Abstract
Purpose
The bone marrow microenvironment is considered a critical component in the dissemination and fate of cancer cells in the metastatic process. We explored the possible correlation between bone marrow mesenchymal stem cells (BM-MSC) and disseminated breast cancer-initiating cells (BCIC) in primary breast cancer patients.
Results
The percentages of BCIC (Aldefluor+CD326+CD44+CD24−) correlated with the percentages of BM-MSC, either CD45−GD2+CD200+CD271+ (Kedall's τ = 0.684, p = 0.004) or CD45−GD2+CD271+ in the bone marrow (Kedall's τ = 0.464, p = 0.042).
Experimental Design
Bone marrow mononuclear cells (BM-MNC) were collected at the time of primary surgery in 12 breast cancer patients. BM-MNC was immunophenotyped and BCIC was defined as epithelial cells (CD326+CD45−) with a “stem-like” phenotype (CD44+CD24low/−, ALDH activity). BM-MSC was defined as CD34−CD45− cells that co-expressed GD2, CD271 and/or CD200 within CD326-depleted BM-MNC.
Conclusions
There was a positive correlation between mesenchymal stem cells expressing GD2 and CD271 and breast cancer-initiating cells in BM of patients with primary breast cancer.
Key words: mesenchymal stem cells, bone marrow, microenvironment, cancer-initiating cells, cancer stem cells
Introduction
Breast cancer-initiating cells (BCIC) can metastasize and form new tumors.1,2 BCIC (CD44+CD24low/−) have been detected within the disseminated tumor cells (CD326+) in bone marrow (BM) of early stage breast cancer patients.1,3 Moreover, breast cancer with high aldehyde dehydrogenase (ALDH) activity, Aldefluor+, may contain the tumorigenic cell fraction and ALDH-positive cells that have been shown to be responsible for metastasis.2,4
Human bone marrow mesenchymal stem cells (BM-MSC) represent a phenotypically heterogeneous population of cells with the potential for multidirectional differentiation to bone, fat, cartilage and other mesenchymal tissues.5 In recent years, much attention has been focused on the plasticity of BM-MSC and other BM-derived progenitor cells, and their contribution to tumor-associated stroma formation and tumor progression.6,7 Moreover, these cells could play a specific role in the BM colonization of disseminated breast cancer cells.8 Finally, other BM precursors, the BM-derived hematopoietic stem cells that co-express the vascular endothelial growth factor receptor-1 (CD34+VEGFR1+) could play a role in establishing a pre-metastatic niche in distant organs in mouse models, though unconfirmed in humans.9
Recently, GD2, CD271 and CD200 were proposed as specific BM-MSC markers10–12 and the role of BM microenvironment and its specific components in supporting BCIC have been investigated.13 In the current study, we test the hypothesis that there is a correlation between either the percentages of BM-MSC or BM CD34+VEGFR1+ cell population and of BCIC in BM of primary breast cancer patients.
Results
We evaluated BM samples from 12 primary breast cancer patients, median age 51 years, range 27–75 years. Tumor histology was of infiltrating ductal carcinoma in 11 cases and of mixed lobular and undifferentiated breast carcinoma in one case; estrogen receptor was positive in ten cases and negative in two cases; HER2 was amplified in one patient. Tumor size was pT1 in seven cases and pT2–pT4 in five cases; nodal status was positive in five cases and negative in seven cases. Five patients received neoadjuvant chemotherapy. After a median follow-up of 12 months (range 6–12 months), one patient had a disease relapse with brain metastases (Table 1).
Table 1.
Patient and disease characteristics
| No. | (%) | |
| Age (years) | ||
| Median (range) | 51 | (27–75%) |
| Histology | ||
| Infiltrating ductal carcinoma | 11 | (92%) |
| Infiltrating lobular carcinoma | 0 | (0%) |
| Other | 1 | (8%) |
| Estrogen receptor status | ||
| Positive | 10 | (83%) |
| Negative | 2 | (17%) |
| HER2 status | ||
| Amplified | 1 | (8%) |
| Normal | 11 | (92%) |
| Tumor size (pT) | ||
| pT1 | 7 | (58%) |
| pT2–pT4 | 5 | (42%) |
| Nodal status | ||
| Positive | 5 | (42%) |
| Negative | 7 | (58%) |
| Neoadjuvant chemotherapy | ||
| Yes | 5 | (42%) |
| No | 7 | (58%) |
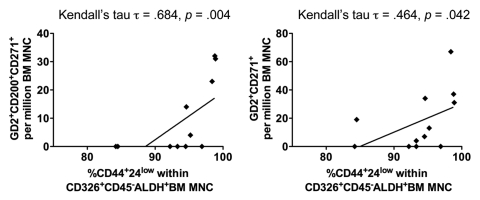
To test the hypothesis that a high proportion of BM-MSC is associated with an increased representation of BCIC within the bone marrow, we evaluated the phenotype of bone marrow mono-nuclear cells (BM-MNC) by flow cytometry (Table 2). All 12 BM samples contained epithelial cells with the BCIC phenotype and ALDH activity (Aldefluor+CD326+CD44+CD24−). BCIC correlated with BM-MSC that co-expressed GD2 and CD271 (either CD45−GD2+CD200+CD271+, Kedall's τ = 0.684, p = 0.004; or CD45−GD2+CD271+, Kedall's τ = 0.464, p = 0.042) (Fig. 1). On the other hand, BM-MSC t hat expressed either GD2 or CD271 did not correlate with BCIC (data not shown). In addition, VEGFR1+CD34+VEGFR2−CD31−expressing cells did not correlate with BCIC (data not shown).
Table 2.
Relative frequencies of hematopoetic stem cells, mesenchymal stem cells and breast cancer initiating cells
| Cells per million BM MNC | ||||||
| Subject | CD34+ | ALDH+ | ALDH+ CD326+CD45− CD44+CD24lo (BCIC) | GD2+ CD271+ (BM MSC) | GD2+CD271+ CD200+ (BM MSC) | CD34+ VEGFR1+ |
| 1 | 3800 | 15900 | 5603 | 19 | 0 | 1794 |
| 2 | 7700 | 39400 | 27355 | 31 | 31 | 2975 |
| 3 | 9900 | 25000 | 15203 | 67 | 23 | 2683 |
| 4 | 13800 | 19700 | 15902 | 37 | 32 | 32 |
| 5 | 9500 | 9700 | 4091 | 0 | 0 | 2347 |
| 6 | 11500 | 4800 | 2449 | 7 | 0 | 2197 |
| 7 | 4400 | 47900 | 16133 | 0 | 0 | 704 |
| 8 | 4700 | 16300 | 4280 | 0 | 0 | 17374 |
| 9 | 7300 | 11100 | 3371 | 34 | 14 | 1716 |
| 10 | 8500 | 14300 | 2914 | 0 | 0 | 723 |
| 11 | 4600 | 14900 | 4766 | 13 | 4 | 1339 |
| 12 | 5300 | 16400 | 3994 | 4 | 0 | 1240 |
Figure 1.
Correlation between the BCIC and BM-MSC. BM samples from primary breast cancer patients were interrogated for ALDH function using the aldefluor® assay (Stem Cell Technologies, Vancouver, BC). Within the aldefluor+ BM-MNC subset, BCIC phenotype was defined as CD326+CD45−CD44+CD24low and its percentage is correlated with BM-MSC that co-expressed GD2 and CD271 (either CD45−GD2+CD200+CD271+ or CD45−GD2+CD271+).
Discussion
BCIC have the capacity to seed in the BM at an early stage.4,8 In our study, all 12 BM samples contained BCIC epithelial cells (CD326+CD44+CD24−) and ALDH activity. A 70–80% incidence of BCIC in BM of primary breast cancer patients was previously reported by Balic et al.3 The presence of BCIC in the BM appeared related to BM-MSC subsets that co-expressed CD271 and GD2. The 1-year median follow-up precludes any conclusion on the metastatic growth potential of this correlation. Larger studies with longer follow-up are needed to correlate the percentage of the BCIC and/or the association to BM-MSC to the tumor status and therapy.
This study was hindered by the small sample size and the relative paucity of cells expressing the BM-MSC phenotype, (on average 18 BM MSC per 106 BM-MNC for the GD2+CD271+ phenotype or 9 BM MSC per 106 BM-MSC for the more restrictive GD2+CD271+CD200+ phenotype). Nevertheless, the positive correlation between BM-MSC subsets and BCIC with ALDH activity could be interpreted as favoring BCIC survival or as an inhibition of tumor growth. Although the bidirectional crosstalk between BM-MSC and tumor cells is known to occur and critical for tumor survival,6,14,15 naïve BM-MSC have been shown to inhibit tumor growth.16–18 In murine models BM-derived haematopoietic progenitor cells (CD34+VEGFR1+) formed a pre-metastatic niche at the sites of metastasis formation before the infiltration of tumor cells.9 This might explain the homing of tumor cells to the BM observed in patients.13 However, no correlation was found between these cells and BCIC in the current study.
Others showed that BM-MSC mixed with otherwise weakly metastatic human breast carcinoma cells caused the latter to increase their metastatic potency.19 Breast cancer cells stimulate de novo secretion of the chemokine CCL5 from BM-MSC, which then acts in a paracrine fashion on the tumor cells to enhance their motility, invasion and metastatic potential. This metastatic ability, that depends on CCL5 signaling through the chemokine receptor CCR5, is reversible and illustrates that the niche or microenvironment determines the fate of stem cells by modifying their biological properties responsible for the ability to invade and metastatic potential.6 Finally, recently, other authors showed that BM-MSC may accelerate human breast tumor growth by generating cytokine networks that regulate the cancer stem cell population.20
Research on cancer dissemination is currently focused on new therapeutic strategies consisting of molecular targeting of distinct oncogenic signaling components activated in the cancer-initiating cells and/or the cells in the microenvironment. To be effective, the putative targeted therapy should prevent disease relapse and enhance patient survival possibly by modulating the critical role of the microenvironment to support survival of cancer initiating cells.
In conclusion, our results suggest that BM-MSC subsets positively correlate with BCIC with ALDH activity in BM of primary breast cancer patients. Additional research is warranted to further investigate the relationship between BM-MSC and disseminated BCIC.
Materials and Methods
Bone marrow specimens were obtained from primary breast cancer patients (Protocol LAB04-0657; Chair: A. Lucci) at The University of Texas MD Anderson Cancer Center. The study was approved by the Institutional Review Board. Following informed consent, 10 mL of bilateral BM aspirates from the upper anterior iliac crests of patients were collected at the time of surgery. BM mononuclear cells (BM-MNC) were obtained by Ficoll density gradient centrifugation and the remaining erythrocytes were lysed with ammonium chloride solution, as previously reported.19
BM samples were interrogated for ALDH function using the Aldefluor® assay (Stem Cell Technologies, Vancouver, BC). Briefly, BM-MNC were suspended in Aldefluor buffer containing an ATP-binding cassette transport inhibitor. A BM-MNC aliquot was reacted with the ALDH inhibitor, diethylamino-benzaldehyde (DEAB), as a negative control. Both the test reaction and the negative control were analyzed on a LSR II flow cytometer (BD Biosciences, San Jose, CA). Alexa700 (Invitrogen, Carlsbad, CA) labeled antiCD44 monoclonal antibody (BD Pharmingen, San Jose, CA) and pre-conjugated antibodies to CD24 (PE-Cy5, BD Pharmingen), CD45 (PE-Cy7, BD Pharmingen) and CD326 (APC, Miltenyi Biotec, Auburn, CA) were used to label cells. Appropriate isotype-matched controls were used. Within the Aldefluor+ BM-MNC subset, BCIC phenotype was defined as CD326+CD45−CD44+CD24low.
Another aliquot of BM-MNC was reacted with antiCD326 antibody coated magnetic beads and then processed in a MACSPro cell separator (MACS, Miltenyi Biotec) to isolate CD326+ cells. Thereafter, the CD326-depleted cells were reacted with the corresponding antibodies and analyzed on the flow cytometer to identify BM-MSC expressing CD200, GD2 or CD271 but not CD45. In addition, the CD326-depleted cells were reacted with the corresponding antibodies and analyzed on the flow cytometer for VEGFR1+CD34+ VEGFR2−CD31− cells.
The Kendall's τ non-parametric correlational test was used to determine the relationship between either BM-MSC or VEGFR1+CD34+ cells and BCIC.
Acknowledgements
This study was funded in part by a grant from the State of Texas Rare and Aggressive Breast Cancer Research Program. M.C. and J.M.R are the recipients of a R01 grant from the National Cancer Institute to study human breast cancer stem cell surrogates (CA138239-02).
Conflicts of Interest
Presented in part at the 100th AACR Annual Meeting, Denver, CO. April, 18–22 2009.
References
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 4.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology. 2008;47:126–131. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- 6.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 7.Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Corcoran KE, Trzaska KA, Fernandes H, Bryan M, Taborga M, Srinivas V, et al. Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS ONE. 2008;3:2563. doi: 10.1371/journal.pone.0002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez C, Hofmann TJ, Marino R, Dominici M, Horwitz EM. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109:4245–4248. doi: 10.1182/blood-2006-08-039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buhring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann NY Acad Sci. 2007;1106:262–271. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- 12.Delorme B, Ringe J, Gallay N, Le Vern Y, Kerboeuf D, Jorgensen C, et al. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631–2635. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- 13.Pantel K, Brakenhoff RH, Brand B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:330–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 14.Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 15.Mishra PJ, Mishra PJ, Glod JW, Banerjee D. Mesenchymal stem cells: flip side of the coin. Cancer Res. 2009;69:1255–1258. doi: 10.1158/0008-5472.CAN-08-3562. [DOI] [PubMed] [Google Scholar]
- 16.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2003;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu YR, Yuan Y, Wang XJ, Wei LL, Chen YN, Cong C, et al. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol Ther. 2008;7:245–251. doi: 10.4161/cbt.7.2.5296. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W. Mesenchymal stem cells in cancer: friends or foes. Cancer Biol Ther. 2008;7:252–254. doi: 10.4161/cbt.7.2.5580. [DOI] [PubMed] [Google Scholar]
- 19.Bauer KD, de la Torre-Bueno J, Diel IJ, Hawes D, Decker WJ, Priddy C, Bossy B, et al. Reliable and sensitive analysis of occult bone marrow metastases using automated cellular imaging. Clin Cancer Res. 2000;6:3552–3559. [PubMed] [Google Scholar]
- 20.Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]