Abstract
Several studies indicate that the DNA mismatch repair (MMR) system may trigger cytotoxicity upon 5-fluorouracil (5-FU) recognition, but signaling pathways regulated by MMR in response to 5-FU are unknown. We hypothesize that recognition of 5-FU in DNA by MMR proteins trigger specific signaling cascades that results in slowing of the cell cycle and cell death. Whole human genome cDNA microarrays were used to examine relative signaling responses induced in MMR-proficient cells after 5-FU (5 µM) treatment for 24 hours. Analysis revealed 43 pathways differentially affected by 5-FU compared to control (p < 0.05), including cyclin and cell cycle regulation involving G1-S cell cycle transition, activation of Src, MAP K, p53 and base excision repair. In particular, 5-FU upregulated cyclins E1 and E2 (≥1.4-fold) and downregulated cdc25C, cyclins B1 and B2, histone H2A, H2B and H3 (≤-1.4-fold) over control. Cell cycle analysis revealed a G1/S arrest by 5-FU that was congruent with increased cyclin E and decreased cdc25C protein expression. Importantly, with knockdown of hMLH1 and hMSH2, we observed that decreased histone H3 expression by 5-FU was dependent on hMLH1. Additionally, 5-FU treatment dramatically decreased levels of several histone H3 modifications. Our data suggest that 5-FU induces a G1/S arrest by regulating cyclin E and cdc25C expression and MMR recognition of 5-FU in DNA may modulate cyclin E to affect the cell cycle. Furthermore, MMR recognition of 5-FU reduces histone H3 levels that could be related to DNA access by proteins and/or cell death during the G1/S phase of the cell cycle.
Key words: 5-fluorouracil, colorectal cancer, DNA mismatch repair, cyclin E, histone H3, cancer treatment, microsatellite instability
Introduction
5-fluorouracil (5-FU) is the principal chemotherapeutic agent used to treat patients with advanced colorectal cancer. In particular, 5-FU-based chemotherapy improves survival in patients with stage III colon cancer,1–3 and in patients with stage II and III rectal cancer.4 Although 5-FU based chemotherapy is the gold standard for advanced stage colorectal cancer patients, individual patient tumor response rate for 5-FU treatment is low (20–30%) but it does have an impact on survival.5,6 There is no current methodology to decide which advanced colorectal cancer patient will have a tumor response to 5-FU treatment. However, loss of DNA mismatch repair (MMR) within the patient's tumor is associated with no survival benefit from 5-FU treatment.7,8
Functional MMR requires hMutSα (a heterodimer of hMSH2 and hMSH6) and hMutSβ (a heterodimer of hMSH2 and hMSH3) to recognize and bind mispairs and/or insertion/deletion loops (IDLs) that occur at microsatellite sequences. hMutLα (a heterodimer of hMLH1 and hPMS2) joins the hMutS complexes to help coordinate DNA excision and repair of the mispair or IDL.9–11 Defects in the MMR genes hMSH2, hMLH1, hMSH6 or hPMS2 cause Lynch syndrome and epigenetic inactivation of hMLH1 by promoter hypermethylation occurs in 15–20% of sporadic colorectal tumors with microsatellite instability (MSI).12–17
Retrospective and prospective studies of patients with colorectal cancer indicate that those with intact MMR in their tumors have improved survival with 5-FU treatment, whereas patients whose tumors lost MMR do not have improved survival.7,8,18 In vitro studies revealed that human colorectal cell lines with intact MMR were selectively killed with 5-FU treatment whereas MSI cells were resistant to 5-FU treatment.19 Additionally, biochemical studies demonstrated that hMutSα directly recognizes and binds 5-FU that is incorporated into DNA with a greater affinity compared to its natural substrate, a base mispair and such recognition was lost with MMR deficiency.20,21 These observations suggest that MMR, at least in part, mediates the cytotoxicity of 5-FU in addition to its known roles affecting RNA.20
It is not clear how the MMR system recognizes 5-FU incorporated into DNA, although the human MMR system can recognize certain DNA adducts such as 6-thioguanine (6-TG) and O6-methylguanine (O6-MeG) caused by alkylation damage.22,23 The downstream signaling pathways triggered by MMR recognition of modified DNA have been partially elucidated for some chemotherapeutic agents. For example, incorporation or formation of O6-MeG into DNA induces DNA mispairing and distorts the DNA double helix that is easily detected by the MMR system.22–24 Introduction of O6-MeG into DNA results in a G2/M cell cycle arrest and apoptosis that are dependent on an intact MMR system and involve the ATM and Rad3-related (ATR) signaling pathway as well as mitochondrial signaling that activates both caspase-dependent and caspase-independent pathways.25–27 However, the signaling pathways triggered by MMR in response to 5-FU-modified DNA have not been elucidated.
We aimed to elucidate key signaling pathways upon MMR recognition of 5-FU that result in slowing of the cell cycle and cell death. In this study, we utilized a whole human genomic cDNA microarray analysis to examine relative signaling responses induced in MMR-proficient colorectal cancer cells in response to 5-FU. We verified microarray observations with protein expression of each gene affected by 5-FU and performed cell cycle analysis. Our data indicate that 5-FU induces a G1/S cell cycle arrest by regulating cyclin E and cdc25C expression in MMR-proficient cells and MMR recognition of 5-FU in DNA modulates cyclin E to affect the cell cycle. Furthermore, we demonstrate that 5-FU reduces expression of histone H3 and its various modifications (acetyl-, methyl- and phospho-histone H3) and the decreased histone H3 expression after 5-FU treatment is dependent upon the presence of hMLH1.
Results
Effect of 5-FU on gene expression in MMR-proficient cells.
Whole human genome cDNA microarrays were used to examine relative signaling responses induced in MMR-proficient SW480 cells after treatment with DMSO control or 5 µM 5-FU for 24 hours. Signaling cascade analysis showed that 43 pathways were differentially affected by 5-FU treatment (p < 0.05) compared with control treatment. The major signaling pathways affected by 5-FU treatment include: mitotic roles of polo-like kinase (PLK), cyclin and cell cycle regulation involving G1-S phase transition, cell cycle, activation of Src, mitogen-activated protein kinase (MAPK) signaling, p53 signaling and base excision repair (Table 1). Among signaling pathways after 5-FU treatment, 4 genes [cyclin E1, cycline E2, p21 and E2F transcription factor (TF) 2] were upregulated (≥1.4-fold) and 9 genes [polo-like kinase-1 (plk-1), CDC20, cyclin B1 and cyclin B2, budding uninhibited by benzimidazoles 1 homolog (BUB 1), cell division cycle 25C (cdc25C), histone H2A, histone H2B and histone H3] were downregulated (≤-1.4 fold) compared to control treatment. The modest but significant changes in expression of genes involved in these pathways may be due to the short treatment (24 hr) and low pharmacological dose (5 µM) of 5-FU, the mean steady state plasma concentration of colorectal cancer patients after 5-FU infusion.28
Table 1.
Signaling pathways affected by 5-FU treatment in MMR-proficient SW480 cells
| Pathway (p-value) | Gene name | GeneBank accession no. | Fold change |
| Mitotic roles of polo-like kinase (p = 0.00002) | Polo-like kinase1 | NM_005030.3 | −1.53 |
| CDC20 (cell division cycle 20 homolog, S. cerevisiae) | NM_001255.1 | −1.42 | |
| Cyclin B2 | NM_004701.2 | −1.45 | |
| Cyclin B1 | NM_031966.2 | −1.94 | |
| Cyclins and cell cycle regulation (p = 0.0002) | Cyclin E2 | NM_057749.1 | 1.77 |
| Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | NM_078467.1 | 1.40 | |
| E2F transcription factor 2 | NM_004091.2 | 1.53 | |
| Cyclin B2 | NM_004701.2 | −1.45 | |
| Cyclin E1 | NM_001238.1 | 1.53 | |
| Cyclin B1 | NM_031966.2 | −1.94 | |
| Cell cycle (p = 0.0003) | BUB1 (budding uninhibited by benzimidazoles 1 homolog, yeast) | NM_004336.2 | −1.49 |
| Polo-like kinase1 | NM_005030.3 | −1.53 | |
| Cyclin E2 | NM_057749.1 | 1.77 | |
| Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | NM_078467.1 | 1.40 | |
| CDC20 (cell division cycle 20 homolog, S. cerevisiae) | NM_001255.1 | −1.42 | |
| Cyclin B2 | NM_004701.2 | −1.45 | |
| Cyclin E1 | NM_001238.1 | 1.53 | |
| Cyclin B1 | NM_031966.2 | −1.94 | |
| Activation of Src by protein-tyrosine phosphatase alpha (p = 0.0006) | Cell division cycle 25C | NM_001790.2 | −1.40 |
| Cyclin B1 | NM_031966.2 | −1.94 | |
| UVB-induced MAP K signaling (p = 0.0010) | Histone 1, H3e | NM_003532.2 | −1.41 |
| p53 signaling pathway (p = 0.0024) | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | NM_078467.1 | 1.40 |
| Cyclin E1 | NM_001238.1 | 1.53 | |
| Base excision repair pathway (p = 0.0036) | Uracil-DNA glycosylase 2 | NM_021147.2 | 1.29 |
| G1-S phase transition (p = 0.0041) | Cyclin E2 | NM_057749.1 | 1.77 |
| Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | NM_078467.1 | 1.40 | |
| E2F transcription factor 2 | NM_004091.2 | 1.53 | |
| Cyclin E1 | NM_001238.1 | 1.53 | |
| Cell cycle: G1/S check point (p = 0.0191) | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | NM_078467.1 | 1.40 |
| Cyclin E1 | NM_001238.1 | 1.53 | |
| Cell cycle: G2/M check point (p = 0.0206) | Polo-like kinase1 | NM_005030.3 | −1.53 |
| Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | NM_078467.1 | 1.40 | |
| Cell division cycle 25C | NM_001790.2 | −1.40 | |
| Cyclin B1 | NM_031966.2 | −1.94 | |
| Influence of Ras and Rho proteins on G1 to S transition (p = 0.0276) | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | NM_078467.1 | 1.40 |
| Cyclin E1 | NM_001238.1 | 1.53 | |
| Cdc25 and chk1 regulatory pathway in response to DNA damage (p = 0.0332) | Cell division cycle 25C | NM_001790.2 | −1.40 |
| Cell division cycle 25A | NM_001789.2 | 1.35 | |
| MAPK signaling (p = 0.0348) | Histone 1, H2ac | NM_003512.3 | −2.06 |
| Histone 2, H2be | NM_003528.2 | −1.71 | |
| Histone 1, H2bj | NM_021058.3 | −2.38 | |
| Histone 1, H2bg | NM_003518.3 | −1.48 | |
| H2B histone family, member S | NM_017445.1 | −1.68 | |
| Histone 1, H2bd | NM_138720.1 | −1.44 | |
| Histone 1, H2be | NM_003523.2 | −1.89 | |
| DNA damage induced 14-3-3sigma signaling (p = 0.0389) | Cyclin E2 | NM_057749.1 | 1.77 |
| Cyclin B2 | NM_004701.2 | −1.45 | |
| Cyclin E1 | NM_001238.1 | 1.53 | |
| Cyclin B1 | NM_031966.2 | −1.94 |
Major signaling cascades affected by 5-FU were listed by p-value (ones with lower p-values were placed higher, p < 0.05) compared to DMSO-treated cells. In each signaling cascade, affected genes were listed according to their expression changes between DMSO and 5-FU as well as a consistency between two replicates. Fold change was calculated by dividing signaling intensity of 5-FU treatment by one of DMSO treatment in 2 separate experiments and averaging them. Genes with ≥1.4 or ≤-1.4 fold changes were listed except uracil-DNA glycosylase 2 which showed 1.29 fold change.
5-FU induces a G1/S phase arrest after 24 hours in MMR-proficient cells.
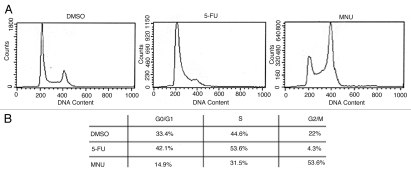
By microarray analysis, we observed that G1-S phase transition and G1/S check point pathways were significantly affected by 5-FU (p < 0.05) and genes involved in those pathways (cyclin E1 and E2, p21 and E2F) were upregulated (Table 1). Cell cycle analysis confirmed those observations (Fig. 1A). 5-FU-treated cells showed a higher proportion of cells in the G1/S phase (96% vs. 78%) in SW480 cells compared to DMSO-treated cells (Fig. 1B), indicating that 5-FU induces the G1/S phase arrest in the MMR-proficient cells. In contrast, MNU arrested the cell cycle at the G2/M phase (54% vs. 22%) compared to DMSO as observed in previous studies.23,29,30
Figure 1.
Cell cycle analysis of MMR-proficient SW480 cells after treatment with DMSO, 5-FU and MNU. Cells were treated with DMSO, 5 µM of 5-FU or 1 mM of MNU for 24 hr and harvested for cell cycle analysis by flow cytometry (A). Note that 5-FU induces a G1/S cell phase arrest compared to MNU, which causes a G2/M phase arrest (B).
Validation of genes affected by 5-FU and influence by competency in MMR function.
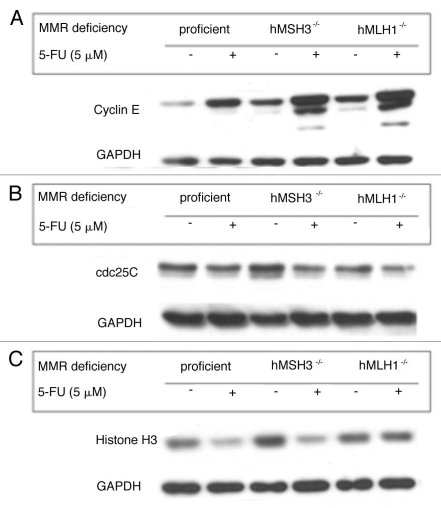
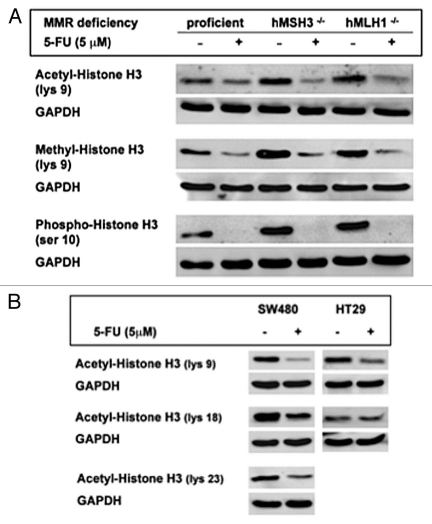
Protein expression was determined by western blotting to verify the expression of genes affected by 5-FU in the microarray analysis. Specifically, to investigate the role of MMR proteins in signaling pathways affected by 5-FU, cells with different MMR backgrounds (SW480: MMR-proficient, HCT116 + ch3: hMLH1- restored but hMSH3-/- and HCT116: hMLH1-/-) were used. As shown in gene expression analysis (Table 1), 5-FU greatly affected expression of proteins that are involved in cell cycle regulation. 5-FU treatment induced increased cyclin E (Fig. 2A) and slightly decreased cdc25C protein expression (Fig. 2B) in SW480 cells, congruent with G1/S arrest by cell cycle analysis after 5-FU treatment. Increased cyclin E and decreased cdc25C expression by 5-FU were also observed in HCT116 + ch3 and HCT116 cells regardless of MMR genetic backgrounds (Fig. 2A and B). However, in Figure 2A when comparing each DMSO controls, MMR-proficient cells had a lower baseline expression and showed a much greater increase in cyclin E expression than MMR-deficient cells after 5-FU treatment. This observation suggests that one or more of the MMR proteins may partially contribute to the reduced baseline and subsequent increase in cyclin E expression in response to 5-FU. Cyclin B1 transcript levels were decreased in SW480 cells in response to 5-FU (Table 1), but cyclin B1 protein expression was increased after 5-FU treatment in all 3 cell lines (Suppl. Fig. S1A). 5-FU-treated SW480 cells did not show any difference in protein expression for plk-1, p21 and E2F-TF-2 compared to DMSO treated cells and BUB 1 expression was not detectable (data not shown). On the other hand, we observed decreased histone H3 protein expression by 5-FU in SW480 cells (Fig. 2C) as observed in the microarray analysis (Table 1). Interestingly, 5-FU treatment also decreased histone H3 expression in hMSH3-/- cells but did not decrease histone H3 expression in hMLH1-/- cells (Fig. 2C). These observations suggest that decreased histone H3 expression by 5-FU may be dependent on hMLH1. However, this observation did not extend to histone H2A and H2B protein expression after 5-FU treatment in any cell line (Suppl. Fig. S1B and C), despite their decreases in the microarray analysis (Table 1).
Figure 2.
Effect of 5-FU on protein expression of cyclin E, cdc25C and histone H3 in colorectal cancer cells with different MMR deficiencies. Significantly-affected genes by 5-FU in the microarray analysis were verified by protein expression after 5-FU treatment in MMR-proficient SW480 cells, hMSH3-deficient HCT116 + chr 3 cells and hMLH1-deficient HCT116 cells. Compared to each counterpart DMSO treatment: (A) 5-FU treatment showed increased cyclin E expression in all cell lines with different MMR deficiencies, (B) 5-FU treatment showed slightly decreased cdc25C expression in all cell lines and (C) 5-FU treatment decreased histone H3 expression in MMR-proficient and hMSH3-deficient cells but it did not decrease histone H3 expression in hMLH1-deficient cells, which suggests that decreased histone H3 expression by 5-FU may be dependent on hMLH1.
Histone H3 and cyclin E expression are regulated by 5-FU in a MMR-dependent manner.
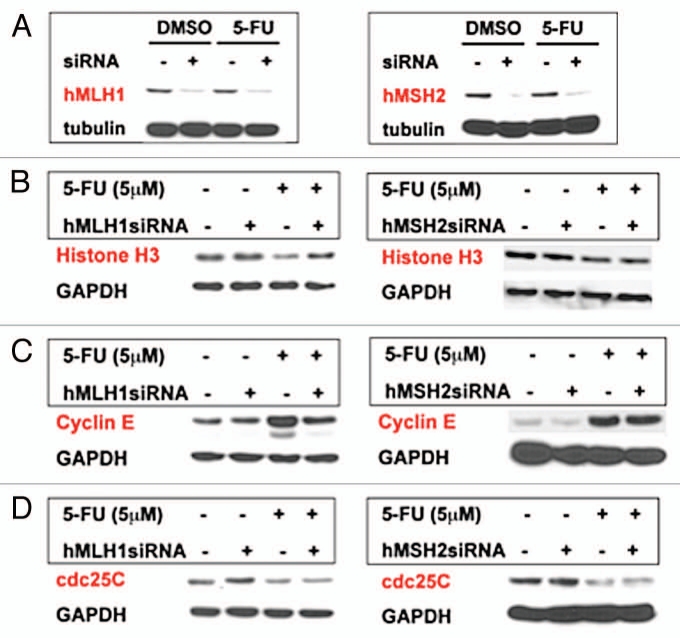
To specifically answer whether decreased histone H3 or increased cyclin E expression after 5-FU treatment (Fig. 2A and C) is dependent on MMR, siRNAs for hMLH1 and hMSH2 (essential components of hMutS and hMutL-α complexes in the MMR system, respectively) were transfected into MMR-proficient cells. In SW480 cells, both hMLH1 and hMSH2 siRNAs significantly decreased hMLH1 and hMSH2 expression (Fig. 3A). As suggested in Figure 2C, knockdown of hMLH1 restored histone H3 expression in response to 5-FU (Fig. 3B). However, knockdown of hMSH2 did not restore histone H3 expression decreased by 5-FU (Fig. 3B). This observation indicates that histone H3 expression is regulated by 5-FU in an hMLH1- dependent manner. Additionally, we found that the increased cyclin E expression after 5-FU treatment was partially hMLH1-dependent as knockdown of hMLH1 lowered but did not completely reduce cyclin E expression to control levels (Fig. 3C). However, decreased cdc25C expression by 5-FU was not dependent on either hMLH1 or hMSH2 (Fig. 3D).
Figure 3.
Expression of histone H3, cyclin E and cdc25C in SW480 cells after MMR recognition of 5-FU. (A) Effectiveness of siRNA knockdown of hMLH1 or hMSH2 proteins. (B) Histone H3 expression and knockdown of hMLH1 or hMSH2. Note the decrease in histone H3 expression with 5-FU treatment and its preservation with knockdown of hMLH1 but not with knockdown of hMSH2. (C) Cyclin E expression and knockdown of hMLH1 or hMSH2. Note the increase in cyclin E expression with 5-FU treatment and its partial reduction with knockdown of hMLH1 but not with knockdown of hMSH2. (D) Cdc25C expression and knockdown of hMLH1 or hMSH2. Decreased cdc25C expression with 5-FU treatment was not restored by knockdown of either hMLH1 or hMSH2.
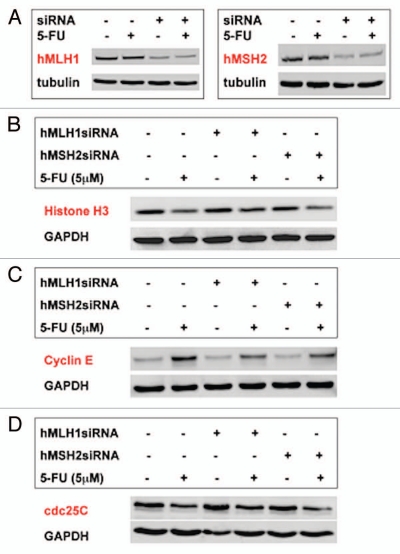
We performed the same siRNA experiments in another MMR-proficient cell line HT29 (Fig. 4A). As demonstrated in SW480 cells, decreased histone H3, increased cyclin E and decreased cdc25C protein expression were also observed in HT29 cells after 5-FU treatment (Fig. 4B–D). Different from SW480 cells, knockdown of hMLH1 partially restored histone H3 expression in response to 5-FU in HT29 cells (Fig. 4B). However, as observed in SW480 cells (Fig. 3B), knockdown of hMSH2 did not restore histone H3 expression in response to 5-FU (Fig. 4B). These observations suggest that decreased histone H3 expression by 5-FU in HT29 cells is partially dependent on hMLH1. Cyclin E expression in response to 5-FU was reduced by knockdown of hMLH1 as well as hMSH2 in HT29 cells although its reduction did not reach cyclin E levels for control, indicating that cyclin E expression in response to 5-FU may be partially regulated by both hMLH1 and hMSH2 in HT29 cells (Fig. 4C). As observed in SW480 cells, decreased cdc25C expression by 5-FU was not dependent on either hMLH1 or hMSH2 in HT29 cells (Fig. 4D).
Figure 4.
Expression of histone H3, cyclin E and cdc25C in HT29 cells after MMR recognition of 5-FU. (A) Effectiveness of siRNA knockdown of hMLH1 or hMSH 2 proteins. (B) Histone H3 expression and knockdown of hMLH1 or hMSH2. Note the decrease in histone H3 expression with 5-FU treatment and its partial restoration with knockdown of hMLH1 but not with knockdown of hMSH2. (C) Cyclin E expression and knockdown of hMLH1 or hMSH2. Note the increase in cyclin E expression with 5-FU treatment and its partial reduction with knockdown of hMLH1 or hMSH2. (D) cdc25C expression and knockdown of hMLH1 or hMSH2. Knockdown of either hMLH1 or hMSH2 did not restore decreased cdc25C expression by 5-FU.
5-FU treatment decreases histone H3 modifications.
Decreased transcript levels of histone H2A, H2B and H3 by 5-FU were observed in the microarray analysis (Table 1) but only histone H3 protein expression was affected by 5-FU in MMR-proficient SW480 cells (Fig. 2C). The decreased histone H3 expression in response to 5-FU was dependent on hMLH1, not hMSH2 (Fig. 3). To further dissect the decreased histone H3 expression, we examined the effect of 5-FU on histone H3 modifications such as acetylation, methylation and phosphorylation. 5-FU treatment greatly decreased expression of acetyl-histone H3 (at lys 9), pan-methyl-histone H3 (at lys 9) and phospho-histone H3 (at ser 10) in the three colon cancer cell lines with different MMR genetic backgrounds when compared to each counterpart controls (Fig. 5A). These observations suggest that these specific histone H3 modifications are not dependent on MMR. These observations were confirmed by siRNA experiments in MMR-proficient cells, as knockdown of hMLH1 or hMSH2 did not restore these decreased histone H3 modifications by 5-FU (Suppl. Fig. S2).
Figure 5.
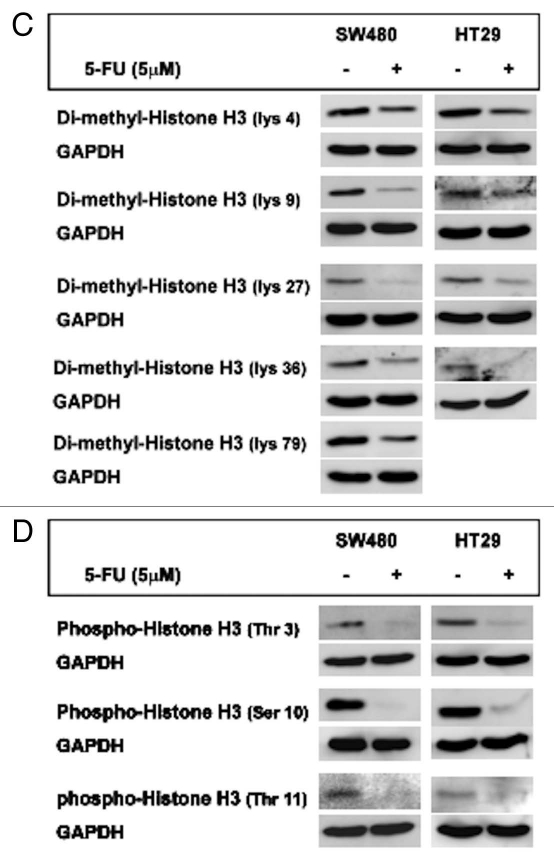
Effect of 5-FU treatment on histone H3 modifications. (A) Decreased histone H3 modifications by 5-FU in cells are not MMR dependent. Expression of acetyl-histone H3 at lys 9, pan-methyl-histone H3 expression at lys 9 and phospho-histone H3 at ser 10 after 5-FU treatment were greatly decreased compared to each DMSO control regardless of status of MMR deficiency. SW480, HCT116 + chr 3 and HCT116 cells were used as MMR-proficient, hMSH3-/- and hMLH1-/- cells, respectively. (B–D) 5-FU treatment inhibits several histone H3 modifications at various sites in MMR-proficient cells. Expression of acetyl histone H3 at lys 9, 18 and 23 (B), expression of dimethy-histone H3 at lys 4, 9, 27, 36 and 79 (C) and expression of phospho-histone H3 at thr 3 and 11 and ser 10 (D) were determined in SW480 and HT29 cells. Note that 5-FU significantly decreased expression of histone H3 modifications at different sites in both cell lines compared to DMSO except acetyl-histone H3 expression at lys 18 in HT29 cells.
Profile of histone H3 modification by 5-FU treatment.
We further examined other potential modifications of histone H3 by 5-FU in MMR-proficient cell lines. We determined the expression of acetyl histone H3 at lys 9, 18 and 23, dimethyhistone H3 at lys 4, 9, 27, 36 and 79 and phospho-histone H3 at thr 3 and 11 and ser 10 and 28 after 5-FU treatment. As shown in Figure 5B, baseline acetyl-histone H3 expression were highest at lys 18 and lys 9 in DMSO-treated SW480 and HT29 cells, respectively. 5-FU treatment decreased expression by more than 50% of acetyl-histone H3 at all sites in SW480 cells. In HT29 cells, 5-FU treatment significantly decreased acetyl-histone H3 level at lys 9 but it did not alter acetyl-histone H3 levels at lys 18 compared to controls. Expression of acetyl-histone H3 at lys 23 was not detectable in HT29 cells.
Dimethyl-histone H3 expression levels were higher at lys 4, 9 and 79 in DMSO-treated SW480 compared to lys 27 and 36, whereas dimethyl-histone H3 expression was significantly higher at lys 4 than other sites in DMSO-treated HT29 cells (Fig. 5C). Expression of dimethyl-histone H3 at lys 79 was not detectable in HT29 cells. 5-FU significantly decreased dimethyl histone H3 expression levels at all sites compared to DMSO-treatment in both cell lines (Fig. 5C).
In both SW480 and HT29 cells, strong expression of phospho-histone H3 at ser 10 was observed with DMSO treatment but a dramatic decrease in its expression was observed with 5-FU treatment (Fig. 5D). Decreased phospho-histone H3 expression at thr 3 and 11 by 5-FU were also observed in both cell lines, although their expression levels with DMSO treatment were significantly lower than ones at ser 10 (Fig. 5D). Phospho-histone H3 expression at ser 28 was not detectable in either cell lines (data not shown). These observations demonstrate that 5-FU inhibits certain histone H3 modifications that likely causes a decrease in its steady-state expression.
Discussion
In this study, we used a whole human genomic cDNA microarray expression profiling with MMR-proficient cells to identify specific signaling pathways triggered by MMR proteins in response to 5-FU that result in cell cycle arrest and cell death. A number of studies have performed DNA microarray analysis in colorectal cancer cells to examine signaling cascades affected by 5-FU using different doses and treatment times of 5-FU in attempt to identify markers capable of predicting 5-FU response.31–36 Despite this, the downstream molecular events underlying cytotoxic effects of 5-FU remain poorly characterized. In particular, no study has been performed to identify the signaling pathways regulated by MMR protein in response to 5-FU although several studies verify the notion that the MMR system triggers cytotoxicity upon 5-FU recognition.7,8,18–21,32,37–39
Our gene expression profiling data revealed that 5-FU treatment significantly affected signaling pathways involved in cell cycle regulation compared to control (Table 1). Specifically, we observed that genes involved in signaling pathways regulating G1/S phase transition and checkpoint were upregulated (cyclin E1, cycline E2, p21 and E2F TF-2) whereas genes associated with signaling pathway regulating G2/M phase transition and checkpoint were downregulated (plk-1, CDC20, cyclin B1 and cyclin B2, BUB 1 and cdc25C). Additionally, microarray analysis revealed that chromosome, nucleosome, replication fork, microtubule cytoskeleton and chromatin were significantly affected cell components by 5-FU (p < 0.001), supporting evidence that a major signaling pathway affected after 5-FU treatment is cell cycle regulation. Furthermore, cell cycle analysis revealed a G1/S arrest in MMR-proficient cells in response to 5-FU (Fig. 1), confirming the results of microarray analysis. The G1/S arrest by 5-FU has been reported in several studies,40–43 and is distinguished from other DNA adducts such as 6-TG and O6-MeG which have shown to induce a G2/M phase arrest in MMR-proficient cells.22,23 Interestingly, MAPK signaling pathway was also significantly affected by 5-FU treatment (p < 0.05) although only histone genes such as histone H2A, histone H2B and histone H3 showed meaningful and significant fold changes (≤-1.4 fold, p < 0.05) by 5-FU treatment in the pathway (Table 1). Decreased histone H2A, histone H2B and histone H3 mRNA levels were also observed in mammalian cells in response to DNA damages via ionizing radiation, which was reported through the G1 checkpoint pathway.44
We observed relatively small fold changes (within ∼±2 fold) in transcriptional levels of genes affected by 5-FU in microarray analysis (Table 1), and confirmed protein expression changes by 5-FU in some genes, such as cyclin E (increased) and cdc25C (decreased) and histone H3 (decreased), whereas most genes revealing transcriptional changes by 5-FU, such as plk-1, E2F TF-2, p21, histone H2A and histone H2B did not show their protein expression changes (Figs. 2 and S1). This may be caused by our treating the cells with a relatively low concentration (5 µM) of 5-FU for a short time (24 hr).
To determine the role of MMR proteins in expression changes of cyclin E, histone H3 and cdc25C in response to 5-FU, we took 2 different approaches: one was to use colcorectal cancer cell lines with different MMR deficiencies (MMR-proficient, hMSH3-/- and hMLH1-/-) and the other was knockdown the major MMR proteins, hMLH1 and hMSH2 using specific siRNA in MMR-proficient cells. Through these approaches, we demonstrated that increased cyclin E expression by 5-FU is partially dependent on hMLH1 but decreased cdc25C expression by 5-FU is not dependent on either hMLH1 or hMSH2 (Figs. 3 and 4). The dramatic decrease in histone H3 expression after 5-FU treatment was also dependent on hMLH1 (but not hMSH2) expression (Figs. 3 and 4). We initially speculated and investigated that there could be a direct interaction between hMLH1 and histone H3 to trigger histone H3 degradation. We individually pulled-down hMLH1 and histone H3 and then immunoblotted with histone H3 or hMLH1 antibodies after treating SW480 and HT29 cells with DMSO or 5-FU, but we did not observe a direct interaction between hMLH1 and histone H3 (data not shown). Thus, although it is clear that MMR recognition of 5-FU in DNA depends on hMLH1, the trigger for histone H3 destruction after this recognition is not clear and further study is necessary to elucidate how it occurs.
In attempt to characterize potential histone H3 modification after MMR recognition of 5-FU in DNA to clue in on regulators that hMLH1 may signal through, we profiled several histone H3 modifications. Histones undergo post-translational modifications which alter their interaction with DNA and nuclear proteins.45,46 The modifications such as acetylation, methylation and phosphorylation occur at the tails of core histones and histone modifications are known to act in diverse biological processes including gene regulation, DNA repair and mitosis.45 Biological roles of each modification of histones at different sites are varied in the context. It has been reported that acetylation of histone H3 at lys 9 is involved in activation of gene expression, methylation of histone H3 at lys 9 is associated with gene silencing and phosphorylation of histone H3 at ser 10 is involved in condensation of chromosomes as well as activation of gene expression.47 Dramatic reduction in expression of histone H3 modifications by 5-FU treatment was observed regardless of status of MMR deficiency (Fig. 5) and knockdowns of hMLH1 and hMSH2 proteins did not restore their expressions (Suppl. Fig. S2), indicating that effect of 5-FU on histone H3 modifications are not dependent on MMR proteins.
We also determined the effect of 5-FU treatment on expression of acetyl histone H3, di-methyl histone H3 and phospho-histone H3 at 3 to 5 different amino acid sites (Fig. 5) in MMR-proficient cells compared to control treatment. 5-FU treatment decreased at least 50% of expression of all detectable histone H3 modifications in both SW480 and HT29 cells except the expression of acetylhistone H3 at lys 18 in HT29 cells which was not changed by 5-FU treatment (Fig. 5). These data demonstrated that levels of histone H3 modifications are different among cell lines and sites of histone H3 (in control treatment), and 5-FU treatment inhibits acetylation, metylation and phosphorylation of histone H3.
Since both histone H3 and cyclin E are regulated once DNA MMR recognizes 5-FU in DNA, we speculate why these are affected. As mentioned, 5-FU in MMR-proficient cells cause a G1/S cell cycle arrest, as compared to a G2/M cell cycle arrest seen with O6-MeG adducts. Unlike the G2 phase where nucleosomes are not assembled and DNA has been recently replicated, the G1 phase maintains nucleosome structures. Upon recognition of 5-FU in DNA, the DNA MMR system would trigger a G1 cell cycle arrest, but in order to allow repair to ensue, nucleosome structures would have to be locally disrupted, for example, to complete repair. We speculate that the destruction of histone H3 allows access to DNA by the repair machinery to attempt repair during G1/S phase.
In summary, we demonstrate that 5-FU induces a G1/S cell cycle arrest by regulating cyclin E and cdc25C expression and MMR recognition of 5-FU in DNA may regulate cyclin E to affect the cell cycle. Furthermore, MMR recognition of 5-FU reduces histone H3 level that could be related to DNA access by repair proteins and/or triggering cell death. MMR handling of 5-FU in DNA may trigger downstream events that contribute to colorectal cancer patient survival.
Materials and Methods
Reagents.
5-FU and N-methyl-N-nitrosourea (MNU) were obtained from Sigma (St. Louis, MO) and dissolved in DMSO (Sigma) at a stock concentration of 1 and 200 mM, respectively and stored at −20°C. Five µM of 5-FU or 1 mM of MNU diluted in growth medium were treated to cells for 24 hour in all the experiments performed in this study.
Cell lines and cultures.
The human colorectal cancer cell lines SW480, HT29 (both MMR-proficient) and HCT116 (hMLH1-/- and hMSH3-/-) cells were obtained from American Type Culture Collection (Rockville, MD) and maintained in either Iscove's modified Dulbeeco's medium (IMDM, Invitrogen, Carlsbad, CA, for SW480 and HCT116 cells) or Dulbeeco's modified Eagle's medium (DMEM, Invtrogen, for HT29 cells) with 10% fetal bovine serum (FBS) and penicillin (100 U/ml)/streptomycin (100 µg/ml) (P/S, Invitrogen) as supplements. The HCT116 cell line containing transferred chromosome 3 (HCT116 + chr3, hMLH-restored but hMSH3-/-) was developed as previously described48 and maintained in IMDM containing 10% FBS, P/S and 400 µg/ml of G418 sulfate (CellGro, Manassas, VA).
Microarray analysis.
1.5 × 106 MMR-proficient SW480 cells were plated in 6 cm cell culture dishes. On the following day, cells were treated with DMSO control or 5 µM 5-FU for 24 hr. Total RNA were isolated from 2 independent experiments using Qiagen RNeasy Plus Mini Kit (Qiagen, Valencia, CA), following the manufacturer's instructions. Isolated total RNA was sent to the UCSD BioMedical Genomics Microarray (BIOGEM) Facility (San Diego, CA) for microarray analysis. cDNA synthesis, labeling and hybridization for Illumina Bead Whole Human Genomic cDNA Microarray (Illumina, San Diego, CA) were performed by BIOGEM according to their protocols. Detailed protocols are available at http://www.micrarrays.ucsd.edu/illumina/. Microarray data analysis was done using the Potkan software program. The analysis was performed by grouping gene expression according to signaling pathways, molecular function, biological process and cell components and comparing DMSO-treated cells with 5-FU treated cells (p < 0.05).
Cell cycle analysis.
Cell cycle analysis was performed by flow cytometric measurement of the DNA content using Propidium Iodide (PI) (Sigma). Briefly, cells were trypsinized at 24 hr after DMSO or 5-FU treatment, washed with Phosphate Buffered Saline (PBS) and centrifuged at 250x g for 10 min. The cells were resuspended at a density of 2 × 106 cells in 1 ml ice-cold PBS. The cells were gently vortexed, slowly added to 9 ml of 70% ethanol and stored at −20°C overnight. On the next day, cells were pelleted by centrifugation, washed with cold PBS and centrifuged at 200x g for 10 min at 4°C. Cell pellets were resuspended in 300 µl of staining solution containing 20 µg/ml PI (Sigma), 0.1% (v/v) Triton X-100 (Sigma) and 0.2 mg/ml DNAse-free RNAse A (Sigma) and incubated for 30 min at 20°C and then placed on ice. Subsequently, samples were analyzed for DNA content by FACS ARIA (Becton Dickinson Immunocytometry Systems (BDIS), San Jose, CA). Analysis of cell cycle data was made with Multicycle Softweare Autofit Version 2.5 (Phoenix Flow Systems, San Diego, CA).
Small interfering RNA (siRNA) transfections.
Silencer human MLH1 and MSH2 siRNAs were purchased from Ambion (Austin, TX). siRNA transfection were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocols. At 48 hr after transfection, the cells were treated with DMSO or 5-FU for 24 hr and then lysed for western blot analysis.
Western blotting.
Cells were washed with cold PBS and lysed with Radio Immuno Precipitation Assay (RIPA) buffer [1% (w/w) Nonidet P-40, 1% (w/v) sodium deoxycholate, 0.1% (w/v) SDS, 0.15 M NaCl, 0.01 M sodium phosphate, pH 7.2, 2 mM EDTA and 50 mM sodium fluoride] containing inhibitors (2 mM DTT, 1 mM PMSF, 10 µg/ml aprotinin, 2 µg/ml leupeptin and 0.2 mM Na-orthovandate) on ice. Lysed cells were centrifuged at 12,000x g for 15 min at 4°C and supernatants were collected as whole cell lysates. The protein content in each lysate was determined by Lowry assay. Cell lysates were resuspended in 4x gel loading buffer, boiled for 5 min and separated by SDS-PAGE. Immunodetection was done using primary antibodies: cyclin B1 (D-11), cyclin E (HE12) and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA), α-tubulin (Sigma) and cdc25C (5H9), histone H2A, histone H2B and histone H3, acetyl-histone H3 antibody sampler kit, methyl-histone H3 antibody sampler kit and phospho-histone H3 antibody sampler kit (Cell Signaling Technology, Denvers, MA) and horseradish peroxidase-conjugated secondary rabbit or mouse antibodies (Santa Cruz Biotechnology). The signal was detected by X-ray film or Fujifilm LAS-4000 luminescent image analyzer (Fujifilm, Tokyo, Japan) using a chemiluminescent solution.
Acknowledgements
Supported by the United States Public Health Service (DK067287 to J.M.C.) and the UCSD Digestive Diseases Research Development Center (DK080506) and the SDSU/USCD Comprehensive Cancer Center Partnership (CA 132379 and CA132384). We thank the support of the following shared resources: UCSD Moores Cancer Center Flow Cytometry Shared Resource and UCSD BIOGEM for microarray analysis.
Abbreviations
- 5-FU
5-fluorouracil
- DNA MMR
DNA mismatch repair
- MSI
microsatellite instability
- MSS
microsatellite stability
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/13447
Author's Contributions
J.M.C. and H.C. designed the project; H.C., J.C. and C.G.L. performed all experiments; H.C. and J.M.C. wrote the manuscript.
Supplementary Material
References
- 1.Laurie JA, Moertel CG, Fleming TR, Wieand HS, Leigh JE, Rubin J, et al. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo. Clinic J Clin Oncol. 1989;7:1447–1456. doi: 10.1200/JCO.1989.7.10.1447. [DOI] [PubMed] [Google Scholar]
- 2.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 3.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122:321–326. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Boland CR, Sinicrope FA, Brenner DE, Carethers JM. Colorectal cancer prevention and treatment. Gastroenterology. 2000;118:115–128. doi: 10.1016/s0016-5085(00)70010-2. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Meta-Analysis Groups, author. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16:301–308. doi: 10.1200/JCO.1998.16.1.301. [DOI] [PubMed] [Google Scholar]
- 6.Carethers JM, Chung H, Tajima A. Intestinal Inflammation and Colorectal Cancer. Prceedings of Falk Symposium #158. Dordrecht. The Netherlands: Springer Publishers; 2007. Mismatch repair competency predicts 5-Fluorouracil effectiveness on patient survival; pp. 72–84. [Google Scholar]
- 7.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carethers JM, Smith EJ, Behling CA, Nguyen L, Tajima A, Doctolero RT, et al. Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology. 2004;126:394–401. doi: 10.1053/j.gastro.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Palombo F, Iaccarino I, Nakajima E, Ikejima M, Shimada T, Jiricny J. hMutS-beta, a heterodimer of hMSH2 and hMSH3, binds to insertion/deletion loops in DNA. Curr Biol. 1996;6:1181–1184. doi: 10.1016/s0960-9822(02)70685-4. [DOI] [PubMed] [Google Scholar]
- 10.Modrich P. Mechanisms in eukaryotic mismatch repair. J Biol Chem. 2006;281:30305–30309. doi: 10.1074/jbc.R600022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 13.Akiyama Y, Sato H, Yamada T, Nagasaki H, Tsuchiya A, Abe R, et al. Germ-line mutation of the hMSH6/GTBP gene in an atypical hereditary nonpolyposis colorectal cancer kindred. Cancer Res. 1997;57:3920–3923. [PubMed] [Google Scholar]
- 14.Nicolaides NC, Papadopoulos N, Liu B, Wei YF, Carter KC, Ruben SM, et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994;371:75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- 15.Papadopoulos N, Nicolaides NC, Wei YF, Ruben SM, Carter KC, Rosen CA, et al. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994;263:1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- 16.Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veigl ML, Kasturi L, Olechnowicz J, Ma AH, Lutterbaugh JD, Periyasamy S, et al. Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Natl Acad Sci USA. 1998;95:8698–8702. doi: 10.1073/pnas.95.15.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jover R, Zapater P, Castells A, Llor X, Andreu M, Cubiella J, et al. Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut. 2006;55:848–855. doi: 10.1136/gut.2005.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carethers JM, Chauhan DP, Fink D, Nebel S, Bresalier RS, Howell SB, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology. 1999;117:123–131. doi: 10.1016/s0016-5085(99)70558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tajima A, Hess MT, Cabrera BL, Kolodner RD, Carethers JM. The mismatch repair complex hMutS alpha recognizes 5-fluorouracil-modified DNA: implications for chemosensitivity and resistance. Gastroenterology. 2004;127:1678–1684. doi: 10.1053/j.gastro.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Meyers M, Wagner MW, Mazurek A, Schmutte C, Fishel R, Boothman DA. DNA mismatch repair-dependent response to fluoropyrimidine-generated damage. J Biol Chem. 2005;280:5516–5526. doi: 10.1074/jbc.M412105200. [DOI] [PubMed] [Google Scholar]
- 22.Hawn MT, Umar A, Carethers JM, Marra G, Kunkel TA, Boland CR, et al. Evidence for a connection between the mismatch repair system and the G2 cell cycle checkpoint. Cancer Res. 1995;55:3721–3725. [PubMed] [Google Scholar]
- 23.Carethers JM, Hawn MT, Chauhan DP, Luce MC, Marra G, Koi M, et al. Competency in mismatch repair prohibits clonal expansion of cancer cells treated with N-methyl-N'-nitro-N-nitrosoguanidine. J Clin Invest. 1996;98:199–206. doi: 10.1172/JCI118767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kat A, Thilly WG, Fang WH, Longley MJ, Li GM, Modrich P. An alkylation-tolerant, mutator human cell line is deficient in strand-specific mismatch repair. Proc Natl Acad Sci USA. 1993;90:6424–6448. doi: 10.1073/pnas.90.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tibbetts RS, Brumbaugh KM, Williams JM, Sarkaria JN, Cliby WA, Shieh SY, et al. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 1999;13:152–157. doi: 10.1101/gad.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hickman MJ, Samson LD. Apoptotic signaling in response to a single type of DNA lesion, O(6)-methylguanine. Mol Cell. 2004;14:105–116. doi: 10.1016/s1097-2765(04)00162-5. [DOI] [PubMed] [Google Scholar]
- 27.Stojic L, Mojas N, Cejka P, Di Pietro M, Ferrari S, Marra G, et al. Mismatch repair-dependent G2 checkpoint induced by low doses of SN1 type methylating agents requires the ATR kinase. Genes Dev. 2004;18:1331–1344. doi: 10.1101/gad.294404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remick SC, Grem JL, Fischer PH, Tutsch KD, Alberti DB, Nieting LM, et al. Phase I trial of 5-fluorouracil and dipyridamole administered by seventy-two-hour concurrent continuous infusion. Cancer Res. 1990;50:2667–2672. [PubMed] [Google Scholar]
- 29.Aquilina G, Crescenzi M, Bignami M. Mismatch repair, G(2)/M cell cycle arrest and lethality after DNA damage. Carcinogenesis. 1999;20:2317–2326. doi: 10.1093/carcin/20.12.2317. [DOI] [PubMed] [Google Scholar]
- 30.Sato K, Kitajima Y, Kohya N, Miyoshi A, Koga Y, Miyazaki K. Deficient MGMT and proficient hMLH1 expression renders gallbladder carcinoma cells sensitive to alkylating agents through G2-M cell cycle arrest. Int J Oncol. 2005;26:1653–1661. [PubMed] [Google Scholar]
- 31.Takahashi Y, Nagata T, Ishii Y, Ikarashi M, Ishikawa K, Asai S. Upregulation of vitamin D3 upregulated protein 1 gene in response to 5-fluorouracil in colon carcinoma SW620. Oncol Rep. 2002;9:75–79. [PubMed] [Google Scholar]
- 32.Mariadason JM, Arango D, Shi Q, Wilson AJ, Corner GA, Nicholas C, et al. Gene expression profiling-based prediction of response of colon carcinoma cells to 5-fluorouracil and camptothecin. Cancer Res. 2003;63:8791–8812. [PubMed] [Google Scholar]
- 33.Clarke PA, George ML, Easdale S, Cunningham D, Swift RI, Hill ME, et al. Molecular pharmacology of cancer therapy in human colorectal cancer by gene expression profiling. Cancer Res. 2003;63:6855–6863. [PubMed] [Google Scholar]
- 34.De Angelis PM, Kravik KL, Tunheim SH, Haug T, Reichelt WH. Comparison of gene expression in HCT116 treatment derivatives generated by two different 5-fluorouracil exposure protocols. Mol Cancer. 2004;3:11. doi: 10.1186/1476-4598-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuyama R, Togo S, Shimizu D, Momiyama N, Ishikawa T, Ichikawa Y, et al. Predicting 5-fluorouracil chemosensitivity of liver metastases from colorectal cancer using primary tumor specimens: three-gene expression model predicts clinical response. Int J Cancer. 2006;119:406–413. doi: 10.1002/ijc.21843. [DOI] [PubMed] [Google Scholar]
- 36.Xi Y, Nakajima G, Schmitz JC, Chu E, Ju J. Multi-level gene expression profiles affected by thymidylate synthase and 5-fluorouracil in colon cancer. BMC Genomics. 2006;7:68. doi: 10.1186/1471-2164-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemminki A, Mecklin JP, Jarvinen H, Aaltonen LA, Joensuu H. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology. 2000;119:921–928. doi: 10.1053/gast.2000.18161. [DOI] [PubMed] [Google Scholar]
- 38.Elsaleh H, Powell B, Soontrapornchai P, Joseph D, Goria F, Spry N, et al. p53 gene mutation, microsatellite instability and adjuvant chemotherapy: impact on survival of 388 patients with Dukes' C colon carcinoma. Oncology. 2000;58:52–59. doi: 10.1159/000012079. [DOI] [PubMed] [Google Scholar]
- 39.Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355:1745–1750. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 40.Yoshikawa R, Kusunoki M, Yanagi H, Noda M, Furuyama JI, Yamamura T, et al. Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer Res. 2001;61:1029–1037. [PubMed] [Google Scholar]
- 41.Liu HC, Chen GG, Vlantis AC, Leung BC, Tong MC, Van Hasselt CA. 5-fluorouracil mediates apoptosis and G1/S arrest in laryngeal squamous cell carcinoma via a p53-independent pathway. Cancer J. 2006;12:482–493. doi: 10.1097/00130404-200611000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Lim YJ, Rhee JC, Bae YM, Chun WJ. Celecoxib attenuates 5-fluorouracil-induced apoptosis in HCT-15 and HT-29 human colon cancer cells. World J Gastroenterol. 2007;13:1947–1952. doi: 10.3748/wjg.v13.i13.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matuo R, Sousa FG, Escargueil AE, Grivicich I, Garcia-Santos D, Chies JA, et al. 5-Fluorouracil and its active metabolite FdUMP cause DNA damage in human SW620 colon adenocarcinoma cell line. J Appl Toxicol. 2009;29:308–316. doi: 10.1002/jat.1411. [DOI] [PubMed] [Google Scholar]
- 44.Su C, Gao G, Schneider S, Helt C, Weiss C, O'Reilly MA, et al. DNA damage induces downregulation of histone gene expression through the G1 checkpoint pathway. EMBO J. 2004;23:1133–1143. doi: 10.1038/sj.emboj.7600120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lafon-Hughes L, Di Tomaso MV, Mendez-Acuna L, Martinez-Lopez W. Chromatin remodelling mechanisms in cancer. Mutat Res. 2008;658:191–214. doi: 10.1016/j.mrrev.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Bartova E, Krejci J, Harnicarova A, Galiova G, Kozubek S. Histone modifications and nuclear architecture: a review. J Histochem Cytochem. 2008;56:711–721. doi: 10.1369/jhc.2008.951251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol. 2007;14:1008–1016. doi: 10.1038/nsmb1337. [DOI] [PubMed] [Google Scholar]
- 48.Koi M, Umar A, Chauhan DP, Cherian SP, Carethers JM, Kunkel TA, et al. Human chromosome 3 corrects mismatch repair deficiency and microsatellite instability and reduces N-methyl-N'-nitro-N-nitrosoguanidine tolerance in colon tumor cells with homozygous hMLH1 mutation. Cancer Res. 1994;54:4308–4312. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.