Abstract
Purpose
Inhibitors of apoptosis proteins (IAP s) have been shown to contribute to resistance of neoplastic cells to chemotherapy and to biologic antineoplastic agents. Consequently, new agents are being developed targeting this family of proteins. In a panel of bladder cancer cell lines, we evaluated a Smac mimetic that antagonizes several IAPs for its suitability for bladder cancer therapy.
Results
Single-agent compound-A had little effect, but compound-A augmented TRAIL- and chemotherapy-induced apoptosis. Immunoblotting showed that combination treatment with compound-A and TRAIL resulted in cleavage of procaspase-3 and procaspase-7, activation of which irreversibly commits cells to apoptosis. Immunoprecipitation of XIAP showed displacement of active caspase-3 fragments from XIAP, supporting the proposed mechanism of action. Furthermore, siRNA-mediated silencing of XIAP similarly sensitized these cells to apoptosis.
Experimental design
A panel of seven bladder cancer cell lines were evaluated for sensitivity to the Smac mimetic compound-A alone, TRAIL alone, chemotherapy alone, compound-A plus TRAIL and compound-A plus chemotherapy by DNA fragmentation analysis. IAP levels and caspase activation were examined by western blotting. Release of caspase-3 from X-linked inhibitor of apoptosis protein (XIAP), the most effective IAP, was assessed by immunoprecipitation and western blotting. Finally, siRNA knockdown of XIAP was correlated with the sensitivity of cells to apoptosis induced by compound-A plus TRAIL by DNA fragmentation and western blotting.
Conclusion
Our results suggest that targeting of XIAP with the Smac mimetic compound-A has the potential to augment the effects of a variety of chemotherapeutic and biologic therapies in bladder cancer.
Key words: bladder cancer, smac mimetic, IAP antagonist, chemotherapy, TRAIL
Introduction
Apoptosis (programmed cell death) is necessary for physiologic homeostasis.1–3 In mammalian cells, apoptosis can occur via either of two distinct pathways: the extrinsic pathway, mediated by death receptors on the surface of cells and the intrinsic pathway, mediated by mitochondria.4 The extrinsic pathway is activated by binding of death receptors, which leads to cleavage of procaspase-8, whereas the mitochondrial pathway is activated by internal signals, which results in release of cytochrome-c and caspase-9.4 Both pathways converge into a common cascade with the activation of caspase-3, which commits the cell to apoptosis.5,6
Disorders of apoptosis have been linked to carcinogenesis as well as resistance to anticancer therapy.7,8 Re-establishing the integrity of apoptotic pathways in apoptosis-resistant cancer cells may increase the effectiveness of conventional chemotherapies as well as open a myriad of alternative therapeutic options.9,10
There are a number of mechanisms that can inhibit apoptotic cascades prior to the irreversible commitment step of caspase-3 activation. One family of apoptosis-inhibitory proteins is the inhibitors of apoptosis proteins (IAPs), characterized by baculoviral IAP repeat domains that are required for inhibition of apoptosis.11,12 IAPs bind to active caspase-3 and caspase-9 and prevent these proteins from cleaving intracellular proteins and initiating the committed apoptosis cascade. The most effective IAP is X-linked IAP (XIAP), first discovered by Liston and colleagues in 1996.11,13,14 XIAP has three baculoviral IAP repeat domains. Second mitochondrial activator of caspases (Smac) binds to these three domains and prevents XIAP from binding to active caspase-3, caspase-7 and caspase-9.
Previous reports have demonstrated that synthetic Smac peptides can activate caspases in cancer cells and potentiate both TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis and chemotherapy-mediated apoptosis.13,15–19 Bacillus Calmette-Guérin (BCG) is the current gold standard therapy for the majority of patients with bladder cancer and is thought to work at least in part via induction of TRAIL-mediated apoptosis. For systemic disease, cisplatinum based chemotherapy has been used successfully in the treatment of bladder cancer. However, with both of these approaches, resistance to initial therapy is a common problem. We show here that a Smac mimetic compound sensitized a panel of bladder cancer cell lines to apoptosis mediated by TRAIL and a variety of chemical and biological cytotoxic agents.
Results
Sensitivity of cells to single-agent compound-A.
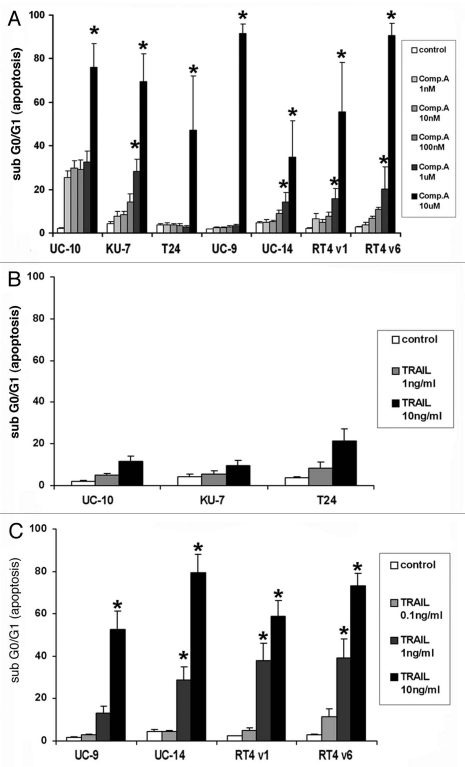
In the panel of seven different bladder cancer cell lines, the Smac mimetic compound-A induced significant apoptosis only in UM-UC-10 cells at concentrations less than 1 µM (Fig. 1A). UM-UC-10 cells appeared to have baseline sensitivity to compound-A without an appreciable increase in apoptosis with increasing concentration through 1 µM. To confirm specific activity, all cells were treated with the inactive enantiomer of compound-A, compound-B and there was no appreciable induction of apoptosis in any cell line at any dose up to 10 µM (data not shown). There was no cell cycle arrest seen in any phase other than G0/G1 after exposure to Compound A; and the percentage of cells that became subG0/G1 corresponded with an equal decrease in percentage of the cells in G0/G1.
Figure 1.
Flow cytometric analysis of DNA fragmentation by PI staining 24 hours after treatment with compound-A and TRAIL as single agents. (A) Compound-A. No statistically significant difference in apoptosis was seen between control and Compound A at concentrations of 1 nM, 10 nM and 100 nM in all cell lines. At concentrations 1 uM and 10 uM a significant increase in the percentage of apoptotic cells was seen in all cell lines. (*indicates p < 0.05). (B) TRAIL, minimally sensitive cell lines. No statistically significant difference in apoptosis was seen between control and either concentration of TRAIL. (C) TRAIL, highly sensitive cell lines. None of the sensitive cell lines demonstrated a significant increase in apoptosis when exposed to the lowest concentration of TRAIL 0.1 ng/mL (p > 0.05) but statistical significance was achieved for the higher concentrations of TRAIL in every cell line. (p < 0.05) All graphs indicate the Mean ± SE M, three independent experiments.
The high degree of apoptosis seen in all the cell lines at the 10-µM concentration of compound-A was due to nonspecific effects rather than IAP inhibition (unpublished observations, McKinlay M, data not shown).
Sensitivity of cells to single-agent TRAIL.
Sensitivity to single-agent TRAIL varied among the cell lines. KU7, UM-UC-10 and T24 cells demonstrated less than 10% apoptosis at a TRAIL dose of 1 ng/ml and less than 25% apoptosis at a TRAIL dose of 10 ng/ml at 24 hours (p > 0.05) (Fig. 1B). We arbitrarily established 25% as the cut point to differentiate between cells less sensitive and more sensitive to TRAIL-induced apoptosis. Conversely, UM-UC-9, UM-UC-14, RT4 v1 and RT4 v6 cells had significant (>50%) apoptosis induced at the 10-ng/ml dose of TRAIL (p < 0.05) (Fig. 1C). Furthermore, these “highly sensitive” cell lines also demonstrated marked apoptosis at the lower 1 ng/ml dose of TRAIL that was statistically significant (p < 0.05). As a result, we also evaluated the degree of apoptosis in these cell lines at a 0.1-ng/ml dose of TRAIL in order to establish a level of apoptosis in highly sensitive cells that is not significantly higher than the baseline level of apoptosis seen in controls (p > 0.05) (Fig. 1C).
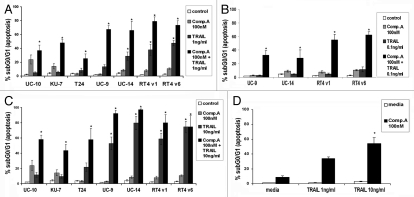
Sensitivity of cells to combination of compound-A and TRAIL.
The combination of 100 nM compound-A and 1 ng/ml TRAIL induced significant apoptosis in all cell lines compared to each compound alone and to control (p < 0.05) (Fig. 2A). The combination of 100 nM compound-A and 10 ng/ml TRAIL induced significant apoptosis in all cell lines and the effect of TRAIL alone at a concentration of 10 ng/ml was marked in the four “highly sensitive” cell lines (p < 0.05) (Fig. 2B). In these same four “highly sensitive” cell lines, the combination of compound-A and 0.1 ng/ml TRAIL resulted in significant apoptosis (p < 0.05), but with TRAIL alone at a concentration of 0.1 ng/ml, there was no significant apoptosis (p > 0.05) (Fig. 2C). The inactive enantiomer compound-B did not demonstrate any induction of apoptosis when combined with TRAIL (data not shown).
Figure 2.
Flow cytometric analysis of DNA fragmentation by PI staining 24 hours after treatment with compound-A in combination with TRAIL. (A) Compound-A 100 nM and TRAIL 1 ng/ml. (*indicates p < 0.05). (B) Compound-A 100 nM and TRAIL 10 ng/ml. (*indicates p < 0.05). (C) Compound-A 100 nM and TRAIL 0.1 ng/ml. (*indicates p < 0.05). (D) One-hour washout experiment showed marked induction of apoptosis after exposure to the combination of compound-A 100 nM and TRAIL 10 ng/ml but little apoptosis for either single agent in KU7 cells. Mean ± SE M, three independent experiments.
In order to mimic intravesical therapy for bladder cancer, washout studies were performed at increasing hourly intervals up to 6 hours. Induction of apoptosis was seen in all cell lines tested at all time intervals from 1 hour to 6 hours (data not shown). The TRAIL-resistant cell line KU7 demonstrated similar levels of apoptosis at 1-hour washout and after continuous incubation for 24 hours (p = 0.003) (Fig. 2D).
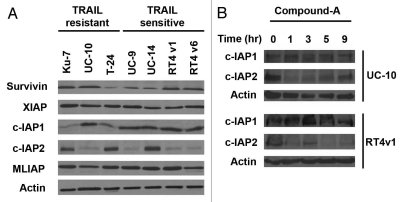
Baseline IAP expression does not predict sensitivity to compound-A or TRAIL.
After noting the wide disparity in sensitivity of cell lines to compound-A as a single agent and TRAIL as a single agent, we hypothesized that baseline IAP expression might predict sensitivity to compound-A and/or TRAIL, with high baseline IAP levels conferring relative sensitivity to compound-A and relative resistance to TRAIL. [Compound-A binds to XIAP, cIAP1, cIAP2 and MLIAP with high affinity]. Therefore, baseline expression levels of the IAPs were evaluated by western blotting. However, no discernible correlation between baseline IAP expression and sensitivity of cell lines to compound-A or TRAIL was identified (Fig. 3A). In addition, IAP expression did not change with increasing duration of exposure to compound-A up to 9 hours in representative cell lines from the less TRAIL sensitive and highly TRAIL sensitive groups (Fig. 3B).
Figure 3.
Western blotting of baseline levels of IAP expression. (A) Variable expression of CIAP 2 and CIAP 1 but no apparent correlation between baseline IAP expression and sensitivity to TRAIL or compound-A as single agents. (B) Expression of cIAP 1 and cIAP 2 did not change with increasing duration of exposure to 100 nM compound-A up to 9 hours in representative cell lines from the less TRAIL sensitive and highly TRAIL sensitive groups. Results shown are representative of those obtained in three independent experiments.
Compound-A enhances cytotoxicity of chemotherapeutic agents.
To evaluate the effect of compound-A with cytotoxic agents commonly used for bladder cancer therapy, we chose to use KU7 cells, which are relatively resistant to most single-agent cytotoxic agents in vitro. Since IAPs are relevant at the caspase-3, caspase-7 and caspase-9 levels, we combined compound-A with other cytotoxic agents in order to identify whether the augmentation of apoptosis seen with TRAIL via the extrinsic pathway also occurred with agents that work via the intrinsic (mitochondrial) pathway.
Staurosporine is known to induce apoptosis through the mitochondrial apoptosis pathway and was markedly cytotoxic at relevant doses in all cell lines evaluated (data not shown). Notably, compound-A combined with the lowest concentration of staurosporine significantly enhanced induction of apoptosis (p = 0.04) (Suppl. Fig. 1).
Cisplatin and gemcitabine are used clinically in the treatment of bladder cancer.24 Therefore, we evaluated the combination of compound-A with each of these agents in vitro. Marked induction of apoptosis was noted with compound-A at 100 nM and cisplatin at 1 µg/ml (p = 0.00003) (Suppl. Fig. 2). Gemcitabine did not demonstrate any cytotoxicity in KU7 cells at any concentration up to 100 µM, although marked cytostatic effects were noted at higher concentrations with accumulation of cells in the G2 stage of the cell cycle (data not shown). However, despite the near total lack of cytotoxic effect of gemcitabine, the combination of 1 µM gemcitabine and 100 nM compound-A resulted in high levels of apoptosis in KU7 cells (p = 0.00001) (Suppl. Fig. 3).
Compound-A potentiates effect of interferon-α.
Since IFNα has been shown to induce TRAIL production in cancer cells and is used in bladder cancer therapy, we assessed IFNα for activity in the panel of bladder cancer cells (25–27). When 100 nM compound-A was combined simultaneously with 10,000 IU of interferonα, no induction of apoptosis was noted after 24 or 48 hours of incubation (data not shown). However, when the cells were exposed to interferonα 24 hours before the addition of compound-A, significant induction of apoptosis was noted (p < 0.005) (Suppl. Fig. 4).
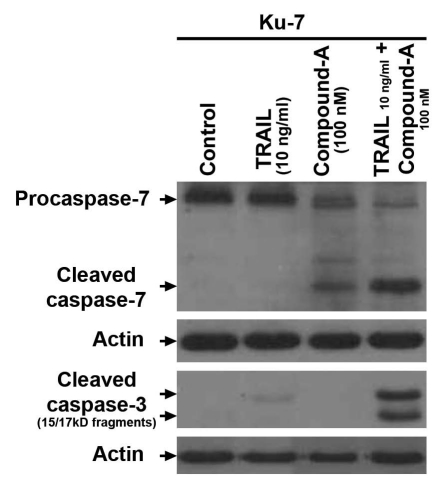
Caspase activation occurs with combination therapy with compound-A and TRAIL.
Activation of effector caspases caspase-3 and caspase-7 irreversibly commits cells to apoptosis. The IAPs block apoptosis via binding to activated caspase-3 and caspase-7.11 Immunoblotting for caspase-3 and caspase-7 in KU7 cells, which are minimally sensitive to TRAIL, demonstrated full cleavage of caspase-3 and caspase-7 only when cells were exposed to both TRAIL and compound-A (Fig. 4). Furthermore, low levels of caspase-7 activation were seen with compound-A as a single agent (Fig. 4), a finding that is consistent with the flow cytometry findings showing modest single-agent activity for compound-A in the cells.
Figure 4.
Immunoblot detection of active caspase fragments as an indicator of caspase-mediated apoptosis. KU7 cells were incubated for 4 hours with 100 nM compound-A, 10 ng/ml TRAIL orboth. Results shown are representative of those obtained in 3 replicates.
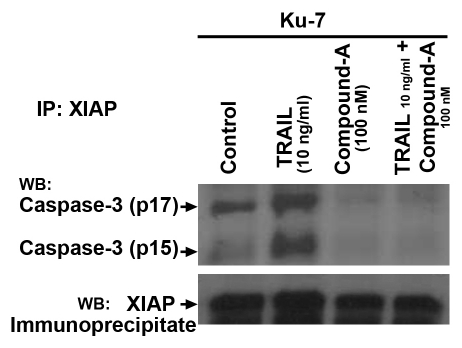
Cleaved caspase-3 is released from XIAP in the presence of compound-A.
Smac and dimeric Smac mimetic compounds such as compound-A bind to XIAP and release cleaved fragments of caspases-3 and caspase-7 to induce apoptosis.11 In order to confirm that compound-A releases active caspase fragments from IAPs, we immunoprecipitated XIAP and then immunoblotted with anti-active caspase-3 antibody. Active caspase-3 was present in the XIAP immunoprecipitate in control cells and cells treated with TRAIL only but not in cells treated with compound-A alone or compound-A plus TRAIL (Fig. 5). These findings indicate that the active caspase fragments that are bound to XIAP in the control and TRAIL-only lanes are released in the presence of compound-A.
Figure 5.
Compound-A alone and in combination with TRAIL disrupts the XIAP inhibition of active caspase-3. KU7 cells were incubated for 4 hours with 100 nM compound-A, 10 ng/ml TRAIL orboth. Immunoblots corresponding to cleaved fragments of Caspase 3 (p15/p17) are clearly seen in the control and TRAIL 10 ng/mL lanes as expected because XIAP binds to active Caspase 3. These bands are not seen in the Compound A and combination lanes because SMAC and SMAC mimetic compounds like Compound A cause release of the bound Caspase 3 proteins. Results shown are representative of those obtained in two independent experiments.
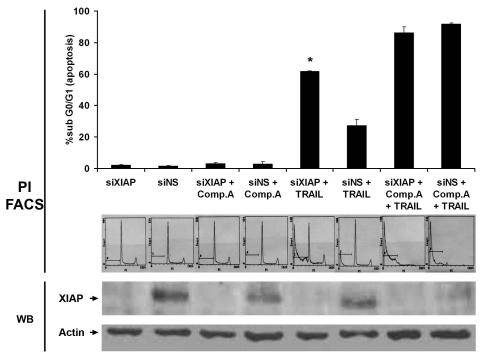
Silencing of XIAP with siRNA sensitizes cells to TRAIL-mediated apoptosis.
To confirm the role of XIAP in TRAIL-induced apoptosis, we silenced XIAP using the transfection agent oligofectamine. After successful silencing, marked induction of apoptosis was seen with TRAIL alone and even higher levels of apoptosis were seen with TRAIL in combination with Compound-A (p = 0.009) (Fig. 6). The additional apoptosis seen with the combination of TRAIL and Compound A suggests that IAPs other than XIAP are bound by Compound A releasing additional activated caspases accounting for the increased apoptosis. Alternatively if silencing of XIAP by oligofectamine was not complete then the additional increased apoptosis may be due to binding of Compound A to the unsilenced XIAP. The immunoblotting for XIAP suggests near complete silencing which suggests a small but notable contribution to caspase-mediated apoptosis by IAPs other than XIAP. Compound A is known to bind to other members of the IAP superfamily (unpublished observations, McKinlay M, data not shown). The effect of silencing on the activation of caspases is as yet unknown.
Figure 6.
Silencing of XIAP with siRNA induces TRAIL sensitivity in KU7 cells. siRNA induced TRAIL sensitivity to a much greater degree than nonspecific siRNA. (*indicates p = 0.009). Low levels of apoptosis are noted in the nonspecific group, suggesting that the act of transient transfection modestly sensitizes cells to TRAIL-induced apoptosis. Top part: Mean ± SE M, three independent experiments. Middle part: Immunoblot bands demonstrating successful silencing of XIAP with oligofectamine. Results shown are representative of those obtained in three independent replicates. Bottom part: Representative fluorescence-activated cell sorting (FACS) histograms displaying percentages of apoptotic cells. Increasing percentages of cells in the subG0/G1 phase are seen in the siXIAP groups. TRAIL, 10 ng/ml; compound-A, 100 nM.
Discussion
Our findings indicate that the Smac mimetic compound-A potentiates the cytotoxicity of a number of cytostatic and cytotoxic agents in a panel of bladder cancer cells.
The role of the IAP family of proteins, which are antagonized by Smac, in inhibition of apoptosis has made them a focus of research in anticancer therapies; numerous preclinical evaluations have confirmed the IAPs as viable targets.16–18,28–31 Previously published reports utilized either IAP-directed antisense RNA constructs or synthetic small molecules that mimic the structure and function of Smac (Smac mimetics). Compound-A has a molecular weight of 1,154 and appears to have excellent cellular uptake, as demonstrated by the induction of irreversible apoptosis after only 1 hour of exposure. Bockbrader et al. concluded that sensitivity to single-agent Smac mimetic therapy was related to baseline IAP expression levels, with cells expressing high levels of IAP being exquisitely sensitive.18 In our panel, however, only the UM-UC-10 (and to lesser extent KU7) cell lines demonstrated any sensitivity to compound-A as a single agent at relevant concentrations; all the other cell lines appeared to have no sensitivity at the maximum concentration tested. Given the varying levels of IAP expression across the panel of cell lines, it appears that in bladder cancer cells the baseline IAP expression does not predict sensitivity to Smac mimetics.
In bladder cancer cell lines, similar to the case in cell lines derived from other tumor types, the IAPs regulate sensitivity to chemotherapeutic agents and biologic agents, including TRAIL and interferon. Antagonism of the IAPs using the Smac mimetic compound-A potentiated the cytotoxicity of gemcitabine and cisplatin. Notably, combination treatment with compound-A and any of the cytotoxic agents tested resulted in an irreversible commitment of these cells to apoptosis in a very short period of time. Interestingly, previous studies have suggested that cisplatin can induce the production of Fas and its ligand, Fas-L, thereby activating the death receptor pathway. Of the combination treatments evaluated, compound-A with TRAIL had the most potent effect in the shortest time period and among the chemotherapeutics tested, low-dose cisplatin showed comparable potency of induction of apoptosis, which might be explained by cisplatininduced Fas-L.
Our findings are particularly relevant in therapy for bladder cancer (estimated 70,000 new cases per year diagnosed each year in the United States) because the standard treatment for the majority of patients with bladder cancer is intravesical therapy with bacille Calmette Guerin (BCG). The mechanism of action for BCG in prolonging the recurrence-free and progression-free intervals is still being elucidated, but recent studies suggest that BCG induces TRAIL production from both T-cells and the tumor itself and that this may be one of the mechanisms by which BCG leads to tumor cell death.32–35 In addition, the combination of interferon-α with standard BCG therapy is sometimes employed for recurrent or refractory cases of superficial bladder cancer. Interferon-α has also been shown to induce TRAIL production that is detectable in the urine after therapy.36 Therefore, an agent such as compound-A that potentiates the effects of TRAIL may be useful in combination with intravesical BCG therapy for bladder cancer patients.
We have shown that antagonism of IAPs with the Smac mimetic compound-A potentiates the cytotoxicity of a number of cytostatic and cytotoxic agents. Compound-A binds with high affinity to XIAP, cIAP1, cIAP2 and MLIAP and has shown marked activity as part of combination treatment in vitro. Thus, this strategy appears to have the potential to broaden the efficacy of already established therapies in bladder cancer and potentially produce responses in previously resistant tumors.
Materials and Methods
Cell lines and culture conditions.
UM-UC-9, UM-UC-10, UM-UC-14 and KU7 cells were obtained from Dr. H. Barton Grossman. (The University of Texas M.D. Anderson Cancer Center; Houston, TX); RT4 v1 and RT4 v6 cells were established in our laboratory; and T24 cells were obtained from the American Type Culture Collection (Rockville, MD).20 All cells except T24 were maintained in minimal essential medium supplemented with heat-inactivated 10% fetal bovine serum, 2% vitamins, 1% penicillin and streptomycin, 1% nonessential amino acids, sodium pyruvate and 0.5 M HEPES. T24 cells were maintained in McCoy's medium supplemented in an identical fashion. All cell lines were routinely authenticated by DNA fingerprinting.
Antibodies and chemicals.
Anti-XIAP for immunoprecipitation and anti-active caspase-3, anti-survivin and anti-caspase-7 for western blotting were obtained from Cell Signaling Technology (Beverly, MA). Anti-MLIAP, anti-cIAP1 and anti-cIAP2 for western blotting were obtained from BD Biosciences Pharmingen (San Diego, CA). Anti-XIAP for western blotting was obtained from R&D Systems (Minneapolis, MN). Horseradish peroxidase-conjugated secondary antibodies for immunoblotting were obtained from Amersham Pharmacia Biotech (Piscataway, NJ). Smac mimetic compound-A and its inactive enantiomer compound-B, described previously, were provided by Tetralogic Pharmaceuticals Corporation (Malvern, PA) Recombinant human TRAIL was purchased from R&D systems.21 Propidium iodide (PI) was obtained from Sigma Chemical Co., (St. Louis, MO). Silencing oligonucleotide for XIAP was obtained from Dharmacon (Chicago, IL). Oligofectamine was purchased from Invitrogen (Carlsbad, CA).
Measurement of DNA fragmentation.
DNA fragmentation was quantified by PI staining and fluorescence-activated cell sorting analysis as previously described.22 Cells were plated in six-well plates and grown to 70% confluence. After incubation with compound-A alone, TRAIL alone, cytotoxic agents alone, compound-A plus TRAIL orcompound-A plus cytotoxic agents at various concentrations for specified time periods, cells were harvested as previously described,18,23 and the cell pellet was resuspended in 500 µl of PI solution (25 µg/ml PI, 1% Triton X-100 and 0.1% sodium citrate in phosphate-buffered NaCl solution). After incubation in PI solution overnight at 4°C, the percentage of cells with subdiploid DNA fragmentation was measured by flow cytometry.
DNA fragmentation with the combination of compound-A and interferonα (IFNα) was also studied. In order to exploit previously reported data showing that IFNα induces TRAIL production in some bladder cancer cell lines, IFNα (Schering, Kenilworth, NJ) was administered 24 hours prior to the addition of compound-A. For these experiments, cells were grown to 60–70% confluence in six-well plates and then incubated with IFNα overnight. Medium was aspirated and fresh medium with IFNα and compound-A was added to the cells, which were then incubated overnight before being harvested as described above.
In washout studies, cells were grown to 70% confluence in six-well plates, incubated with compound-A and TRAIL for specified intervals, washed with untreated medium and then incubated for 24 hours in untreated medium before being harvested as described.
All flow cytometry experiments were performed in triplicate.
Immunoblotting.
Cells were treated with various concentrations of compound-A or compound-B alone or in combination with 1 or 10 ng/ml TRAIL for 4 hours. Cells were collected after brief trypsinization and lysed as described previously.23 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using an equal amount of protein lysate from each sample. The proteins were transferred to nitrocellulose membranes, which were then blocked with 5% nonfat milk in a TBS solution containing 0.1% Tween 20 for 1 hour. The blots were probed overnight with primary antibodies, washed and probed with species-specific secondary antibodies coupled to horseradish peroxidase. Enhanced chemiluminescence reagent (West Pico Pierce, Inc., Rockville, IL) was used to detect immunoreactive material. While immunoblotting for caspase activation was performed in several different cell lines, we have shown only the immunoblots from KU7 cells since they did not exhibit TRAIL-mediated apoptosis at baseline. Caspase activation with TRAIL alone was noted in TRAIL-sensitive cells. All immunoblotting experiments were performed in triplicate.
XIAP silencing.
KU7 cells were plated in six-well plates and grown to 40–50% confluence; washed with serum-free, antibiotic-free medium; and incubated with oligofectamine (Invitrogen) combined with anti-XIAP small interfering RNA (siRNA) (Dharmacon) for 6 hours. Thirty percent minimal essential medium was added to each treatment group after incubation with the siRNA and cells were allowed to grow for 24 hours. Cells were then incubated with 10 ng/ml TRAIL for 4–6 hours before being harvested for immunoblotting and PI-fluorescence-activated cell sorting analysis as described above. All RNA silencing experiments were performed in triplicate.
Immunoprecipitation.
Cells were treated with 100 nM compound-A alone or in combination with 10 ng/ml TRAIL for 4–6 hours. Cells were pelleted as described above and resuspended in total lysis buffer (1 complete mini-tab, 25 mM TRIS, 1% Triton X-100, 0.1% sodium orthovanadate, 0.1% sodium fluoride and 0.1% glycerol phosphate). Cell lysates were mixed with equal volumes of 2x SDS-PAGE sample buffer (50 mmol/L Tris-HCl, 2% SDS, 0.1% bromophenol blue, 10% glycerol and 5% β-mercaptoethanol). Samples were boiled at 100°C for 5 minutes to denature proteins. SDS-PAGE was performed on total cellular proteins transferred to nitrocellulose membrane in standard fashion. The membranes were blocked with 5% nonfat milk dissolved in TBS and probed following standard immunoblotting protocol as described above. Immunoprecipitation experiments were performed in duplicate.
Figure preparation.
All images were composited in Adobe Photoshop software.
Statistics.
Statistical analysis was performed using GraphPad Prism Software (GraphPad, San. Diego, CA USA). As appropriate, raw data or percentages were compared by unpaired Student's t-test. Statistical significance for this study was set at p < 0.05.
Abbreviations
- BCG
bacille calmette-guérin
- IAPs
inhibitors of apoptosis proteins
- XIAP
X-linked inhibitor of apoptosis protein
- Smac
second mitochondrial activator of caspases
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- siRNA
small interfering RNA
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/13237
Financial Disclosure
A.M.K. and D.J.M. received support from the National Institutes of Health (NIH) M.D. Anderson Cancer Center SPORE in Bladder Cancer (P50-CA91846-01). This work was also supported by a research grant to A.M.K. from TetraLogic Pharmaceuticals. S.C. and M.M. are employees of TetraLogic Pharmaceuticals.
Statement of Translational Relevance
Bladder cancer therapy has suffered from lack of significant progress over the past three decades, since the advent of intravesical bacille Calmette-Guérin (BCG) therapy and development of systemic cisplatin-based chemotherapy. Resistance to initial therapy is a common problem, which highlights the need for combination therapy with novel agents that are compatible with existing therapeutic agents. TNF-related apoptosis inducing ligand (TRAIL)-mediated death of bladder cancer cells (via BCG) is one of the major mechanisms known to date by which bladder cancer cells are killed. Our present study suggests the suitability of Smac mimetics in combination with TRAIL or cisplatin to sensitize bladder cancer cells to therapy. We shed light on the mechanisms whereby the combination of Smac mimetic with TRAIL augments the cleavage of caspase-3 and caspase-7 and releases caspase-3 from X-linked inhibitor of apoptosis protein. These data support development of therapeutic strategies using Smac mimetic in combination with existing therapeutic regimens for bladder cancer treatment.
Supplementary Material
References
- 1.Kerr JF. Shrinkage necrosis: a distinct mode of cellular death. J Pathol. 1971;105:13–20. doi: 10.1002/path.1711050103. [DOI] [PubMed] [Google Scholar]
- 2.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 4.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 5.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 6.Wolf BB, Schuler M, Echeverri F, Green DR. Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J Biol Chem. 1999;274:30651–30656. doi: 10.1074/jbc.274.43.30651. [DOI] [PubMed] [Google Scholar]
- 7.Makin G, Hickman JA. Apoptosis and cancer chemotherapy. Cell Tissue Res. 2000;301:143–152. doi: 10.1007/s004419900160. [DOI] [PubMed] [Google Scholar]
- 8.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen JT, Wells JA. Direct activation of the apoptosis machinery as a mechanism to target cancer cells. Proc Natl Acad Sci USA. 2003;100:7533–7538. doi: 10.1073/pnas.1031631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17–22. doi: 10.1016/s1535-6108(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 11.Deveraux QL, Reed JC. IAP family proteins—suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 12.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 13.Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406:855–862. doi: 10.1038/35022514. [DOI] [PubMed] [Google Scholar]
- 14.Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, et al. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 15.Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, et al. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 16.Arnt CR, Chiorean MV, Heldebrant MP, Gores GJ, Kaufmann SH. Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. J Biol Chem. 2002;277:44236–44243. doi: 10.1074/jbc.M207578200. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 18.Bockbrader KM, Tan M, Sun Y. A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene. 2005;24:7381–7388. doi: 10.1038/sj.onc.1208888. [DOI] [PubMed] [Google Scholar]
- 19.Cummins JM, Kohli M, Rago C, Kinzler KW, Vogelstein B, Bunz F. X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res. 2004;64:3006–3008. doi: 10.1158/0008-5472.can-04-0046. [DOI] [PubMed] [Google Scholar]
- 20.Kamat AM, Sethi G, Aggarwal BB. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through downregulation of nuclear factor-kappaB and nuclear factorkappaB-regulated gene products in IFNalpha-sensitive and IFNalpha-resistant human bladder cancer cells. Mol Cancer Ther. 2007;6:1022–1030. doi: 10.1158/1535-7163.MCT-06-0545. [DOI] [PubMed] [Google Scholar]
- 21.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 22.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 23.Lashinger LM, Zhu K, Williams SA, Shrader M, Dinney CP, McConkey DJ. Bortezomib abolishes tumor necrosis factor-related apoptosis-inducing ligand resistance via a p21-dependent mechanism in human bladder and prostate cancer cells. Cancer Res. 2005;65:4902–4908. doi: 10.1158/0008-5472.CAN-04-3701. [DOI] [PubMed] [Google Scholar]
- 24.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Benito M, Balsas P, Bosque A, Anel A, Marzo I, Naval J. Apo2L/TRAIL is an indirect mediator of apoptosis induced by interferon-alpha in human myeloma cells. FEBS Lett. 2005;579:6217–6222. doi: 10.1016/j.febslet.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Papageorgiou A, Lashinger L, Millikan R, Grossman HB, Benedict W, Dinney CP, et al. Role of tumor necrosis factor-related apoptosis-inducing ligand in interferon-induced apoptosis in human bladder cancer cells. Cancer Res. 2004;64:8973–8979. doi: 10.1158/0008-5472.CAN-04-1909. [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell MA, Krohn J, DeWolf WC. Salvage intravesical therapy with interferon-alpha 2b plus low dose bacillus Calmette-Guerin is effective in patients with superficial bladder cancer in whom bacillus Calmette-Guerin alone previously failed. J Urol. 2001;166:1300–1304. [PubMed] [Google Scholar]
- 28.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Feng Q, Kim JM, Schneiderman D, Liston P, Li M, et al. Human ovarian cancer and cisplatin resistance: possible role of inhibitor of apoptosis proteins. Endocrinology. 2001;142:370–380. doi: 10.1210/endo.142.1.7897. [DOI] [PubMed] [Google Scholar]
- 30.Lima RT, Martins LM, Guimaraes JE, Sambade C, Vasconcelos MH. Specific downregulation of bcl-2 and xIAP by RNAi enhances the effects of chemotherapeutic agents in MCF-7 human breast cancer cells. Cancer Gene Ther. 2004;11:309–316. doi: 10.1038/sj.cgt.7700706. [DOI] [PubMed] [Google Scholar]
- 31.McManus DC, Lefebvre CA, Cherton-Horvat G, et al. Loss of XIAP protein expression by RNAi and antisense approaches sensitizes cancer cells to functionally diverse chemotherapeutics. Oncogene. 2004;23:8105–8117. doi: 10.1038/sj.onc.1207967. [DOI] [PubMed] [Google Scholar]
- 32.Kemp TJ, Ludwig AT, Earel JK, Moore JM, Vanoosten RL, Moses B, et al. Neutrophil stimulation with Mycobacterium bovis bacillus Calmette-Guerin (BCG) results in the release of functional soluble TRAIL/Apo-2L. Blood. 2005;106:3474–3482. doi: 10.1182/blood-2005-03-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kocak H, Oner-Iyidogan Y, Kocak T, Oner P. Determination of diagnostic and prognostic values of urinary interleukin-8, tumor necrosis factor-alpha and leukocyte arylsulfatase-A activity in patients with bladder cancer. Clin Biochem. 2004;37:673–678. doi: 10.1016/j.clinbiochem.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Ludwig AT, Moore JM, Luo Y, Chen X, Saltsgaver NA, O'Donnell MA, et al. Tumor necrosis factor-related apoptosis-inducing ligand: a novel mechanism for Bacillus Calmette-Guerin-induced antitumor activity. Cancer Res. 2004;64:3386–3390. doi: 10.1158/0008-5472.CAN-04-0374. [DOI] [PubMed] [Google Scholar]
- 35.Mehmut M, Takeda K, Abe M, Ogata H, Hirose S, Okumura K, et al. Fas ligand and TNF-related apoptosis-inducing ligand induction on infiltrating lymphocytes in bladder carcinoma by bacillus Calmette-Guerin treatment. Urol Int. 2005;75:80–87. doi: 10.1159/000085934. [DOI] [PubMed] [Google Scholar]
- 36.Luo Y, Chen X, Downs TM, DeWolf WC, O'Donnell MA. IFNalpha 2B enhances Th1 cytokine responses in bladder cancer patients receiving Mycobacterium bovis bacillus Calmette-Guerin immunotherapy. J Immunol. 1999;162:2399–2405. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.