk on TNF-α–induced IL-8 and MCP-1 production, Z-YVAD-fmk inhibited LPS-induced IL-8 and MCP-1 production more significantly by 30% and 20%, respectively.
Involvement of Caspase-5 in Tunicamycin-Induced Apoptotic Cell Death
It has been proposed that caspase-5 may play functional roles similar to that of caspase-4.5 Our previous study demonstrated that casapse-4 is heavily involved in ER stress–mediated apoptosis in hRPE cells16; thus, it is important to determine the contribution of caspase-5 to ER stress–mediated apoptosis under the same conditions. Cell death detection ELISA kits were used to measure apoptotic cell death. At 72 hours after treatment, tunicamycin (10 μM) induced substantial hRPE cell apoptotic death compared with the untreated control cells that had undetectable levels of cell death. Using ELISA readings after tunicamycin treatment as 100% cell death-positive control, the caspase-4 inhibitor Z-LEVD (2 μM) and caspase-5 and -1 inhibitor Z-WEHD (2 μM) inhibited the induced hRPE apoptosis by 59% and 32%, respectively (Fig. 7A).
Figure 7.
ER stress-induced hRPE apoptotic cell death. hRPE cells were cultured with or without tunicamycin (10 μM) in the presence or absence of caspase-4 inhibitor Z-LEVD-fmk (Z-LEVD, 2 μM) or caspase-5 and -1 inhibitor Z-WEHD-fmk (Z-WEHD, 2 μM) for 48 or 72 hours. Apoptosis was determined by the absorbance difference between A405nm and A490nM using a cell death detection ELISA kit (A). For comparison, tunicamycin-treated cells were assigned as 100% apoptotic cell death (positive control). (B, C) Quantification of the effects of ER stress-induced hRPE cell death by TUNEL assays. (B) TUNEL staining (dark brown), shown at 400× magnification. The hRPE cells were stained by vimentin (red). Left, middle: unstimulated hRPE cells (normal cultures and culture in TUNEL assay); right: hRPE cells treated with tunicamycin showing nuclear condensation and cell shrinkage. (C) Data are expressed as percentage of TUNEL-positive hRPE cells. Values represent mean ± SEM. ***P <0.001, **P < 0.01, and *P < 0.05, compared with tunicamycin treatment without inhibitors. The findings are representative of three independent experiments.
To further confirm the role of caspase-5 in tunicamycin-induced apoptosis, TUNEL staining was performed (Fig. 7C). After treating hRPE cells with tunicamycin (10 μM) for 48 hours, 24% of the cells exhibited apoptotic cell death, as detected by TUNEL counting the percentage of positive cells. In the presence of the caspase-4 inhibitor Z-LEVD-fmk (2 μM), tunicamycin-induced apoptotic cell death was reduced by 62%, whereas dual inhibition of casapse-5 and caspase-1 only resulted in 20% inhibition.
Discussion
Human caspase-5 and -4 are the two most closely related caspases. Similar to our previous results on caspase-4,16 the present study demonstrates that caspase-5 mRNA expression, protein production, activation, and catalytic activity were all inducible by a variety of proinflammatory agents, such as IL-1β, TNF-α, LPS, and monocyte coculture in addition to the previously reported, and here reconfirmed, agents LPS and IFN-γ. Expression of caspase-5 was subject to stimulation by the ER stress inducers thapsigargin and tunicamycin. These results were similar to those we previously reported for caspase-4 in hRPE cells.16 However, the contribution to ER stress–induced hRPE apoptosis by caspase-5 is much less than that of caspase-4. Although caspase-4 inhibitor Z-LEVD-fmk blocked 59% of tunicamycin-induced hRPE cell death as detected by DNA fragmentation and 62% reduction in TUNEL positivity, capase-5 and -1 dual inhibitor Z-WEHD-fmk inhibited DNA fragmentation by 32% and TUNEL positivity by 20%, suggesting that the contribution of caspase-5 to ER stress–induced hRPE cell death is much less than that of caspase-4.
Of note, Western blot analysis of caspase-5 consistently showed an intermediate product with molecular weight of 30 kDa, consistent with previous observations.3,12 The nature of the intermediate product is unclear. It could be the pro-large subunit as described for caspase-5 closely related caspase-13.25 In addition, a 42-kDa band immediately below the 47-kDa pro-caspase-5 band was observed, as previously reported.3 This protein is likely produced from pro-caspase-5 variant b, which has an expected molecular weight of 5 kDa less than pro-caspase variant a. Variants a and b are both predominant isoforms of human caspase-5.4
By using inhibitors and shRNA knockdown, we further confirmed that caspase-5 was functionally involved in the regulation of hRPE IL-8 and MCP-1 chemokine expression. LPS-induced IL-8 secretion and MCP-1 secretion were sensitive to the inhibition by Z-WEHD-fmk, an inhibitor for caspase-5 and -1. When caspase-5 was knocked down by shRNA, TNF-α–induced IL-8 and MCP-1 production was markedly reduced by 56% and 35%, respectively. hRPE IL-8 and MCP-1 expression were also inhibited by caspase-1 inhibitor Z-YVAD-fmk and caspase pan inhibitor Z-VAD-fmk, although the inhibition by Z-YVAD-fmk was more effective in LPS than TNF-α induction, suggesting that the signaling pathways induced by TNF-α are differentially regulated by caspase-5 and -1. We have shown previously that the induction of hRPE IL-8 and MCP-1 production in hRPE cells is mediated by multiple signaling pathways, including the activation of NF-κB.22,26 Activation of NF-κB has been reported by caspase-1 by a receptor interacting protein 2–dependent pathway8 or by interaction with the TRAIL-DR5 system.27 Therefore, one possible pathway for caspase-5– and -1–mediated upregulation of IL-8 and MCP-1 expression could be by the activation of NF-κB.
We demonstrated that caspase-5 and caspase-1 mutually regulated each other in their protein expression and activation. Regulating caspase-1 activation by the caspase-5 murine homolog caspase-11 has been well documented,5,28 but there has been no report on the regulation of human caspase-1 expression and activation by caspase-5. In this study, we showed that shRNA knockdown of caspase-5 reduced not only caspase-5 but also caspase-1 expression and activation. Conversely, the caspase-1 inhibitor Z-YVAD-fmk also inhibited the expression and activation of caspase-5. The latter finding was consistent with a previous study that showed caspase-1 to be required for the complete maturation of caspase-5.12 Our results suggest that caspase-5 and caspase-1 may work in concert in the inflammasome to modulate inflammation and immunity in hRPE cells.
There have been no reports about the effects of anti-inflammatory medicines on caspase-5 expression and activation. As shown in this study, proinflammatory agent-induced caspase-5 expression was mitigated by adding anti-inflammatory agents. Dex has numerous anti-inflammatory effects, which include the suppression of cytokine-mediated responses. Intraocular Dex concentrations achieved clinically through systemic and topical delivery range from 10−8 to 10−6 M.29 A few reports30–32 demonstrate that Dex reduces caspase-1 activation in a variety of cell types. In addition to Dex, IL-10 has been reported to relieve inflammation, improve cell survival, and inhibit caspase-1 activation in human monocytes.33 Another study showed that IL-10 reduced caspase-1 expression in rat cortical astrocytes.34 The results presented here showed that both Dex and IL-10 strongly reduce the simulated expression and activation of caspase-5; thus, IL-10 could also be a valuable therapeutic agent in ocular inflammation. In our previous study,16 neither Dex (10 μM) nor IL-10 (100 U/mL) induced noticeable apoptosis under the same conditions. Our results may indicate a new potential role of these drugs in ocular therapy. TA is a synthetic crystalline corticosteroid with potent anti-inflammatory properties. Recently, TA was delivered by intravitreal injection for the treatment of posterior ocular diseases, such as AMD,35sympathetic ophthalmia,36 retinal vein occlusion,37 diabetic macular edema,38 and proliferative vitreoretinopathy.39 After intraocular injections of 20 to 25 mg TA in patients, the average concentrations of TA in aqueous humor are lower than 0.01 mg/mL during the first 12 months of injections, while the measured levels of serum TA are negligible within 4 to 92 days.40,41 In contrast to more water-soluble Dex, which is only weakly cytotoxic in hRPE cells, some studies indicate that significant TA toxicity of ARPE19 cells can be observed at a 1.0-mg/mL concentration,42 possibly because of oxidative injury.43–45 However, TA at 0.1 mg/mL, the maximal amount used in this study, has been shown to have minimal toxicity.43 Furthermore, TA at a concentration 10 times lower (0.01 mg/mL) already exhibited a significant inhibitory effect on caspase-5 activation. Thus, our results support the clinical use of TA, which has the advantage of being delivered in a sustained-release crystalline form.
The data presented here demonstrated that in LPS-primed hRPE cells, ATP and its analog, BzATP, caused time-dependent, transient increases in caspase-5 and -1 activation. ATP is present in millimolar concentrations in the cytosol of all eukaryotic cell types, and the extracellular levels are maintained at extremely low levels by ubiquitous ecto-ATPases and ecto-phosphatases in physiologic conditions.46 Extracellular ATP ubiquitously functions as an important mediator for cell-cell communication. Under pathologic conditions, such as inflammation, ATP concentrations may increase substantially by its release from damaged cells. RPE cells have a functional ATP P2X7 receptor that directly induces apoptosis and releases ATP into the subretinal space in response to chemical ischemia, cell swelling, osmotic stress, growth factors, glutamate, and other stimuli.47,48 Human RPE cells are also capable of degrading extracellular ATP, which may be released by leukocytes and RPE cells in retinal diseases. Recent evidence suggests that extracellular ATP accumulation at the sites of inflammation is considered to be a danger signal that alerts the immune system by binding to the P2X7 purinoreceptor, thereby activating NALP3 and caspase-1.24 Therefore, the current finding of involvement of caspase-5 and -1 in hRPE response to ATP may be clinically relevant.
The pathologic significance of caspase-5 has become more evident by a series of pathogenetic investigations.49–52 A polyadenosine repeat A(10) in the caspase-5 coding sequence is often mutated, causing a frame shift in various microsatellite instability–positive cancers, including leukemia and gastrointestinal, endometrial, breast, and lung carcinomas. In addition, a polymorphism of caspase-5 has also been linked to ovarian cancer53 and aging.54 The A(10) repeat does not exist in caspase-4. These studies suggest the clinical importance of caspase-5, for example, in tumorigenesis, perhaps related to a proapoptotic function. Our data show that caspase-5 is not as potent as caspase-4 in ER stress-induced apoptosis. Whether the proinflammatory role of caspase-5 is related to these reduced function mutations is unknown.
Human RPE cells, located at the blood-retina burrier, are putative important immunoregulatory cells that play important key roles in innate and adaptive immunity in a variety of retinal pathologic processes. Many reports have shown RPE cells to be ideal targets for infectious agents.55–58 Pathogen replication and elaboration of toxin by these agents can induce RPE cell death,55,58 which remains a potential risk factor for AMD pathogenesis.59 Inflammatory processes are also implicated in many diseases in which innate immunity contributes to pathogenesis. For example, in diabetic retinopathy, upregulation of IL-1β and caspase-1 activity occurs in retinal capillary cells.60,61 Research results up to now are just beginning to reveal how caspase-5 and caspase-1 may be involved in retinal diseases. The functional roles of caspase-5 remain poorly characterized in hRPE cells. In other cell types, NOD2, NLR protein NALP1, and caspase-1 form a complex mediating innate immune responses.62 Because caspase-5 is known to be associated with caspase-1 in NALP1 inflammasome,5 it is reasonable to propose that caspase-5 is also a downstream effector of the hRPE NLR–mediated innate immune response. The involvement of caspase-5 and the NLR signal system in retinal diseases warrants further investigation.
Footnotes
Supported by National Institutes of Health Grants EY-09441, N007361, and EY007003 and by a Research to Prevent Blindness Senior Scientific Award (VME).
Disclosure: Z.-M. Bian, None; S.G. Elner, None; H. Khanna, None; C.A. Murga-Zamalloa, None; S. Patil, None; V.M. Elner, None
References
- 1. Munday NA, Vaillancourt JP, Ali A, et al. Molecular cloning and pro-apoptotic activity of ICErelII and ICErelIII, members of the ICE/CED-3 family of cysteine proteases. J Biol Chem. 1995;270:15870–15876 [DOI] [PubMed] [Google Scholar]
- 2. Faucheu C, Blanchet AM, Collard-Dutilleul V, Lalanne JL, Diu-Hercend A. Identification of a cysteine protease closely related to interleukin-1 beta-converting enzyme. Eur J Biochem. 1996;236:207–213 [DOI] [PubMed] [Google Scholar]
- 3. Lin XY, Choi MS, Porter AG. Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipopolysaccharide and interferon-gamma. J Biol Chem. 2000;275:39920–39926 [DOI] [PubMed] [Google Scholar]
- 4. Eckhart L, Kittel C, Gawlas S, et al. Identification of a novel exon encoding the amino-terminus of the predominant caspase-5 variants. Biochem Biophys Res Commun. 2006;348:682–688 [DOI] [PubMed] [Google Scholar]
- 5. Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22 [DOI] [PubMed] [Google Scholar]
- 6. Scott AM, Saleh M. The inflammatory caspases: guardians against infections and sepsis. Cell Death Differ. 2007;14:23–31 [DOI] [PubMed] [Google Scholar]
- 7. Cornelis S, Kersse K, Festjens N, Lamkanfi M, Vandenabeele P. Inflammatory caspases: targets for novel therapies. Curr Pharm Des. 2007;13:367–385 [DOI] [PubMed] [Google Scholar]
- 8. Lamkanfi M, Kalai M, Vandenabeele P. Caspase-12: an overview. Cell Death Differ. 2004;11:365–368 [DOI] [PubMed] [Google Scholar]
- 9. Eckhart L, Ballaun C, Hermann M, et al. Identification of novel mammalian caspases reveals an important role of gene loss in shaping the human caspase repertoire. Mol Biol Evol. 2008;25:831–841 [DOI] [PubMed] [Google Scholar]
- 10. Nasir J, Theilmann JL, Vaillancourt JP, et al. Interleukin-1beta-converting enzyme (ICE) and related cell death genes ICErel-II and ICErel-III map to the same PAC clone at band 11q22.2–22.3. Mamm Genome. 1997;8:611–613 [DOI] [PubMed] [Google Scholar]
- 11. Nadiri A, Wolinski MK, Saleh M. The inflammatory caspases: key players in the host response to pathogenic invasion and sepsis. J Immunol. 2006;177:4239–4245 [DOI] [PubMed] [Google Scholar]
- 12. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426 [DOI] [PubMed] [Google Scholar]
- 13. Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574 [DOI] [PubMed] [Google Scholar]
- 14. An E, Lu X, Flippin J, et al. Secreted proteome profiling in human RPE cell cultures derived from donors with age related macular degeneration and age matched healthy donors. J Proteome Res. 2006;5:2599–2610 [DOI] [PubMed] [Google Scholar]
- 15. Bian ZM, Elner SG, Elner VM. Regulated expression of caspase-12 gene in human retinal pigment epithelial cells suggests its immunomodulating role. Invest Ophthalmol Vis Sci. 2008;49:5593–5601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bian ZM, Elner SG, Elner VM. Dual involvement of caspase-4 in inflammatory and ER stress-induced apoptotic responses in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:6006–6014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elner VM, Strieter RM, Elner SG, Baggiolini M, Lindley I, Kunkel SL. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990;136:745–750 [PMC free article] [PubMed] [Google Scholar]
- 18. Elner SG, Elner VM, Pavilack MA, et al. Modulation and function of intercellular adhesion molecule-1 (CD54) on human retinal pigment epithelial cells. Lab Invest. 1992;66:200–211 [PubMed] [Google Scholar]
- 19. Bian ZM, Elner SG, Yoshida A, Elner VM. Human RPE-monocyte coculture induces chemokine gene expression through activation of MAPK and NIK cascade. Exp Eye Res. 2003;76:573–583 [DOI] [PubMed] [Google Scholar]
- 20. Evanoff HL, Burdick MD, Moore SA, Kunkel SL, Strieter RM. A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1). Immunol Invest. 1992;21:39–45 [DOI] [PubMed] [Google Scholar]
- 21. Elner VM, Strieter RM, Pavilack MA, et al. Human corneal interleukin-8. IL-1 and TNF-induced gene expression and secretion. Am J Pathol. 1991;139:977–988 [PMC free article] [PubMed] [Google Scholar]
- 22. Bian ZM, Elner SG, Yoshida A, Kunkel SL, Su J, Elner VM. Activation of p38, ERK1/2 and NIK pathways is required for IL-1beta and TNF-alpha-induced chemokine expression in human retinal pigment epithelial cells. Exp Eye Res. 2001;73:111–121 [DOI] [PubMed] [Google Scholar]
- 23. Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694 [DOI] [PubMed] [Google Scholar]
- 24. Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;4:198–208 [DOI] [PubMed] [Google Scholar]
- 25. Humke EW, Ni J, Dixit VM. ERICE, a novel FLICE-activatable caspase. J Biol Chem. 1998;273:15702–15707 [DOI] [PubMed] [Google Scholar]
- 26. Bian ZM, Elner VM, Yoshida A, Kunkel SL, Elner SG. Signaling pathways for glycated human serum albumin-induced IL-8 and MCP-1 secretion in human RPE cells. Invest Ophthalmol Vis Sci. 2001;42:1660–1668 [PubMed] [Google Scholar]
- 27. Choi C, Kutsch O, Park J, Zhou T, Seol DW, Benveniste EN. Tumor necrosis factor-related apoptosis-inducing ligand induces caspase-dependent interleukin-8 expression and apoptosis in human astroglioma cells. Mol Cell Biol. 2002;22:724–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509 [DOI] [PubMed] [Google Scholar]
- 29. Kurtz RM, Elner VM, Bian ZM, Strieter RM, Kunkel SL, Elner SG. Dexamethasone and cyclosporin A modulation of human retinal pigment epithelial cell monocyte chemotactic protein-1 and interleukin-8. Invest Ophthalmol Vis Sci. 1997;38:436–445 [PubMed] [Google Scholar]
- 30. Irazuzta J, Pretzlaff RK, DeCourten-Myers G, Zemlan F, Zingarelli B. Dexamethasone decreases neurological sequelae and caspase activity. Intensive Care Med. 2005;3:146–150 [DOI] [PubMed] [Google Scholar]
- 31. Irahara M, Ando M, Kol S, Adashi EY. Expression and hormonal regulation of rat ovarian interleukin-1beta converting enzyme, a putative apoptotic marker: endocrine- and paracrine-dependence. J Reprod Immunol. 1999;45:67–79 [DOI] [PubMed] [Google Scholar]
- 32. Yao J, Johnson RW. Induction of interleukin-1 beta-converting enzyme (ICE) in murine microglia by lipopolysaccharide. Brain Res Mol Brain Res. 1997;51:170–178 [DOI] [PubMed] [Google Scholar]
- 33. Kim HJ, Hart J, Knatz N, Hall MW, Wewers MD. Janus kinase 3 down-regulates lipopolysaccharide-induced IL-1 beta-converting enzyme activation by autocrine IL-10. J Immunol. 2004;172:4948–4955 [DOI] [PubMed] [Google Scholar]
- 34. Fernandes A, Vaz AR, Falcão AS, Silva RF, Brito MA, Brites D. Glycoursodeoxycholic acid and interleukin-10 modulate the reactivity of rat cortical astrocytes to unconjugated bilirubin. J Neuropathol Exp Neurol. 2007;66:789–798 [DOI] [PubMed] [Google Scholar]
- 35. Jonas JB, Kreissig I, Degenring RF. Factors influencing visual acuity after intravitreal triamcinolone acetonide as treatment of exudative age related macular degeneration. Br J Ophthalmol. 2004;88:1557–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jonas JB. Intravitreal triamcinolone acetonide for treatment of sympathetic ophthalmia. Am J Ophthalmol. 2004;137:367–368 [DOI] [PubMed] [Google Scholar]
- 37. Park CH, Jaffe GJ, Fekrat S. Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal vein occlusion. Am J Ophthalmol. 2003;136:419–425 [DOI] [PubMed] [Google Scholar]
- 38. Massin P, Audren F, Haouchine B, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218–224 [DOI] [PubMed] [Google Scholar]
- 39. Jonas JB, Hayler JK, Panda-Jonas S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative vitreoretinopathy. Br J Ophthalmol. 2000;84:1064–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Degenring RF, Jonas JB. Serum levels of triamcinolone acetonide after intravitreal injection. Am J Ophthalmol. 2004;137:1142–1143 [DOI] [PubMed] [Google Scholar]
- 41. Jonas JB. Intraocular availability of triamcinolone acetonide after intravitreal injection. Am J Ophthalmol. 2004;137:560–562 [DOI] [PubMed] [Google Scholar]
- 42. Shaikh S, Ho S, Engelmann LA, Klemann SW. Cell viability effects of triamcinolone acetonide and preservative vehicle formulations. Br J Ophthalmol. 2006;90:233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chung H, Hwang JJ, Koh JY, Kim JG, Yoon YH. Triamcinolone acetonide-mediated oxidative injury in retinal cell culture: comparison with dexamethasone. Invest Ophthalmol Vis Sci. 2007;48:5742–5749 [DOI] [PubMed] [Google Scholar]
- 44. Yeung CK, Chan KP, Chan CK, Pang CP, Lam DS. Cytotoxicity of triamcinolone on cultured human retinal pigment epithelial cells: comparison with dexamethasone and hydrocortisone. Jpn J Ophthalmol. 2004;48:236–242 [DOI] [PubMed] [Google Scholar]
- 45. Narayanan R, Mungcal JK, Kenney MC, Seigel GM, Kuppermann BD. Toxicity of triamcinolone acetonide on retinal neurosensory and pigment epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:722–728 [DOI] [PubMed] [Google Scholar]
- 46. Pearson JD, Coade SB, Cusack NJ. Characterization of ectonucleotidases on vascular smooth-muscle cells. Biochem J. 1985;230:503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dutot M, Liang H, Pauloin T, et al. Effects of toxic cellular stresses and divalent cations on the human P2X7 cell death receptor. Mol Vis. 2008;14:889–897 [PMC free article] [PubMed] [Google Scholar]
- 48. Tovell VE, Sanderson J. Distinct P2Y receptor subtypes regulate calcium signaling in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:350–357 [DOI] [PubMed] [Google Scholar]
- 49. Schwartz S, Jr, Yamamoto H, Navarro M, Maestro M, Reventós J, Perucho M. Frameshift mutations at mononucleotide repeats in caspase-5 and other target genes in endometrial and gastrointestinal cancer of the microsatellite mutator phenotype. Cancer Res. 1999;59:2995–3002 [PubMed] [Google Scholar]
- 50. Takeuchi S, Takeuchi N, Fermin AC, Taguchi H, Koeffler HP. Frameshift mutations in caspase-5 and other target genes in leukemia and lymphoma cell lines having microsatellite instability. Leuk Res. 2003;27:359–361 [DOI] [PubMed] [Google Scholar]
- 51. Soung YH, Jeong EG, Ahn CH, et al. Mutational analysis of caspase 1, 4, and 5 genes in common human cancers. Hum Pathol. 2008;39:895–900 [DOI] [PubMed] [Google Scholar]
- 52. Notaridou M, Quaye L, Dafou D, et al. Common alleles in candidate susceptibility genes associated with risk and development of epithelial ovarian cancer. Int J Cancer. 2011;128:2063–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Quaye L, Dafou D, Ramus SJ, et al. Functional complementation studies identify candidate genes and common genetic variants associated with ovarian cancer survival. Hum Mol Genet. 2009;18:1869–1878 [DOI] [PubMed] [Google Scholar]
- 54. Ulybina YM, Kuligina ESh, Mitiushkina NV, et al. Evidence for depletion of CASP5 Ala90Thr heterozygous genotype in aged subjects. Exp Gerontol. 2010;45:726–729 [DOI] [PubMed] [Google Scholar]
- 55. Moyer AL, Ramadan RT, Thurman J, Burroughs A, Callegan MC. Bacillus cereus induces permeability of an in vitro blood-retina barrier. Infect Immun. 2008;76:1358–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Detrick B, Nagineni CN, Grillone LR, Anderson KP, Henry SP, Hooks JJ. Inhibition of human cytomegalovirus replication in a human retinal epithelial cell model by antisense oligonucleotides. Invest Ophthalmol Vis Sci. 2001;2:163–169 [PubMed] [Google Scholar]
- 57. Nagineni CN, Detrick B, Hooks JJ. Toxoplasma gondii infection induces gene expression and secretion of interleukin 1 (IL-1), IL-6, granulocyte-macrophage colony-stimulating factor, and intercellular adhesion molecule 1 by human retinal pigment epithelial cells. Infect Immun. 2000;68:407–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vann VR, Atherton SS. Neural spread of herpes simplex virus after anterior chamber inoculation. Invest Ophthalmol Vis Sci. 1991;32:2462–2472 [PubMed] [Google Scholar]
- 59. Miller DM, Espinosa-Heidmann DG, Legra J, et al. The association of prior cytomegalovirus infection with neovascular age-related macular degeneration. Am J Ophthalmol. 2004;138:323–328 [DOI] [PubMed] [Google Scholar]
- 60. Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mohr S, Xi X, Tang J, Kern TS. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes. 2002;51:1172–1179 [DOI] [PubMed] [Google Scholar]
- 62. Hsu LC, Ali SR, McGillivray S, et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A. 2008;105:7803–7808 [DOI] [PMC free article] [PubMed] [Google Scholar]