Abstract
Mucins are high molecular weight; multifunctional glycoproteins comprised of two structural classes - the large transmembrane mucins and the gel-forming or secreted mucins. The primary function of mucins is to protect and lubricate the luminal surfaces of epithelium-lined ducts in the human body. Recent studies have identified a differential expression of both membrane bound (MUC1, MUC4 and MUC16) and secreted mucins (MUC2, MUC5AC, MUC5B and MUC6) in breast cancer tissues when compared with the non-neoplastic breast tissues. Functional studies have also uncovered many unique roles of mucins during the progression of breast cancer, which include modulation in proliferative, invasive and metastatic potential of tumor cells. Mucins function through many unique domains those can form complex association with various signaling molecules including growth factor receptors and intercellular adhesion molecules. While there is growing information about mucins in various malignancies including breast cancer, no focused review is there on the expression and functional roles of mucins in breast cancer. In this present review, we have discussed the differential expression and functional roles of mucins in breast cancer. The potential of mucins as diagnostic and prognostic markers and as therapeutic targets in breast cancer have also been discussed.
Keywords: Mucins, Polymorphic epithelial mucins, MUC1 (DF3), MUC4, MUC16 (CA125), Cancer, Breast cancer, Cell survival, Apoptosis
1. Introduction
Breast cancer (BC) is the second leading cause of cancer-related deaths in women worldwide after lung cancer. In the year 2010, BC accounted for an estimated 28% of all new cancer cases in the United States, while nearly 15% deaths from this malignancy occurred in the same period. While most BCs are sporadic in nature, approximately, 5% of BC patients have a hereditary predisposition to develop this malignancy. Evidences are emerging those suggest that BC is a heterogeneous disease at the molecular level having a number of distinct entities with specific pathologic features and biologic behaviors. Traditional grading systems based on characteristics of the nucleus have given a way to more molecular approach that relies on distinct gene signatures to separate the different BC subtypes. The recent advances in molecular classification of BC have been studied by several groups of investigator. These approaches are an attempt to avoid over or under-treatment and personalize therapy based on the predicted behavior of a given subtype.
Molecular markers are particularly helpful as an alternative to conventional diagnostic modalities as expression of mucins generally precedes morphological change by a considerable lag period. According to currently available information, the development of BC represents a continuum of events and is believed to progress from non-neoplastic epithelium through the stages of usual epithelial hyperplasia (UEH also called ductal hyperplasia DH), atypical ductal hyperplasia (ADH), carcinoma in situ (CIS) and finally invasive carcinoma. Molecular studies seem to support the hypothesis that the transition between UEH and ADH represents the boundary between benign hyperplasia and CIS (the stage preceding invasive carcinoma). Molecular markers that can distinguish these two lesions could thus have the potential to be immensely useful to identify patients at an elevated risk for BC and therefore requiring enhanced surveillance.
The overexpression, mutation, and deletion of specific genes are major mechanisms underlying the progression and metastasis of BC. Mucins (denoted by the gene symbol MUC) encompass a family of high molecular weight, heavily O-glycosylated proteins those are differentially expressed in several epithelial malignancies. These proteins have been demonstrated to play a pivotal role in the development of BC. Mucins are normally expressed by epithelial cells and contribute to the lubrication of hollow tubular surfaces such as ducts and the passages in the respiratory and gastrointestinal systems. They also serve as a mechanical barrier to extrinsic physical and biological assaults. Mucins are broadly classified structurally into two main classes: membrane-bound mucins (MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC15, MUC16, MUC17, and MUC20) and secreted or gel-forming mucins (MUC2, MUC5AC, MUC5B, MUC6, MUC7, MUC8 and MUC19). All mucins share certain common structural features, but are distinct in the sequence, domain organization, length, and number of their respective tandem repeat sequences. The structure and general biology of mucins have been reviewed in several excellent review articles.
An altered expression of mucin has been reported to be associated with cancer progression, which in turn, influences cellular growth, differentiation, transformation, adhesion, invasion, and immune surveillance. Mucin 1 (MUC1) is mostly-studied mucin in BC. However, recent studies have demonstrated that other mucins, including MUC2, MUC3, MUC4, MUC5AC, MUC5B, MUC6, and MUC7 are also differentially expressed in BC cells. In this present review, we have discussed the current knowledge concerning the expression, clinical relevance and functional role of mucins in BC. Investigation of several research groups reveal that mucins have important roles in pathological state and have immense potential as diagnostic or prognostic markers and as therapeutic targets in BC.
2. Expression of mucins in normal breast and their aberrant expression in benign and malignant breast diseases
2.1. Expression of mucins in normal breast
Several mucins have been reported to be expressed by the non-neo-plastic breast (summarized in Figure 1 and Table 1). MUC1, the best studied mucin in BC, is expressed in nearly 59% of normal breast tissues (without an adjoining malignancy) with a similar degree of positivity in the malignant ducts adjacent to the normal tissue. MUC4 (92%-100% positivity in a single study and to a lesser extent MUC5AC (4% positivity) and MUC6 (9%-14% positivity) are also expressed in the ductal epithelium of the healthy breast. MUC2 expression is however entirely absent. MUC5B, while not expressed in the non-neoplastic ductal epithelium, was detected in cancer adjacent normal tissues (42% positive cases in one study). At the sub-cellular level, MUC1, MUC5AC and MUC6 are expressed mostly in the cytoplasm; while MUC5B is expressed in the apical portion of the non-neoplastic ductal cells of the breast.
Figure 1. Expression of mucins during the progression of breast cancer.
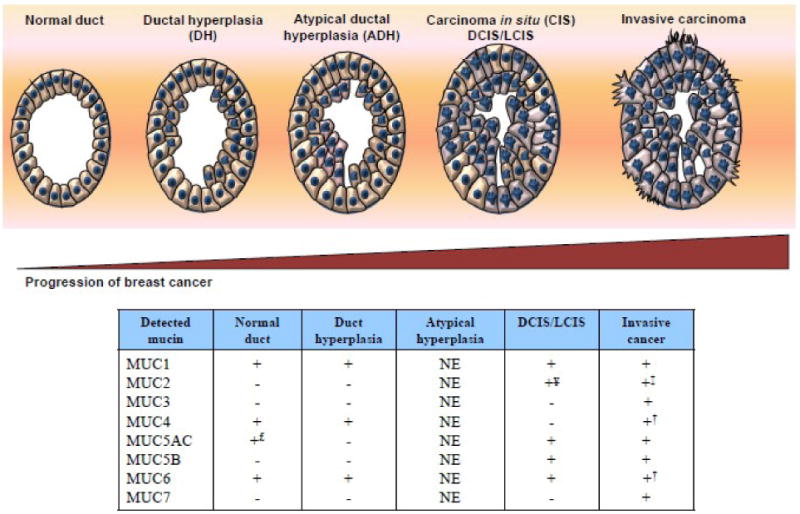
Breast cancer is currently believed to develop through a series of well-defined stages: Normal duct > DH (ductal hyperplasia) > Atypical ductal hyperplasia (ADH) > Carcinoma in situ (CIS): DCIS/LCIS (ductal or lobular carcinoma in situ) > Invasive carcinoma (ductal or lobular). Both secreted and membrane-bound mucins have been demonstrated to be expressed during the progression from normal ducts to invasive adenocarcinoma. (+ indicates mucin detected and – indicates mucin undetected, NE not examined, £ <5% cells positive, ‡ expressed in mucinous carcinoma (in addition to ductal carcinoma), † Expressed in lobular carcinoma (in addition to ductal carcinoma), ¥ Expressed in LCIS (in addition to DCIS).
Table 1. Studies investigating mucins as diagnostic and prognostic markers in breast cancer.
| Ref. | Year | Type of study | Type of tissue | Technique | Mucin genes investigated |
|---|---|---|---|---|---|
| [21] | 2001 | Retrospective | Formalin fixed | IHC | MUC1,MUC5AC and MUC6 |
| [37] | 2005 | Retrospective¥ | Formalin fixed (TMAs) | IHC | MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6 |
| [22] | 2009 | Retrospective | Formalin fixed (Commerical TMAs) | IHC | MUC4 |
| [26] | 1993 | Retrospective | Formalin fixed | IHC | MUC2 |
| [31] | 1998 | Retrospective | Formalin fixed | IHC | MUC6 |
| [24] | 2006 | Retrospective | Formalin fixed | IHC | MUC5B |
| [27] | 2008 | Prospective¶ | Formalin fixed | IHC | MUC1, MUC2, MUC5AC and MUC6 |
| [10] | 2003 | Retrospective | Formalin fixed | IHC | MUC1 |
| [34] | 2009 | Retrospective | Formalin fixed | IHC | MUC1 |
| [46] | 2007 | Retrospective | Formalin fixed (Commercial TMAs] | IHC | MUC1 |
| [35] | 2006 | Prospective | Formalin fixed | IHC | MUC1 |
| [53] | 1992 | Retrospective | Formalin fixed | IHC | MUC1 |
| [45] | 2008 | Retrospective | Formalin fixed | IHC | MUC16 |
| [25] | 2007 | Prospective | Fresh surgical specimen | IF | MUC1 |
| [33] | 2006 | Prospective | Fresh frozen SLN | RT-PCR | MUC1 |
| [70] | 2003 | Prospective | Bone marrow | RT-PCR | MUC2,MUC3,MUC5B, MUC6 and MUC7 |
| [36] | 2001 | Prospective | Fresh frozen ALN | Real time PCR | MUC1 |
| [71] | 2007 | Prospective | Peripheral venous blood | IMS followed by mPCR | MUC1 |
| [72] | 2009 | Prospective | Peripheral venous blood | IMS followed by RT-PCR | MUC1 |
| [52] | 1996 | Retrospective | Serum | RIA | MUC1 |
| [64] | 1988 | Prospective | Serum | RIA | MUC1 (CA15.3] |
| [62] | 2009 | Retrospective | Serum | ELISA | MUC1 (CA15.3] |
| [57] | 1993 | Retrospective | Serum | ELISA | MUC1 (CA15.3] |
| [54] | 2009 | Prospective§ | Serum | ELISA | MUC1 (CA15.3] |
| [66] | 1995 | Prospective | Serum | ELISA | MUC1 (CA15.3] |
| [53] | 1992 | Retrospective | Human milk fat globulin | 43 ELISA | MUC1 |
| [32] | 1992 | Retrospective | Tumor DNA | RFLP | MUC1 |
separation (IMS]; Radioimmunoassay (RIA]; Restriction fragment length polymorphism (RFLP]; Reverse transcription PCR (RT-PCR]; Sentinel lymph node (SLN]; Tissue microarray (TMA].
2.2. Expression of mucins in benign and potentially malignant breast diseases
Present models of BC development suggest that it develops through the stages of hyperplasia (two types - usual and atypical), carcinoma in situ and invasive adenocarcinoma. However, while every carcinoma develops from a carcinoma in situ, not every carcinoma in situ develops into a carcinoma. Molecular markers are thus particularly helpful as an alternative to conventional diagnostic modalities to identify potentially malignant lesions as their expression precedes morphological changes by a considerable lag period. Several studies have demonstrated that the expression of mucins is altered in benign and pre or potentially malignant breast diseases. For instance, the expression of the membrane mucin MUC5B is upregulated in fibroadenomas, a fibrocystic disease of the breast and in sclerosing papillomas. MUC6, on the other hand, is expressed in fibrocystic disease without atypia, and its expression increases in cases with accompanying atypical features (41% positivity in cases without atypia vs. 100% in those with atypia). MUC1, MUC2, MUC5AC, MUC5B, and MUC6 are also expressed in pre-malignant breast lesions like ductal carcinoma in situ (DCIS), while MUC2 expression is upregulated in lobular carcinoma in situ (LCIS), the precursor to invasive lobular carcinoma. MUC1 and MUC6 (but not MUC5AC) are also expressed in simple ductal hyperplasia without atypia. A case report noted that there was positive staining for MUC1 (92%-100% positive cells) but not for MUC2 or MUC5AC in two cases of ductal adenoma of the breast. Weak MUC6 positivity (5% positive cells) was also seen in one case in the same study, while the other was entirely negative.
Using monoclonal antibodies that recognize epitopes in either the tandem repeat (TR) region (C595, HMFG2 and SM3) or the cytoplasmic tail (CT33) of MUC1, it was observed that normal breast ductal epithelium was variably immunopositive (range: 8%-92%). Majority (>70%) of the normal breast tissue sections exhibited an apical, predominantly linear MUC1 staining with the remaining cases showing a non-apical cytoplasmic staining. MUC1 positivity, particularly with the CT33 antibody was maintained in a range of benign (fibroadenoma, non-proliferative lesions, usual epithelial hyperplasia) and pre-malignant lesions (atypical hyperplasia). The incidence of MUC1 immunopositivity, however, was significantly lower with the SM3 anti-TR antibody (ranging from 4%-14%) compared to the other two TR antibodies, HMFG2 (36%-65%) and C595 (44%-61%). Significantly, the highest reactivity (between 71%-96%) was noted with the antibody directed against the MUC1 cytoplasmic tail (MUC1-CT) in both normal breast tissues and those from patients with benign breast diseases. These differences in reactivity to MUC1 antibodies are suggested to be a result from the differential reactivity of anti-MUC1 antibodies against normal and under-glycosylated forms of mucin those are expressed by non-neoplastic/benign cells and cancer cells respectively. While MUC1 does not appear to be useful to distinguish between benign an pre-malignant breast tumors, the secreted mucin MUC6 exhibited significant specificity for atypical fibrocystic disease (100% positivity compared to 41% positivity for fibrocystic disease without atypia) and invasive carcinoma (positivity ranged from 92% for lobular to 100% for ductal carcinoma)
2.3. Expression of mucins in malignant breast tumors
Both membrane-bound and secreted mucins are upregulated in ductal adenocarcinoma of the breast (summarized in Table 3 and 4 respectively). In BC tissues, MUC1 (74%-77% positivity), MUC3 (91% positivity), and MUC4 (79%-95% positivity) among the membrane bound mucins and MUC2 (19% positivity), MUC5AC (7%-37% positivity), MUC5B (19% positive), and MUC6 (20%-100% positivity) among the secreted mucins, show a significant up-regulation compared to the normal breast epithelium. In addition to up-regulation of gene transcription, the copy number of MUC1 was also shown to be significantly increased in BC cells (but not in the non-neoplastic breast ductal cells). When staining of mucin was correlated with the expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER-2/ErbB2), and p53, only the cytoplasmic expression of MUC1 correlated positively with ER expression, while a membranous distribution of MUC3 staining and MUC6 positivity correlated negatively with ER status in most of those cases. Hormone receptor status and expression of HER-2/ErbB2 have been demonstrated to be significant prognostic indicators or surrogate markers for response to anti-hormonal (Tamoxifen and Fulvestrant) and anti-ErbB2 (Trastuzumab) therapy respectively. MUC3 expression has also been demonstrated to be upregulated in MCF-7 BC cells following treatment with steroids (progesterone, β-estradiol, testosterone, and hydrocortisone) in vitro suggesting a potential association between alteration in hormone levels and aberrant expression of mucins in BC. Further, it appears that there is no significant relationship between mucin expression with expression of either PR, HER-2, or p53 in BC. It has also been shown that tumor cells initially loose MUC4 expression, but MUC4 is re-expressed in tumor cells in lymph node metastases. This indicates that selective modulation of the expression of MUC4 at different stages confers a metastatic advantage to the BC cells.
Table 3.
Membrane bound mucins in the diagnosis and prognosis of breast cancer.
| Ref. | Mucin | N | Antibody (mAb) | Positivity in Cancer | Controls (% +ve) | Pathologic correlation | Clinical correlation |
|---|---|---|---|---|---|---|---|
| [21] | MU C1 | 68 cancer, 29 Nor, 15 DHWA, 2 DCIS |
SM3 | 74%; C+M | 1.Nor: 59%;C 2.NAT: 59% 3.DHWA: 67% 4.DCIS: 100% |
None to tumor size/stage/grade/E R/HER-2/p53 | None to age. Others NE |
| [10] | MUC1 | 42 FA, 23 NPL, 25 UEH, 7ADH €, 27 Nor | C595, HMFG2,S M3, CT33 | NE | 1. CT33: 92%,M 2. C595: 44%,M 3. HMFG2: 36%,M 4. SM3: 8%,M |
NE | NE |
| [37] | MUC1 | 1447 cancer 0 Nor |
Ma695¥ | 1. Purely L/A: 4.5% 2. L+C: 18.4% 3. C with CMA: 77% |
NE | 1. ER status: +ve correlation: 3. Tumor grade: a. C+M staining: more in high-grade tumors b. L/L+C staining: more in low-grade tumors |
1. Distant metastasis and LN spread: -ve correlation overall. But L staining pattern correlates with vascular invasion, LN spread and distant metastasis. 2. Survival: No relation to overall MUC1 expression. However, C+M staining was associated with lower survival (OS+DFS) than tumors with L staining pattern. 3. Recurrence: ↑ in MUC1-tumors |
| [36] | MUC1 | Axillary LN (17 known +ve, 21 known – ve BC, 51 Nor nodes) | NA (Real time PCR) | 67% sensitive and 82% specific for micrometastasis in LN | NA | NE | NE |
| [25] | MUC1 | 20 cancer (120 margins) | ND | Combination with E-cadherin was 80% sensitive, 100% specific with 100% PPV and 99% NPV by IF on EIOTPs. | Formalin fixed sections were used to confirm EIOTP results | NE | NE |
| [34] | MUC1 | 82 cancer | DF3 (CA15.3), VU-4-H5, PankoMab | Comparison of mAbs: 1. PankoMab: stronger staining in ER+ tumors 2. VU-4-H5: strong staining in LN deposits and higher grade tumors 3. DF3: NS |
NE | 1. PankoMab ↓ staining with ↑ grade (NS) 2. DF3: ↑ staining in Grade 2 than in grade 3 tumors (NS) 3. VU-4H-5:In ER-LN- cases, staining G1>G2; In ER+LN+ cases, staining G2>G3. |
NE |
| [43] | MUC1 | 17 Mucinous and 46 ductal carcinoma | NCL-MUC1-CORE, DF3, HMFG-2, MY.1E12, NCL-MUC1, HMFG-1 | 1. NCL-MUC1-CORE: 98% (IDC) vs. 76% (MC) 2. DF3: 100% (IDC) vs 100% (MC) 3. HMFG-2: 92% (IDC) vs. 88% (MC) 4. MY.1E12: 100% (IDC) vs. 100% (MC) 5. NCL-MUC1: 95% (IDC) vs. 88% (MC) 6.HMFG-1:98% (IDC)vs.65% (MC) |
100% (NAT) | 1. Nor: L staining of ducts and lobules 2. Cancer: C+M (C>M) |
NE |
| [35] | MUC1 | 5 spindle cell BC | - | 100% | NE | 20% were HER2+, 40% PR+ and 90% ER+ | NE |
| [37] | MUC3 | 1447 cancer 0 Nor |
1143/B7 | 91% C:91% C+M:21% |
NE |
1. ER status: -ve correlation with the expression of MUC3 3. Tumor grade: More expression correlates with higher grade |
1. Distant metastasis and LN spread: +ve correlation with vascular invasion, and LN spread 2. Survival: None 3. Recurrence: ↑ in MUC3+ tumors |
| [37] | MUC4 | 1447 cancer 0 Nor |
1G8 | 95% | NE | 1. ER status: None 3. Tumor grade: +ve correlation |
1. Distant metastasis and LN spread: None 2. Survival: None 3. Recurrence: None |
| [22] | MUC4 | 1. Lysates: 8 Nor and 70 tumors. 2. Tissues: 110 Nor, 40 pre-malignant, 286 P & 48 Met BC |
1G8 | 1.IB: 84% 2. IHC: 58% PADC showed ↓ in expression (relative to paired Nor tissue) and 11% had ↑ expression (79% +ve overall). 3. Lobular Ca (68% by IHC) |
Nor breast1. IB: 100%§ 2. IHC: 92% Hyperplasia (100%) DCIS (86%) |
ER/PR/HER-2/p5 3 status: None (both in IB and IHC) | Distant metastasis: had ↑ MUC4 expression than matched primary tumor. |
| [38] | MUC1, 2,4, 5A C and 6 | Six 1° and three metastati c breast SRCC | MUC1 (Ma695) MUC2 (CCP58) MUC4 (1G8) MUC5AC (45M1) MUC6 (CLH5) |
1. MUC1 (100% of P 100% Met) 2. MUC2 (33% of P & 0% Met) 3. MUC6 (17% of P &0% Met) 4. MUC4 and MUC5AC were -ve |
NE | No correlation between MUC16 positivity and nuclear grade | NA |
| [45] | MUC16 | 37 IMPC | CA125 | 40%, L+M | NE | NE | NE |
| [71] | MUC1 | 48 cancer† Unknow number of healthy blood samples | IMS (using anti-MUC1 mAbs) →Multiple × PCR for MUC-1, HER-2, Claudin-7 and GA733-2 | 1. 58% of BC patients with detectable DTCs were MUC1+2. Patients who were MUC1- in the beginning were also –ve on follow-up at 1 year. | None | NE | NE |
| [72] | MUC1 | 63 cancer 14 Nor Blood samples |
IMS (using anti-MUC1 mAbs) to isolate DTCs→ Multiplex PCR for MUC-1, HER-2, MGB-1, GA733-2 and SPDEF | ND | NE | NE | 1. MUC1 positivity ↑ from stage 1 (0%) →Stage 2 (6%) → Stage 3 (33%) 2. It also ↑ from no LN involvement (7%) → N1 (16%) → N2 (33%) 3. Higher positivity in Met (32%) than non-Met (9%) BC. 4. 56% +ve for serum CA15.3 were also MUC1+, while only 8% of CA15.3-samples were MUC1+ |
Recognizes the glycosylated form of MUC1. Similar results were also obtained with an mAb that recognizes the unglycosylated form of MUC1 (Ma552)
Wang et al.[94] using the Ma695 mAb reported a similar observation that MUC1+ breast cancers were more likely to be ER+ and of a lower grade than MUC1- tumors.
Expression was significantly downregulated in tumor tissue compared to adjacent normal breast tissue by IB.
All patients had metastatic BC and had been treated with either Herceptin or chemotherapy prior to drawing of the blood sample. Breast cancer (BC); Primary Adenocarcinoma (PADC); Apical (A); Cytoplasmic (C); Circumferential membrane accentuation (CMA); Granular (G); Invasive micropapillary carcinoma (IMPC); Luminal (L); Membrane (M)Number of samples (N);Lymph node (LN); Primary (P); Normal (Nor) Metastatic (Met) Not examined (NE); Not statistically significant (NS); DCIS, ductal carcinoma in situ; DHWA, ductal hyperplasia without atypia; monoclonal antibody (mAb); polyclonal antibody (pAb); Overall survival (OS), Disease free interval (DFI); Immunoblotting (IB); Immunofluorescence (IF); Enhanced intraoperative touch preps (EIOTP);
Fibroadenoma (F), Non-proliferative lesions (NPL), Usual epithelial hyperplasia (UEH), Atypical ductal hyperplasia (ADH).
Table 4. Secreted mucins in the diagnosis and prognosis of breast cancer.
| Ref. | Mucin | N | Anti body | mAb or pAb | Positivity in cancer | Positivity in normal or benign tissues | Pathologic correlation | Clinical correlation |
|---|---|---|---|---|---|---|---|---|
| [26] | MUC 2 | 200 cancer 81 CIS |
4F1 | mAb | 1. Ductal Ca.: 19% (C+G) 2. Lobular Ca: 8% (C+G) 3. Mucinous Ca: 100% (C+G) |
1. Absent except weak cytoplasmic staining in one case. 2. DCIS (14%), LCIS (7%) |
1. ER/post-menopausal status/grade: None 2. Size: ↑ expression in tumors <10mm or >50mm in size |
1. DFS: Shorter (49 months) in MUC2+ than in MUC2- (75 months) tumors 2. OS: None |
| [43] | MUC 2 | 17 MC, 46 IDC | Ant-MRP | pAb | 1. IDC: 15% 2. MC: 94% |
Absent in NAT | NE | NE |
| [21] | MUC 5AC | 68 cancer, 29 normal, 15 DHWA, 2 DCIS |
CLH2 | mAb | 7%¥; C+G | 1.Normal: 4%; C+G 2. NAT: 3% 3.DHWA: None 4.DCIS: 50% |
None to tumor size/stage/grade/E R/HER-2/p53 | None to age. Others NE |
| [37] | MUC 5AC | 1447 cancer 0 normal |
CLH2 | mAb | 37% | NE | None | +ve correlation with post-menopausal status only. |
| [43] | MUC 5AC | 17 MC, 46 IDC | NCL-MUC5A C | mAb | 1. IDC: 4% 2 MC: 12% |
Absent in NAT | NE | NE |
| [24] | MUC 5B | 42 cancer 24 normal 13 DCIS 14 benign diseases |
- | pAb | 1. Ductal Ca: 19% (high) and 62%(intermediate) stained. 2. Ductal Ca: C +P Colloid Ca: A |
1. Normal breast epithelium: 0% 2. NAT: 42% 3. DCIS (54%); Benign disease (93%) 3. Distribution: Mostly A (C in some NAT) |
1. ER/PR status/tumor size/grade/stage: NS 2. LN metastasis: +ve when 1° tumor was +ve |
NE |
| [21] | MUC 6 | 68 cancer, 29 normal, 15 DHWA, 2 DCIS |
CLH6 | mAb | 23%; C | 1.Normal: 14%;C2. NAT: 14% 3.DHWA: 13% 4.DCIS: 50% |
None to tumor size/stage/grade/E R/HER-2/p53 | None to age. Others NE |
| [37] | MUC 6 | 1447 cancer 0 normal | CLH5 | mAb | 20% § | NE |
1. ER status: -ve correlation with MUC6 expression 3. Tumor grade: None |
1. Distant metastasis LN spread and recurrence: none 2. Survival: Appears to improve survival |
| [31] | MUC 6 | 60 cancer 11 normal 28 fibrocystic disease |
- | pAb | 1.Intraductal Ca: 82% 2.Ductal infiltrating Ca: 100% 3.Lobular Ca: 92% (Diffuse C) |
1. Normal: 9% 2. Fibrocystic disease without atypia: 41% 3. Atypical fibrocystic disease: 100% |
ER status: None (Other factors NE) | NE |
| [43] | MUC 6 | 17 MC, 46 IDC | NCL-MUC6 | mAb | 1. IDC: 15 2. MC: 72% |
Absent in NAT | NE | NE |
| [70] | MUC 2 MUC 3 MUC 5BMUC 6 MUC 7 |
Breast tissues and BM from 46 cancer patients PBMCs from 15 normal donors |
NA(RT-PCR) | N A |
BC tissue using RT-PCR 1. MUC5B-7/15 (47% sensitive) 2. MUC2-1/11 (9% sensitive) 3. MUC3-1/11 (9% sensitive) 4. MUC6-3/14 (21% sensitive) 5. MUC7-5/12 (42% sensitive) Nested PCR for MUC5B-52% sensitive |
PBMCs using RT-PCR 1. MUC2, MUC5B, MUC6-none(100% specific) 2. MUC3-4/14 (29% specific) 5. MUC7-10/13 (77% specific) Nested PCR for MUC5B-none (100% specific) |
1. Tumor size: ↑ +ve with ↑size. T1 (14%) < T2 (22%) < T3 (50%) 2. Stage: ↑ +ve with ↑ stage. Stage I (15%) < Stage IIa (25%) < Stage IIb (9%) < Stage III (50%) 3. LN: NS |
Sensitivity of MUC5B nested PCR was comparable to RT-PCR for CEA (17% sensitive) and inferior to CK19 (41% sensitive) for detecting DTCs |
|
Validation set Nested PCR for MUC5B applied to BM of pre-operative BC patients (19.5% +ve) |
All the five MUC5AC positive cancers were also positive for MUC1 and 4/5 were positive for MUC6.
High expression in mucinous breast tumors compared to other tumor types
Breast cancer (BC); Carcinoma in situ (CIS); Apical (A); Cytoplasmic (C); Circumferential membrane accentuation (CMA); Granular (G); Luminal (L); Perinuclear (P); Number of samples (N);Lymph node (LN); Normal adjacent tissue (NAT); Not examined (NE); DCIS, ductal carcinoma in situ; DHWA, ductal hyperplasia without atypia; monoclonal antibody (mAb); polyclonal antibody (pAb); Overall survival (OS), Disease free survival (DFS); Mucinous carcinoma (MC), Invasive ductal carcinoma (IDC)
Mucinous carcinomas constitute a distinct and significantly rare pathological entity accounting for only about 2% of breast malignancies. Although rare, but mucinous carcinomas are clinically highly relevant owing to the observation that purely mucinous carcinomas have a significantly better prognosis than ductal carcinoma mainly due to lower incidence of lymph node metastasis. A comparison of selected mucin expression in mucinous and ductal carcinomas revealed that MUC2 and MUC6 expression is significantly more common in mucinous carcinomas (94% and 71% respectively) than in ductal adenocarcinomas (15% and 15% respectively). MUC1, which was highly expressed in both types of BC (65%-100% in mucinous and 92%-100% in ductal carcinoma and MUC5AC, which was rarely expressed (12% and 4% positivity in mucinous and ductal cancer respectively, however, did not show any difference between these two histologic subtypes. An interesting observation in this study was the difference in the proportion of strongly immune-reactive cancer cells using antibodies recognizing the differentially glycosylated forms of MUC1. For instance, NCL-MUC1-CORE, an antibody that recognizes the mucin core peptide (TRPAPG) showed a significantly lower immunopositivity in both mucinous and ductal carcinoma (incidence of strong immunopositivity 48% and 24% respectively) than DF3, which also recognizes the mucin core peptide (TRPAPGS) but whose immunoreactivity is enhanced by presence of carbohydrate side chains (incidence of strong immunoreactivity being 87% and 88% for mucinous and ductal cancer respectively). Notably, the incidence of strong immunostaining (>50% of cancer cells positive) with both the above mentioned antibodies was decreased upon pre-treatment of the tissues with sialidase to 0% and 15% for NCL-MUC1-CORE and to 35% and 30% for DF3 for mucinous and ductal cancer respectively. In comparison, immunoreactivity to antibodies that recognize the fully glycosylated forms of MUC1 (mAbs HMFG-1 and HMFG-2) was significantly increased following sialidase treatment. 18% to 100% and 78% to 96% change for mucinous and ductal carcinoma respectively with HMFG-1 and 41% to 82% and 33% to 85% for mucinous and ductal carcinoma respectively with HMFG-2. This modulation of immunoreactivity of mucin antibodies by silaidase treatment underscores the important role of glycosylation in mucin biology, with particular relevance to mucin based biomarkers and therapeutic studies.
Invasive micropapillary carcinoma of the breast (IMPC) is another rare variant of invasive ductal carcinoma characterized by the presence of micropapillary structures surrounded by clear spaces. Clinically, they are extremely aggressive and characterized by a high incidence of axillary lymph node metastasis. Immunohistochemical analysis of 37 cases of IMPC (invasive micropapillary carcinoma) using a commercial monoclonal antibody against MUC16 (CA125), only 9 (24%) cases showed a strong expression with the remaining being only focally positive.
While ductal carcinoma is the predominant histologic type of BC encountered, signet ring cell carcinoma (SRCC) is a comparatively rarer entity. Metastatic SRCCs present a diagnostic dilemma as the primary site of origin is often difficult to identify. It has been noted that the mucin expression profile of SRCCs could help to pinpoint the site of the primary tumor. A comparison of primary and metastatic SRCCs arising from the stomach, colorectum, or breast revealed that each had a unique mucin expression profile. 100% of primary and metastatic SRCCs from the breast were positive (14%) for MUC1, and no positivity were found in SRCC arising from the stomach and also no detectable MUC1 expression in SRCCs arising from the colon.
Not just the expression, but the sub-cellular localization of mucins also appears to be altered in BC. MUC1 staining varies from cytoplasmic (with SM3 mAb) to a heterogenous combination of luminal and cytoplasmic with or without a membranous accentuation (with Ma695 mAb). The pattern of staining appears to have important pathobiological implications, as tumors with primarily cytoplasmic MUC1 staining are often ER positive, while a cytoplasmic staining with membrane accentuation is a feature of high-grade tumors, and a luminal or a combination of luminal and cytoplasmic staining is more common in low-grade tumors. MUC2 and MUC5AC exhibit a cytoplasmic and granular staining pattern in ductal adenocarcinomas. In contrast, MUC5B has a cytoplasmic and perinuclear distribution in ductal carcinoma and a predominantly apical distribution in colloid carcinoma.
Immune complexes comprised of IgM or IgG antibody and MUC1 have also been reported in the serum of BC patients although no significant difference in levels was found when compared to healthy women or those with benign breast disease.
3. Importance of mucins in the diagnosis and prognosis of breast cancer
The differential expression of mucins has been investigated for its potential diagnostic and prognostic relevance in BC and other malignancies. Potential of mucins as diagnostic and prognostic markers in other malignancies has been reviewed by many investigators previously. These studies have been mainly targeted MUC1, although MUC4 and other mucins are being increasingly investigated as potential targets.
3.1. Tissue-based profiling of mucins in the diagnosis or prognosis of breast cancer
Detection of mucins, primarily based on immunohistochemical analysis using specific antibodies, has been shown to correlate with several clinicopathologic characteristics in BC patients. For instance, a significant correlation was observed between the sub-cellular distribution of MUC1 and prognosis in BC patients. Using the Ma695 monoclonal antibody, a luminal staining for MUC1 was positively correlated to the presence of vascular invasion, lymph nodal spread and distant metastasis. On the other hand, presence of both cytoplasmic and membrane staining was associated with a significantly lower survival than cases with luminal staining. Further, positivity for MUC1 (by immunofluorescence), when combined with that for E-cadherin was 80% sensitive and 100% specific in identifying cancer-positive margins in an intra-operative setting. A real-time PCR based assay for MUC1 was 67% sensitive and 82% specific in identifying micro-metastasis in axillary lymph nodes of BC patients. In a study of 200 BC and 81 cases of CIS, MUC2 expression was weak or absent completely from normal breast tissues. In comparison, 100% of mucinous carcinomas, but only 8% of lobular and 19% of ductal carcinomas were positive for the mucin. Breast cancer patients whose tumors were positive for MUC2 had a significantly shorter survival (49 months) compared to those with MUC2 non-expressing tumors (75 months). This study suggests that differential expression of MUC2 could be potentially useful in the prognostication of patients with a suspected diagnosis of BC. MUC6 expression on the other hand appears to improve survival, although it is expressed only in about 20% of BCs. Both MUC3 and MUC4 appear to correlate with prognosis. While the expression of the former was shown to be significantly higher in patients with vascular invasion, nodal metastasis and correlated with the recurrence of BC, MUC4 staining showed a significant positive correlation with higher tumor grade. MUC6, by virtue of its strong expression in atypical fibrocystic disease of the breast (a precursor to invasive ductal carcinoma) appears to hold significant clinical potential as a marker for the early identification of pre-malignant breast lesions. Given the propensity for variations in the number of tandem repeats (termed as variable number of tandem repeat polymorphism or VNTR polymorphism) and the degree of glycosylation, the availability of domain and glycosylation specific antibodies against mucins offers a unique array of reagents for potential use as biomarkers. Prospective, multicenter trials are then needed using the most promising antibodies to investigate whether a combination of mucin markers is able to aid both the early detection and predict prognosis of BC patients.
3.2. Mucins as serum based biomarkers for breast cancer
Mucins have not only been investigated to explore in tissue based diagnostics but also have emerged as novel blood based biomarkers for the detection and prognostication of BC. The tumor marker antigen CA15.3 which corresponds to an immuno-dominant epitope in the extracellular portion of the membrane bound mucin MUC1, is shed into the bloodstream and can be detected with a number of monoclonal antibodies. The epitope is a stretch of seven amino acids (PDTRPAP) that are in turn part of the 20-amino acid tandem repeat sequence of MUC1. The specificity of different anti-MUC1 mAbs for either the core peptide or the carbohydrate side chains has been examined. Several commercial kits are currently available to measure CA15.3 levels and have shown a high degree of specificity in both diagnosing BC and detecting recurrence of the malignancy following treatment (Table 2). CA15.3 (MUC1) levels appear to correlate with stage but not with histologic type of BC. In one study, 6/108 (6%) stage 1, 5/52 (10%) stage 2 and 9/39 (18%) BC patients had elevated serum CA15.3 levels (≥30 U/ml) at diagnosis. During a 12-month follow-up during which serum CA15.3 levels were measured once every 3 months, the levels of CA15.3 declined to normal levels in all the patients except three (one from each stage) who were then diagnosed with metastatic disease. However, CA15.3 (MUC1) levels were not significantly different between patients with ductal, lobular or tubular type of BC. A comparison of the CA15.3 assay with one employing the epithelial membrane core antigen (EMCA, also against MUC1) found that serial measurement of serum MUC1 levels with a threshold of 33 U/ml (prior to start of therapy and then at 2, 4, and 6 months after starting therapy) significantly correlated with progression of the disease (non-progression being defined as having either a complete/partial response or stable disease). The sensitivity and specificity of CA15.3 in detecting progressive disease being 85% and 91% at 2 months, 96% and 96% at 4 months, and 92% and 100% at 6 months respectively. Similar results were also reported by other investigator. In a retrospective analysis, an initial surge (>10% increase from baseline levels) in serum CA15.3 levels in BC patients following chemotherapy was shown to be significantly correlated with the risk of disease progression as measured by a shortened progression free survival (24 months in those with a surge vs. 35 months in those without any surge). Significantly, the surge in CA15.3 levels appeared to be independent of the type of prior therapy (anthracyclines, taxanes, gemcitabine, endocrine-based or trastuzumab) given to the patients.
Table 2. Comparison of 15 commercial immunoassays for detection of CA15.3 (MUC1) in serum [54].
| Antibody assays | Manufacturer | Manufacturer cutoff (U/mL) | FN Group 1 (expected to be LOW) | FP in Group 2 (expected to be HIGH) | FP in Group 3 (expected to be HIGH) | AUC* | Optimum cut-off (U/ml) § |
|---|---|---|---|---|---|---|---|
| Architect CA15-3 | Biomira | 23.4 | 7 | - | 1 | 0.99 9 | 31 |
| Axsym CA 15-3 | CanAg | 38 | - | - | 1 | 0.99 8 | 39 |
| CA15-3 Kryptor | Centocor/Fujirebio | 31.3 | - | 2 | 1 | 1 | 25¥ |
| Advia Centaur CA 15-3 | Centocor/Fujirebio | 25 | 1 | - | - | 1 | 29 |
| Advia Centaur BR CA15-3 | Centocor/Fujirebio | 30 | - | - | - | 1 | 23¥ |
| Acess BR monitor | Centocor/Fujirebio | 30 | - | - | - | 1 | 25.1¥ |
| Vidas CA15-3 | CanAg | 31.3 | - | 6 | 3 | 0.99 4 | 25.6 |
| ELSA CA15-3 | Centocor/Fujirebio | 30 | - | 3 | 2 | 0.99 4 | 24.7 |
| Liaison CA15-3 | Centocor/Fujirebio | 35 | - | - | - | 1 | 31.5¥ |
| CA15-3 IRMA | Centocor/Fujrebio | 30 | - | 2 | - | 0.99 9 | 25 |
| Immulite 2000/2500 BR-MA | Centocor/Fujirebio | 28 | - | 6 | - | 0.98 6 | 25 |
| IRMA MUC1 CA15-3 | CanAg | 35 | - | - | 1 | 1 | 34 |
| Vitros CA 15-3 | Biomira | 38.6 | - | - | 1 | 1 | 30.2¥ |
| Elecys CA 15-3 II | Centocor/Fujirebio | 32.4 | - | 2 | 1 | 1 | 23 |
| AIA Pack 27.29 | Centocor/Fujirebio | 31.3 | - | - | - | 1 | 27¥ |
| Overall | FPR= 1.5% | FNR: 3.1% | FNR: 1.9% |
False negative rate (FNR); False positive rate (FPR); Area under the curve (AUC)
AUC was used as a method to distinguish between patients without recurrence (Group 1) from those with recurrent breast cancer (Groups 2 and 3).
Achieves 100% specificity;
Achieves 100% sensitivity
Group 1: Breast cancer (BC) patients undergoing follow-up after treatment who showed no recurrence during observation for a period of at least 4 years after initial diagnosis.
Group 2: BC patients who had blood sample drawn between 0-90 days before the first diagnosis of metastasis.
Group 3: BC patients who had blood sample drawn ≥90 days after the detection of metastasis.
CA15.3 levels in serum are also elevated in 50%-80% of BC patients with metastatic BC. Using ELISA and normality defined as <25 U/ml, only 1/14 (7%) patients with local recurrence of BC had elevated CA15.3 levels in a single center retrospective study. In comparison, 22/23 (96%) patients with both local recurrence and distant metastasis had elevated circulating CA15.3 levels. In a large study of 3,953 patients with BC followed for detection of disease recurrence (following therapy), 274 of the 784 patients (35%) who had recurrence of the disease had at least one abnormally elevated CA15.3 measurement (>30 U/ml). Further, elevated CA15.3 levels were associated with a 30% higher risk of recurrence during follow-up. The level of CA15.3 (MUC1) in patients with metastatic BC appears to correlate not with the level of HER-2 expression but with the hormone receptor (HR) status of the primary tumor. In a study examining the effect of HR (ER and PR) and HER-2 status on CA15.3 levels in metastatic BC patients, the incidence of CA15.3 positivity was observed to be higher in patients with hormone receptor positive tumors (69% in HR+HER-2- and 56% in HR+HER2+ patients vs. 46% in HR-HER2+ and 41% in HR-HER2- patients). This is supported by the observation that a high level of MUC1 expression in BC tissues correlated positively with the occurrence of axillary node metastasis.
A prospective study comparing the abilities of CA15.3, mucin-like carcinoma associated antigen (MCA), and CEA to predict the onset of metastasis in BC patients observed that CA15.3, at a cut-off of >27 U/ml, could predict metastasis in 36% of BC patients compared to 64% for MCA and 33% for CEA. CA15.3 elevation in the serum appeared to be more sensitive (78%-96% sensitive with a range of 56-140 U/ml) to detect patients with mixed metastasis (both bony and soft tissue metastasis) than those with either an isolated bony or soft tissue metastasis (32%-75% sensitivity for bone metastasis, range: 21-40 U/ml and 47%-83% sensitivity for soft tissue metastasis, range: 22-67 U/ml). While MCA was a better early marker of metastasis than either CA15.3 or CEA, neither marker was very good at predicting disease relapse. Other studies however found no correlation between serum CA15.3 levels and the site of metastasis of BC. In a study of 144 BC patients, 73 of whom were clinically free of the disease at the time of entry into the study, none of the five patients with elevated CA15.3 (cut-off 35 U/ml) relapsed during a follow-up time ranging from 14-18 months.
The levels of CA15.3 (MUC1) also appear to be significantly influenced by the presence of one or more single nucleotide polymorphisms (SNPs) in the MUC1 gene. In a study among Dutch women to investigate the effect of the 568 A/G polymorphism in MUC1 on serum CA15.3 levels, it was observed that women who had the GG genotype (21% of healthy, 24% of benign breast disease and 27% of BC patients) had significantly higher levels of serum CA15.3 compared to those with the AG or AA genotype. These differences were attributed to the variable number of tandem repeats, with patients having the GG genotype having a larger number of repeats (and hence a higher expression of MUC1) than those with other genotypes. These studies raise an important need in the area of mucin based BC diagnosis. In addition to inter-laboratory standardization, establishment of unique cut-offs based on the presence of a given SNP are required to increase the sensitivity of serum CA15.3 (MUC1) in detecting BC.
When taken together, the existing evidence from clinical studies suggests that serum levels of MUC1/CA15.3 correlates with stage of the disease and is positively correlated to the expression of hormone receptors by BC cells. This suggests that serum CA15.3 measurement could be potentially useful to select patients for clinical trials of novel targeted therapies in BC. In addition, tissue staining and subcellular localizations for mucins appears to correlate with survival, an observation that needs to be investigated further, particularly in combination with other modalities like imaging. However, a major drawback of CA15.3 as a biomarker is its false elevations in patients with non-malignant systemic diseases such as liver failure, diabetes and fatty liver. Further, in a proportion of patients, the primary and/or metastatic tumor may not secrete the antigen (MUC1), leading to false negative results. The role of CA15.3 as a biomarker in BC has been extensively reviewed in a recent article.
3.3. Mucins as markers of circulating tumor cells in the blood
The detection of circulating tumor cells (CTCs) in the blood and disseminated tumor cells (DTCs) in the bone marrow have been used to identify micro-metastasis, and therefore, predict prognosis in BC patients (reviewed recently by Ross and Slodkowska). A study aimed at examining the mucin expression profile of DTCs in the bone marrow of preoperative BC patients showed that MUC2, MUC3, MUC5B, MUC6, and MUC7 were detectable in at least one bone marrow specimen (1/11 BC patients positive for MUC2 and MUC3, 7/15 for MUC5B, 3/14 for MUC6, and 5/12 for MUC7). MUC5B was the most discriminating mucin marker, distinguishing between healthy and cancer patients with a sensitivity of 47% and specificity of 100%. Although MUC7 is as sensitive as MUC5B (42%), it lacks specificity (77%). Based on the results in the bone marrow, a nested RT-PCR assay was designed to detect MUC5B transcripts in the peripheral blood of BC patients. This test was moderately sensitive (52%) but highly specific (100%), suggesting it could be used clinically to identify the appearance of DTCs in BC patients. When applied to the bone marrow samples of BC patients collected at the time of surgery, however, nested PCR for MUC5B was as sensitive (19.5%) as that for carcino-embryonic antigen (CEA, 17%) and inferior to PCR for CK19 (41% sensitivity) in identifying DTCs. While the diagnostic potential of MUC5B remains to be examined further, the study uncovered that MUC5B expression in the bone marrow positively correlates with the size of the tumor and stage (but not the nodal status). These results suggest a role for the mucin in the metastasis of BC cells. The expression of membrane-bound and secreted mucins in normal, pre-malignant, and various malignant breast tumor tissues are summarized in Table 3 and Table 4, respectively. Immunomagnetic separation using one or more anti-MUC1 antibodies is now commonly used to isolate CTCs (or DTCs) from the peripheral blood of BC patients. In one study, PCR analysis of isolated CTCs revealed that 58% of BC patients with detectable DTCs were positive for MUC1 expression. Further, the percentage of MUC1-positive DTCs increased progressively with increasing stage of the disease (0%, 6%, and 33% positive cases in stage 1, 2, and 3 BC, respectively), nodal involvement (7%, 16%, and 33% positivity in patients without nodal involvement, or with N1 and N2 disease, respectively) and metastasis.
4. Mucin mediated cellular signaling events in breast cancer
In addition to the emerging role in the diagnosis and prognosis of BC, mucins have been demonstrated to be involved in several signaling pathways in malignant cells. Among all the cell surface-associated mucins, MUC1 is the best characterized with respect to its role in signal transduction in BC cells. Overexpression of MUC1 in BC cells has been demonstrated to block cell death in response to oxidative stress, DNA damage, and hypoxia and induce anchorage-independent growth and tumorigenicity.
The migration of tumor cells to the site of metastases depends upon its interaction with the vascular endothelium. Intercellular adhesion molecule 1 (ICAM-1), a protein that is expressed on endothelial cells and leucocytes, plays a major role in stabilizing the interaction of BC cells with the vascular endothelium. ICAM-1 present on endothelial cells can bind to MUC1 on the surface of tumor cells. This, in turn, activates a signaling cascade mediated via the MUC1 cytoplasmic tail (MUC1-CT) and includes calcium dependent signaling, involving Src kinase, phosphoinositol 3-kinase (PI-3K), phospholipase C (PLC), and lipid rafts (Figure 2A). Due to this, ICAM-1 has been proposed as a transmembrane ligand for MUC1.
Figure 2. Mucin mediated cellular signaling events in breast cancer. MUC1-ICAM1 interaction.
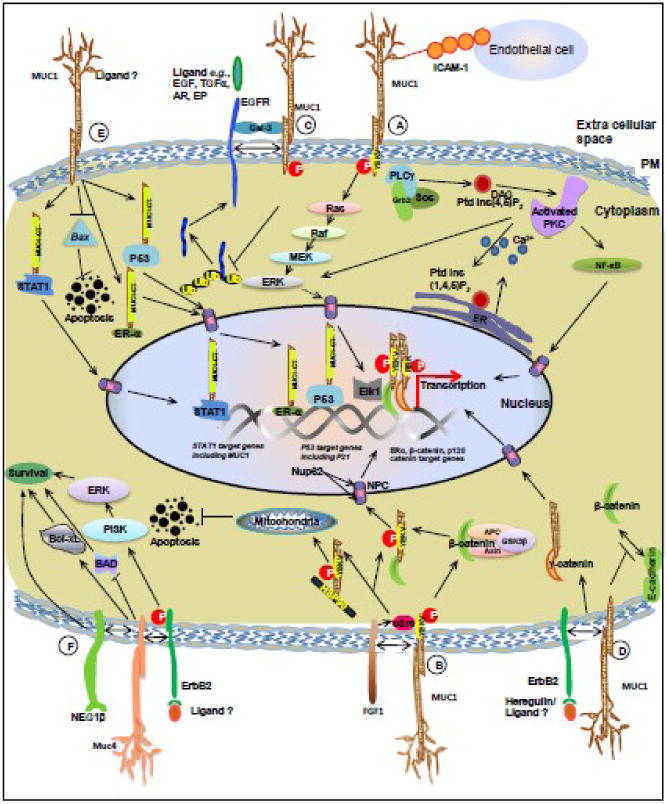
MUC1–ICAM-1 interactions regulate different heterotypic cell– cell adhesions. Phosphorylated MUC1-CT can contribute to Grb2–Sos-mediated activation of the Ras–ERK pathway or activation of phospholipase Cγ (PLCγ)-mediated signaling events. (B) MUC1-FGF1 interaction: FGF1 induces phosphorylation of the MUC1-CT on the YEKV motif via activation of the non-receptor tyrosine kinase c-Src which results in nuclear translocation of MUC1-CT to drive the transcription of β-catenin, estrogen receptor α (ERα) and p53 target genes. The MUC1-CT–HSP70/HSP90 interaction facilitates translocation of MUC1-CT to mitochondria, and attenuates activation of the intrinsic apoptotic pathway in the response to stress. (C) MUC1-EGFR interaction: MUC1 binds to ErbB1 (EGFR) following activation by EGF. This interaction is bridged by the secreted glycoprotein galectin-3. The interaction (between MUC1 and EGFR) inhibits EGF-stimulated ubiquitination and internalization of ErbB1, thus driving EGF-mediated downstream signaling events. (D) MUC1-HER2 interaction: Stimulation of BC cells with heregulin (HRG), a soluble ligand for HER2, leads to phosphphorylation of the MUC1-CT (at the YEKV motif). The phosphorylated MUC1-CT interacts with molecules such as γ-catenin and p120 catenin, following which the complex is translocated into the nucleus by Nup62, a nucleoprotein located on the nuclear pore. (E) Nuclear localization of MUC1-CT: The MUC1-CT interacts with the nucleoprotein Nup62, an interaction that regulates the transport of the MUC1-CT into the nucleus. In the nucleus, the MUC1-CT interacts with and regulates transcription of ERα, β-catenin, p120 catenin, STAT1 and p53 target genes. (F) MUC4 mediated cell signaling in breast cancer cells: MUC4 can bind to the receptor tyrosine kinase HER2 and alter its cellular signaling by activation of the PI3-kinase and Erk pathways. MUC4 induced phosphorylation of HER2 leads to the inactivation of pro-apoptotic proteins such as Bad and an increase in expression of pro-survival proteins like Bcl-xL. (Abbreviations: Gal-3-Galectin -3, NPC-Nuclear Pore Complex, MUC1-mucin 1, MUC4-mucin 4, DAG-diacylglycerol, ER- endoplasmic reticulum, NF-kB- nuclear factor kB; PKC-protein kinase C, PM- plasma membrane, EGF-Epidermal Growth Factor, TGFβ-Transforming Growth Factor β, AR-Amphiregulin, EP-Epiregulin).
MUC1 has been shown to bind with fibroblast growth factor receptor (FGFR). FGF, a growth factor involved in angiogenesis, development and wound repair induces phosphorylation of the MUC1 cytoplasmic tail (MUC1-CT) on a YEKV motif via activation of the non-receptor tyrosine kinase c-Src. This results the MUC-CT being transported into the nucleus where it drives the transcription of β-catenin, estrogen receptor α (ERα) and p53 target genes. The phosphorylated MUC1-CT also interacts with the molecular chaperone Hsp90 to be delivered to the mitochondria. These signals, in turn, prevent activation of the intrinsic apoptosis pathways in time of stress, and thus contribute to the survival of BC cells (Figure 2B).
MUC1 has also been shown to bind with ErbB1 (EGFR/HER1) on the cell surface following activation by its ligand epidermal growth factor (EGF). This interaction, in turn, inhibits EGF-stimulated ubiquitination and internalization of ErbB1, thus allowing prolonged activation of EGF-mediated downstream signaling events (Figure 2C). Galectin-3, a secreted glycoprotein interacts with the MUC1-CT (at Asn-36) and through this interaction, acts as a bridge to stabilize the MUC1-ErbB1 interaction. The N-glycosylated MUC1-CT also helps to stabilize the galectin-3 mRNA levels by suppressing the expression of micro RNA miR-322.
Transforming growth factor α (TGF α), through its binding to and activation of EGFR, is another potent inducer of cellular transformation. Mouse Muc1 has been shown to modulate TGFα-dependent BC progression in the WAP-TGFα transgenic animals, when it crossed with Muc1-/-. The WAP-TGFα/Muc1+/+ transgenic mice had a significantly higher incidence (100%) of mammary tumors when compared to Muc1-null (WAP-TGFα/Muc1-/-) (37%) after one year. Further analysis revealed that the activation of cyclin D1 was significantly suppressed in tumors derived from the Muc1 null transgenic animals (compared to the Muc1 expressing mice), suggesting a potential mechanism underlying Muc1 driven breast tumorigenesis. In another study, the down regulation of MUC1 significantly decreased the interaction between the nucleus-localized EGFR and the cyclin D1 promoter followed by a significant down regulation of cyclin D1 protein expression. These studies suggest that MUC1 could modulate BC cell proliferation through regulating EGF mediated cyclin D1 transcription.
Cleavage of a single polypeptide generates the two subunits of MUC1, an N-terminal mucin subunit (MUC1-N) and a C-terminal cytoplasmic tail (MUC1-CT). Shedding of the MUC1-N subunit leaves MUC1-CT to transduce intracellular signals that confer cellular growth and survival. The MUC1-CT is translocated to the nucleus, where it interacts with estrogen receptor α (ERα) and activates downstream target genes. Importantly, the MUC1-CT activates the expression of genes, predicting not only a response to tamoxifen (estrogen receptor antagonist), but also the overall survival of BC patients. The set of genes induced by the MUC1-CT in BC cells has been termed the “MUC1 tumorigenesis signature” and include ADA, ASPM, BUB1B, CDC20, CENPE, CST3, CTSC, DHCR7, ECT2, FADS1, FAM64A, FDPS, GBP2, IDI1, IFI44L, IMPA1, ISG15, KIF20A, MKI67, MTHFD1, NET1, NSDHL, PGD, PSAT1, RNASE4, RRM2, SIDT2, SLIT2, SOAT1, SQLE, STAT1, TFRC, UBD, UBL3, and VCAM1 (the names correspond to their Entrez gene IDs).
MUC1 has also been shown to be involved in HER2 (also known as ErbB2) mediated signaling events. Stimulation of BC cells with heregulin (HRG) leads to phosphphorylation of the MUC1-CT (at the YEKV motif). The phosphorylated MUC1-CT then interacts with molecules such as γ-catenin and p120 catenin, following which the complex is transported into the nucleus by Nup62, a nucleoprotein located on the nuclear pore. Within the nucleus, MUC1-CT interacts with the transcription factors ERα, β-catenin, p120 catenin, STAT1 (Signal Transducer and Activator of Transcription 1) and p53 and drives the transcription of several genes that promote tumor cell growth and invasiveness (Figure 2D).
Recent studies have shown that the MUC1-CT associates with STAT1 in response to interferon γ in non-malignant epithelial cells, and these two proteins interact constitutively in BC cells (Figure 2E). This MUC1-STAT1 interaction, in turn, activates STAT1 target genes, including MUC1. A correlative study of MUC1 and STAT1 expression in tumor sections showed that patients whose tumors co-expressed both markers had a significantly higher risk of recurrence and death. This correlation was independent of ER status and size of the primary tumor. However, BC patients with grade 2 and 3 tumors that co-expressed MUC1 and STAT1 had a significantly higher risk of death compared to those that expressed only one antigen, suggesting the synergistic effect of the two proteins in promoting BC aggressiveness.
β-catenin is an oncogenic protein that contributes to the metastasis of several types of malignant epithelial cells. MUC1 interacts with β-catenin and results in its redistribution to the margin of invading cells, in turn leading to increased invasiveness of the tumor cells. The expression of β-catenin is tightly regulated by several proteins including the Adenomatous polyposis coli (APC). APC is a tumor suppressor gene whose expression is downregulated through promoter methylation or loss of heterozygosity in nearly 25-60% of primary breast tumors. EGF stimulation increases the interaction of MUC1 with APC and promotes β-catenin mediated gene transcription. MUC1 also associates with p53, a tumor suppressor that is often inactivated in BC. Specifically, the MUC1-CT binds directly to the regulatory domain of p53 and co-activates the transcription of genes that promote p53-dependent growth arrest.
MUC4 is a transmembrane mucin (like MUC1) and frequently displays an altered expression in many cancers. It has been proposed to act as an anti-adhesive barrier on the surface of epithelial tumor cells. Muc4 also can bind to the receptor tyrosine kinase HER2 and alter its cellular signaling (Figure 2F). Sialomucin complex (SMC), the rat homologue of human MUC4, has been demonstrated to mask the surface antigens on target tumor cells, and thus suppresses tumor cell killing by cytotoxic lymphocytes. SMC and MUC4 have both been demonstrated to interact with HER2, although whether the interaction is direct or through an intermediate adaptor protein is unknown. Muc4 expression promotes HER2 and HER3 translocation to the cell surface and thereby augment the number of available receptors for signaling through the phosphoinositol 3 kinase (PI3K) (Figure 2). The PI3K pathway is one of the important regulators of cell proliferation and survival in BC development, and it has been showed that PI3K is essential for HER2/HER3 mediated breast tumor cell proliferation. Aberrant expression of Muc4 in almost all cancer cell lines induces the phosphorylation of HER2 and subsequent inactivation of the pro-apoptotic proteins such as Bad and increased expression of pro-survival proteins like Bcl-xL.
Studies in BC and other malignancies suggest that mucins play a pivotal role in several aspects of the behavior of a cancer cell. Most studies in BC have focused on the role of MUC1 and only recently the roles of other mucins are being uncovered. Future studies are expected to accumulate more information regarding the functional significance of these glycoproteins in the initiation, progression and metastasis of BC.
5. Importance of mucins in the therapy of breast cancer
MUC1 has been the most widely targeted mucin for the therapy of BC. Several approaches, including vaccination, gene therapy, immunotherapy, radio-immunotherapy, and conjugation with immunotoxins, have been used with limited success (summarized in Table 5). Recently, antibodies developed against tumor-specific variants of MUC1 (e.g., the 12ESC-6 mAb), and against other mucins including MUC4 and MUC16 (cytoplasmic tail), have opened avenues for targeting multiple mucin epitopes simultaneously for a better anti-tumor effect. Several research groups have successfully used the MUC1 promoter in transcriptional targeting strategies of various cancers, including BC. The purpose of using this MUC1 promoter is to deliver and express imaging reporter genes specifically in breast tumor metastases in living subjects.
Table 5. Mucin based therapeutic strategies for breast cancer.
| Ref. | Nature of therapeutic strategy | Name of vaccine | Mucin targeted | Other immunogens | Vector | No. of patients | Vaccination schedule | Response (against mucin antigen) | Adverse effects |
|---|---|---|---|---|---|---|---|---|---|
| [108] | Vaccine | PAN VAC | MUC1 | 1. CEA 2. T-cell co-stimulatory molecules (B7.1, ICAM-1 and LFAA-3)† |
1. Vaccini a virus (Wyeth strain) – primary immunization 2. Replication deficient avian pox virus-booster doses |
25 (8 female, 17 male). 2 breast cancer. | Single dose of 1° vaccination (s.c.) followed by boosters on day 15,29,43. Then boosters q28 days till patient was on the study | 1. Immune response: 4/14 patients showed production of MUC1 specific T-cells post-vaccination 2. Clinical response: 21% reduction in unidimensional sum of tumor size (according to RECIST criteria) associated with immune response to MUC1 and CEA. Ultimately CA27.29 (TM) returned to above baseline levels. This patient had improved response to post-vaccination chemotherapy ‡ for 15 months associated with marked reduction in CA27.29 levels. |
Apart from injection site reaction, grade ≥2 toxicity was seen in <3% patients |
| [107] | Vaccine | N/A | MUC1 | KLH (conjugated to MUC1) and QS-21ψ | N/A | Nine breast cancer patients with no evidence of disease (NED) at time of entry into study | 5 s.c. doses of MUC1-KLH+QS-21 given at weeks 1,2,3,7 and 19 | 1. ↑ in IgG (mainly IgG1 and G3) in all patients post-vaccination. 7/9 patients had significant ↑ in IgM levels that bound to MCF-7 cells. In contrast, only 3/9 patients had showed a positive binding of IgG to MCF7 cells.2. 2/9 patients developed recurrence during vaccination. Since all patients were NED at start of study, tumor response was not the end point. | 1. Most common: skin reaction (at injection site). 2. Other reactions: fever, myalgi as, NVD€, fatigue and headache |
| [109] | Vaccine | Theratrope ™ (Biomira Corp., Edmonton, Canada) | Underglycosylated, mucin associated glycoprotein STn | None (patients also received concomitant hormone therapye) | None | None (in vitro assay of mechanism underlying response to vaccine) | N/A | 1. ER+STn+MUC1+ (but not ER-STn +MUC1+ or ER+STn-MUC1+) cells showed significant increase in monocyte mediated ADCC when treated with anti-STn or anti-MUC1 antibodies in presence of the aromatase inhibitor. 2. In absence of antibodies, formestan inhibited monocyte mediated ADCC |
None |
| [110] | Vaccine | N/A | MUC1 VNTR | GM-CSF€ | pUC plasmid | None (SCID mice inoculated with human PBLs) | Two i.p. inections on day 7 and day 21 after MCF-7 injection s.c. | 1. Incidence of tumors in animals injected with rBCG-MVNTR4-CSF (25% on day 35, 63% on day 70) or rBCG-MVNT8-CSF (25% on day 35, 38% on day 70) was significantly lower than that in control mice (100%) e 2. Significant induction of MUC1 specific CTL response as measured by Ellispot test in mice receiving the MUC1 vaccine. |
None |
| [112] | Gene therapy | N/A | MUC1 (DF3) | Replication deficient adenovirus (adenovirus 5) | None (cell lines: MCF7, ZR-75-1, BT-20, MDA-MB231) and athymicnude mice | N/A | 1. β-galactosidase expression, driven by the DF3 promoter was noted only in the MUC1 positive cells. 2. Cells infected with adenovirus containing HSV-tka enzyme under the DF3 promoter showed significant cell death upon treatment with ganciclovir 3. Mice injected with MCF-7 cells intraperitoneally followed by Ad-DF3-tk/Ganciclovir i.p. showed significant reduction in the size of tumors and incidence of ascites. |
None. However, when the adenoviruses (Ad-DF3-tk) were given i.v., no therapeutic effect was evident. | |
| [111] | Gene therapy | N/A | MUC-1 | None (cells were co-infected with retrovir uses encoding various ILs like IL-2, IL-4, IL-12, and IFN-γ) | Retrovirus (TFG-mIL-12) | None (conducted in MUC-1 transgenic mice using mouse mammary adenoca rcinoma cell line (410.4) in aBALB/c background) | N/A | 1. The cells that had been modified to secrete IL-12d had significantly lower tumorigenecity (compared to cells expressing either only MUC-1 or MUC-1 + IL-2/IL-4/IFN-γ). 2. T-cell mediated cytotoxicity developed in the transgenic mice which had rejected IL-12 secreting 410.4 cells, associated with immunity to development of breast cancer (upon subsequent injection of MUC-1 expressing 410.4 cells): Potential for autologous therapy in breast cancer patients. |
None |
| [114] | RIT* | N/A | Anti-mucin mAb (2G3) labeled with I131 MUC1 | None | N/A | 11 | 1. 3/9 patients receiving escalated doses (>50 mCi) showed a partial response (reduction in ascites). 2. Drawback: Specific uptake of the radiolabeled antibody by the tumor was seen only in 2/5 patients who were biopsied (suggests that the antitumor effect was mostly due to retention of the radioactivity within the peritoneum) |
Not significant | |
| [113] | Immunotherapy | N/A | (DF3 and its bispecific antibody DF3×H2 2) | None | N/A | None (cell lines: ZR75-1) | N/A | 1. ADCP, but not ADCCb was observed with both DF3 and DF3xH22 (more with the former mAb). 2. ADCP was inhibited by ↑ by IFN-γ than GM-CSFc |
N/A |
| [115] | Immunotoxin | BM7-PE | MUC-1 (mAb BM7) | BM7 conjugated to | N/A | None (nude rats); MT-1 and MA-11 human breast cancer cell lines | N/A | 1. In vitro: IC-50 for BM7 was 4-25 ng/ml. 2. In vivo: Treatment with BM7-PE significantly ↑ symptom-free survival to 41.3 days when given from day 1 (following injection of tumor cells intracardiac) but produced no significant benefit when given 7 days after initial injection (control rats had mean symptom free survival of 25.8 days) 3. In vivo: 80μg (but not 40μg) BM7-PE prevented tumor formation in 3/5 rats (p=0.003) when administered 7 days after intratibial injection of tumor cells. |
The response to BM7-PE appeared to be dependent on the express ion of MUC1 by the tumor cells. |
Granulocyte Monocyte Colony Stimulating factor (GM-CSF)
Intercellular adhesion molecule-1 (ICAM-1), lymphocyte function associated-antigen 3 (LFAA-3)
paclitaxel and bevacizumab,
Pseudomonal exotoxin A
Key hole limpet is an immunogenic protein isolated from the blood of the organism by the same name while QS-21 is obtained from the bark of Quillaja saponaria, native to South America.
nausea, vomiting and diarrhea
Radioimmunotherapy (RIT)
HSV-tk is an enzyme that phosphorylates and activates Ganciclovir. The drug, which is non-toxc to mammalian cells, becomes toxic when activated by HSV-tk
Antibody dependent cell phagocytosis (ADCP) and antibody dependent cell cytotoxicity (ADCC)
granulocyte-monocyte colony stimulating factor (GM-CSF)
IL-12 is a potent T-cell stimulating cytokine that induces secretion of IFN-γ and TNF-α from the T-cells. It also promotes development of Th1 CD4+ cells (involved in cell mediated immune response) and tumor infiltrating lymphocytes
Aromatase inhibitor Formestane
Bacillus Calmette Guerin (BCG) bacilli transformed with a plasmid encoding the VNTR region 4 or 8 of MUC1 in frame with GM-CSF.
Trastuzumab (Herceptin™), a monoclonal antibody targeting the extracellular portion of the HER-2 receptor is one of the drugs at the forefront of HER-2 positive BC. Recent reports indicate that both MUC1 and MUC4 can confer resistance to trastuzumab treatment in BC cells. Similarly, overexpression of mucins has recently been demonstrated to be associated with the resistance of cancer cells to chemotherapeutic drugs. While these findings need to be confirmed and the underlying mechanisms need to be elucidated, these studies nonetheless affirm the importance of mucin expression as important determinants of response of cancer cells to chemotherapy.
Immunotherapy, particularly against MUC1 has been extensively explored for possible application in the treatment of BC. Their failure however to translate pre-clinical success into clinical efficacy in human patients has cast doubts on the practical application of these approaches. The limited success of immunotherapy against mucin antigens can be attributed to several potential mechanisms. The immunogen used for many of these studies (using MUC1) is the tandem repeat peptide, a stretch of 20 amino acids. This region, which is highly glycosylated in normal breast epithelial cells is under-glycosylated in tumors and hence an attractive target for therapy. However, tumors are heterogenous in the extent of glycosylation which might account for the variable response to antibodies raised against the TR region. A second possibility is that the TR region in tumors may interact with other molecules and such interactions can potentially block its interaction with the anti-MUC1 antibodies. The small size of the TR is another limiting factor as it results in the generation of fewer immunogenic epitopes and further, some of the haplotypes of MHC molecules generated against these epitopes have been suggested to be incapable of mounting an effective immune response. Strategies relying on generation of dendritic cells (DCs: antigen presenting cells that process and present antigens to the T-cells) specific for the tumor antigen have also suffered from several difficulties including inadequate loading of antigens onto DCs, specific targeting DCs to sites of tumor spread and ensuring their survival long enough (after pulsing with antigen) to mediate an immune response. Protein transduction domains (PTDs) also called cell permeable peptides or membrane translocating sequences (MTS) are small peptides capable of transporting much larger molecules across the cell membrane through a mechanism independent of the classical endocytosis pathway. PTDs such as the tat protein of human immunodeficiency virus (HIV) have been demonstrated to efficiently transport immunogens (like MUC1) into DCs. Further, tat conjugated peptides have been shown to allow the efficient processing and presentation of the antigen by DCs to the helper and cytotoxic T-cells (via MHC class-II and class-I molecules respectively). The N-terminal region of MUC1 (amino acids 2-147) fused to the PTD domain of the HIV tat protein was shown to induce a more effective Th1 response in vivo (assessed by induction of interferon-gamma and tumor necrosis factor-alpha) and cytotoxic T-cell response in vitro compared to the MUC-1 N-terminal peptide alone (minus tat fusion). Significantly, administration of dendritic cells pulsed with the MUC1 N-terminal tat fusion significantly delayed the development of breast tumors in a spontaneous mouse model of BC (MUC1/PyMT double transgenic mouse) compared to animals who received DCs pulsed with the MUC1 N-terminal peptide alone. This study, although in the preclinical stage, but points toward the exciting possibilities for immunotherapy of MUC1 expressing BCs.
6. Conclusions and perspectives
Breast cancer is the second leading cause of cancer deaths and is a problem worldwide. Although efforts are being made to identify factors those are responsible for its aggressiveness of this disease but the exact succession of molecular events underlying the development of this devastating disease has remained unclear. Mucins have emerged as important molecules in the progression and metastasis of BC. Changes in their expression, glycosylation and presence of multiple splice variants are currently under active investigation to better understand their role in BC pathogenesis. Current and emerging evidences suggest that mucins are differentially expressed during progression and metastasis of BC, and thus, could be extremely useful in either early detection or predicting prognosis or both of BC patients. Recent developments in molecular biology have used mucins as molecular beacons to identify occult sites of micrometastasis. For instance, an adenovirus mediated tumor targeting system has recently been developed that employs the firefly luciferase gene driven by the MUC1 promoter. The luciferase gene permits imaging of the target cells while MUC1 targets the cancer cells that express a high level of the mucin. Pre-clinical studies with this bioluminescent probe in mice have revealed that it is highly specific in detecting experimentally induced lymph node and hepatic metastasis in BC. As the current strategies for identifying nodal, particularly sentinel node spread of the cancer (a “sentinel node” is defined as the first lymph node that drains lymph from the site of malignancy) rely on injection of lymphotropic dyes or radiolabelled colloids into the tumor and are thus quite invasive, the development of molecular probes using mucins as targets could offer sensitive, minimally invasive alternatives to accurate staging and early detection of micrometastasis. However, both these techniques are quite invasive and have the potential for complications for the patient
Furthermore, mucins particularly the MUC1, due to its differential expression, has emerged as promising target for vaccine development and targeted therapy for BC, Recent studies have unraveled that in addition to serving as biophysical barriers involved in protection and lubrication of epithelial surfaces, mucins are active partners in cellular signaling, important regulators of gene expression, and also determinants of drug resistance in BC. In order to mediate such diverse biological processes, mucins interact with a multitude of proteins and such interactions could be potential targets for a new wave of therapeutic intervention (reviewed recently in). A significant first step in this direction is the development of a cell-permeable peptide inhibitor G0-201 that blocks MUC1-CT oligomerization. In BC cells this inhibitor was demonstrated to block MUC1-CT nuclear localization, induce growth arrest and cell death in vitro, and inhibit tumorigenicity in vivo. It is conceivable that therapies targeting such oncogenic interactions of mucins with other molecules will become available in the near future. Information regarding the molecular structure of mucins is currently nonexistent. With the realization of the involvement of various mucin domains in diverse biological processes, future studies should focus on elucidating the structure of mucins for understanding the interaction of mucins with other proteins at molecular level. Such information will be of prime importance for rational design of drugs (or /inhibitors) targeting the interaction of mucins.
Cancer stem cells (reviewed recently in) are thought to be responsible for not only the initiation of cancer but also mediate disease aggressiveness, metastasis and promote resistance to chemotherapy. Recently, MUC1 was shown to be expressed in mammary stem/progenitor cells. This reveals a potentially novel mechanism underlying the role of mucins in modulating aggressiveness of cancer in general and BC in particular. MUC1 could be explored, in future, as a specific therapeutic target against mammary cancer stem cells for tumor relapse.
MicroRNAs comprise a group of gene repressors that work by the process of posttranscriptional repression. They have emerged as novel modulators of tumor initiation and progression. Growing evidences indicate that microRNAs play a fundamental role in BC progression including modulating cell proliferation, differentiation, apoptosis and influence treatment relevant characteristics including chemoresistance. In cancer, microRNAs may function both as oncogenes and tumor suppressors, hence often termed as ‘oncomiRs’. The micro RNA mir-1226 was shown in a recent study to downregulate the expression of MUC1 and thereby induce cell death, while miR-145, whose expression is down regulated in tumor tissues has been demonstrated to inhibit tumor cell growth and invasion by targeting MUC1. Further, miR-125b has also been shown to suppress translation of the MUC1 oncoprotein and in this way might function as a tumor suppressor in BC. Interestingly mucins can also regulate the expression of microRNAs. The MUC1-CT was found to suppress the expression of the microRNA miR-322 which led to the stabilization of galectin-3 mRNA levels These recent advances in the regulation of mucins by micro RNAs and some microRNAs by mucins add a new layer of complexity to mucin biology. The role of miRNAs in regulating other mucins and resulting biologic significance remains a question to be elucidated in future studies.
Acknowledgments
The authors on this work are supported by grants from the Department of Defense (BC074639, BC083295, and BC09742), the National Institutes of Health (RO1 CA78590, EDRN UO1 CA111294, RO1 CA133774, RO1 CA131944, P50 CA127297 and RO3 CA139285) and the Susan Komen Foundation (KG070826). We also thank Drs. S. Kaur, V.S Gnanapragassam, S. Das, and also D. Haridas for critical reading and suggestions on this review article.
Abbreviations
- BC
breast cancer
- UEH
usual epithelial hyperplasia (also called ductal hyperplasia DH)
- ADH
atypical ductal hyperplasia
- CIS
carcinoma in situ
- SRCC
signet ring cell carcinoma
- FGF
fibroblast growth factor
- EGFR
epidermal growth factor receptor (also called ErbB1)
- MUC1-CT
MUC1 cytoplasmic tail
- EGF
epidermal growth factor
- PTD
Protein transduction domains
- MTS
membrane translocating sequences
- MCA
mucin-like carcinoma associated antigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010 doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Simpson PT, Reis-Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol. 2005;205:248–254. doi: 10.1002/path.1691. [DOI] [PubMed] [Google Scholar]
- 3.Callagy G, Cattaneo E, Daigo Y, Happerfield L, Bobrow LG, Pharoah PD, Caldas C. Molecular classification of breast carcinomas using tissue microarrays. Diagn Mol Pathol. 2003;12:27–34. doi: 10.1097/00019606-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Cianfrocca M, Gradishar W. New molecular classifications of breast cancer. CA Cancer J Clin. 2009;59:303–313. doi: 10.3322/caac.20029. [DOI] [PubMed] [Google Scholar]
- 5.Jonsson G, Staaf J, Vallon-Christersson J, Ringner M, Holm K, Hegardt C, Gunnarsson H, Fagerholm R, Strand C, Agnarsson BA, Kilpivaara O, Luts L, Heikkila P, Aittomaki K, Blomqvist C, Loman N, Malmstrom P, Olsson H, Johannsson OT, Arason A, Nevanlinna H, Barkardottir RB, Borg A. Genomic subtypes of breast cancer identified by array comparative genomic hybridization display distinct molecular and clinical characteristics. Breast Cancer Res. 2010;12:R42. doi: 10.1186/bcr2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de RM, Perou CM. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 7.Perou CM, Sorlie T, Eisen MB, van de RM, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 8.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de RM, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein LP, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinder SE, Ellis IO. The diagnosis and management of pre-invasive breast disease: ductal carcinoma in situ (DCIS) and atypical ductal hyperplasia (ADH)--current definitions and classification. Breast Cancer Res. 2003;5:254–257. doi: 10.1186/bcr623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrianifahanana M, Moniaux N, Schmied BM, Ringel J, Friess H, Hollingsworth MA, Buchler MW, Aubert JP, Batra SK. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033–4040. [PubMed] [Google Scholar]
- 12.Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta. 2006;1765:189–222. doi: 10.1016/j.bbcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 14.Senapati S, Das S, Batra SK. Mucin-interacting proteins: from function to therapeutics. Trends Biochem Sci. 2010;35:236–245. doi: 10.1016/j.tibs.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 17.Fowler J, Vinall L, Swallow D. Polymorphism of the human muc genes. Front Biosci. 2001;6:D1207–D1215. doi: 10.2741/A674. [DOI] [PubMed] [Google Scholar]
- 18.Diaz LK, Wiley EL, Morrow M. Expression of epithelial mucins Muc1, Muc2, and Muc3 in ductal carcinoma in situ of the breast. Breast J. 2001;7:40–45. doi: 10.1046/j.1524-4741.2001.007001040.x. [DOI] [PubMed] [Google Scholar]
- 19.Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29:2893–2904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira MB, Dias AJ, Reis CA, Schmitt FC. Immunohistochemical study of the expression of MUC5AC and MUC6 in breast carcinomas and adjacent breast tissues. J Clin Pathol. 2001;54:210–213. doi: 10.1136/jcp.54.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Workman HC, Miller JK, Ingalla EQ, Kaur RP, Yamamoto DI, Beckett LA, Young LJ, Cardiff RD, Borowsky AD, Carraway KL, Sweeney C, Carraway KL., III The membrane mucin MUC4 is elevated in breast tumor lymph node metastases relative to matched primary tumors and confers aggressive properties to breast cancer cells. Breast Cancer Res. 2009;11:R70. doi: 10.1186/bcr2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de B C, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995;109:723–734. doi: 10.1016/0016-5085(95)90379-8. [DOI] [PubMed] [Google Scholar]
- 24.Sonora C, Mazal D, Berois N, Buisine MP, Ubillos L, Varangot M, Barrios E, Carzoglio J, Aubert JP, Osinaga E. Immunohistochemical analysis of MUC5B apomucin expression in breast cancer and non-malignant breast tissues. J Histochem Cytochem. 2006;54:289–299. doi: 10.1369/jhc.5A6763.2005. [DOI] [PubMed] [Google Scholar]
- 25.Blair SL, Wang-Rodriguez J, Cortes-Mateos MJ, Messmer D, Sandoval S, Messmer B, Trogler W, Kummel A. Enhanced touch preps improve the ease of interpretation of intraoperative breast cancer margins. Am Surg. 2007;73:973–976. [PubMed] [Google Scholar]
- 26.Walsh MD, McGuckin MA, Devine PL, Hohn BG, Wright RG. Expression of MUC2 epithelial mucin in breast carcinoma. J Clin Pathol. 1993;46:922–925. doi: 10.1136/jcp.46.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terada T. Ductal adenoma of the breast: immunohistochemistry of two cases. Pathol Int. 2008;58:801–805. doi: 10.1111/j.1440-1827.2008.02315.x. [DOI] [PubMed] [Google Scholar]
- 28.Demichelis SO, Alberdi CG, Servi WJ, Isla-Larrain MT, Segal-Eiras A, Croce MV. Comparative immunohistochemical study of MUC1 and carbohydrate antigens in breast benign disease and normal mammary gland. Appl Immunohistochem Mol Morphol. 2010;18:41–50. doi: 10.1097/PAI.0b013e3181ac1c20. [DOI] [PubMed] [Google Scholar]
- 29.Gendler SJ, Spicer AP, Lalani EN, Duhig T, Peat N, Burchell J, Pemberton L, Boshell M, Taylor-Papadimitriou J. Structure and biology of a carcinoma-associated mucin, MUC1. Am Rev Respir Dis. 1991;144:S42–S47. doi: 10.1164/ajrccm/144.3_pt_2.S42. [DOI] [PubMed] [Google Scholar]
- 30.Hanisch FG, Stadie TR, Deutzmann F, Peter-Katalinic J. MUC1 glycoforms in breast cancer--cell line T47D as a model for carcinoma-associated alterations of 0-glycosylation. Eur J Biochem. 1996;236:318–327. doi: 10.1111/j.1432-1033.1996.00318.x. [DOI] [PubMed] [Google Scholar]
- 31.de BC, Guma M, Barranco C, Garrido M, Kim YS, Real FX. MUC6 expression in breast tissues and cultured cells: abnormal expression in tumors and regulation by steroid hormones. Int J Cancer. 1998;77:193–199. doi: 10.1002/(sici)1097-0215(19980717)77:2<193::aid-ijc4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 32.Borg A, Zhang QX, Olsson H, Wenngren E. Chromosome 1 alterations in breast cancer: allelic loss on 1p and 1q is related to lymphogenic metastases and poor prognosis. Genes Chromosomes Cancer. 1992;5:311–320. doi: 10.1002/gcc.2870050406. [DOI] [PubMed] [Google Scholar]
- 33.Cannone M, Oliveri C, Roz E, Rispoli F, Ferrarese S, Alexiadis S, Barberis MC. Molecular markers of breast cancer cells identified in fine needle aspiration samples from resected sentinel lymph nodes. Acta Cytol. 2006;50:271–276. doi: 10.1159/000325953. [DOI] [PubMed] [Google Scholar]
- 34.Dian D, Janni W, Kuhn C, Mayr D, Karsten U, Mylonas I, Friese K, Jeschke U. Evaluation of a novel anti-mucin 1 (MUC1) antibody (PankoMab) as a potential diagnostic tool in human ductal breast cancer; comparison with two established antibodies. Onkologie. 2009;32:238–244. doi: 10.1159/000209280. [DOI] [PubMed] [Google Scholar]
- 35.Ding HY, Gao LX. Spindle cell carcinoma of breast with neuroendocrine differentiation. Zhonghua Bing Li Xue Za Zhi. 2006;35:13–17. [PubMed] [Google Scholar]
- 36.Mitas M, Mikhitarian K, Walters C, Baron PL, Elliott BM, Brothers TE, Robison JG, Metcalf JS, Palesch YY, Zhang Z, Gillanders WE, Cole DJ. Quantitative real-time RT-PCR detection of breast cancer micrometastasis using a multigene marker panel. Int J Cancer. 2001;93:162–171. doi: 10.1002/ijc.1312. [DOI] [PubMed] [Google Scholar]
- 37.Rakha EA, Boyce RW, bd El-Rehim D, Kurien T, Green AR, Paish EC, Robertson JF, Ellis IO. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005;18:1295–1304. doi: 10.1038/modpathol.3800445. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen MD, Plasil B, Wen P, Frankel WL. Mucin profiles in signet-ring cell carcinoma. Arch Pathol Lab Med. 2006;130:799–804. doi: 10.5858/2006-130-799-MPISCC. [DOI] [PubMed] [Google Scholar]
- 39.Lacunza E, Baudis M, Colussi AG, Segal-Eiras A, Croce MV, Abba MC. MUC1 oncogene amplification correlates with protein overexpression in invasive breast carcinoma cells. Cancer Genet Cytogenet. 2010;201:102–110. doi: 10.1016/j.cancergencyto.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Jones SE. A new estrogen receptor antagonist--an overview of available data. Breast Cancer Res Treat. 2002;75 1:S19–S21. doi: 10.1023/a:1020357631871. [DOI] [PubMed] [Google Scholar]
- 41.Singh AP, Chaturvedi P, Batra SK. Emerging roles of MUC4 in cancer: a novel target for diagnosis and therapy. Cancer Res. 2007;67:433–436. doi: 10.1158/0008-5472.CAN-06-3114. [DOI] [PubMed] [Google Scholar]
- 42.Lacroix-Triki M, Suarez PH, MacKay A, Lambros MB, Natrajan R, Savage K, Geyer FC, Weigelt B, Ashworth A, Reis-Filho JS. Mucinous carcinoma of the breast is genomically distinct from invasive ductal carcinomas of no special type. J Pathol. 2010;222:282–298. doi: 10.1002/path.2763. [DOI] [PubMed] [Google Scholar]
- 43.Matsukita S, Nomoto M, Kitajima S, Tanaka S, Goto M, Irimura T, Kim YS, Sato E, Yonezawa S. Expression of mucins (MUC1, MUC2, MUC5AC and MUC6) in mucinous carcinoma of the breast: comparison with invasive ductal carcinoma. Histopathology. 2003;42:26–36. doi: 10.1046/j.1365-2559.2003.01530.x. [DOI] [PubMed] [Google Scholar]
- 44.Gunhan-Bilgen I, Zekioglu O, Ustun EE, Memis A, Erhan Y. Invasive micropapillary carcinoma of the breast: clinical, mammographic, and sonographic findings with histopathologic correlation. AJR Am J Roentgenol. 2002;179:927–931. doi: 10.2214/ajr.179.4.1790927. [DOI] [PubMed] [Google Scholar]
- 45.Moritani S, Ichihara S, Hasegawa M, Endo T, Oiwa M, Yoshikawa K, Sato Y, Aoyama H, Hayashi T, Kushima R. Serous papillary adenocarcinoma of the female genital organs and invasive micropapillary carcinoma of the breast. Are WT1, CA125, and GCDFP-15 useful in differential diagnosis? Hum Pathol. 2008;39:666–671. doi: 10.1016/j.humpath.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Jarrett J, Huang CC, Satcher RL, Jr, Levenson AS. Identification of estrogen-responsive genes involved in breast cancer metastases to the bone. Clin Exp Metastasis. 2007;24:411–422. doi: 10.1007/s10585-007-9078-6. [DOI] [PubMed] [Google Scholar]
- 47.Isla LM, Demichelis S, Crespo M, Lacunza E, Barbera A, Creton A, Terrier F, Segal-Eiras A, Croce MV. Breast cancer humoral immune response: involvement of Lewis y through the detection of circulating immune complexes and association with Mucin 1 (MUC1) J Exp Clin Cancer Res. 2009;28:121. doi: 10.1186/1756-9966-28-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chakraborty S, Bonthu N, Swanson BJ, Batra SK. Role of mucins in the skin during benign and malignant conditions. Cancer Lett. 2010 doi: 10.1016/j.canlet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rachagani S, Torres MP, Moniaux N, Batra SK. Current status of mucins in the diagnosis and therapy of cancer. Biofactors. 2009;35:509–527. doi: 10.1002/biof.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh AP, Senapati S, Ponnusamy MP, Jain M, Lele SM, Davis JS, Remmenga S, Batra SK. Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer. Lancet Oncol. 2008;9:1076–1085. doi: 10.1016/S1470-2045(08)70277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wittel UA, Goel A, Varshney GC, Batra SK. Mucin antibodies - new tools in diagnosis and therapy of cancer. Front Biosci. 2001;6:D1296–D1310. doi: 10.2741/wittel. [DOI] [PubMed] [Google Scholar]
- 52.Bon GG, Verheijen RH, Zuetenhorst JM, van Kamp GJ, Verstraeten AA, Kenemans P. Mucin-like carcinoma-associated antigen serum levels in patients with adenocarcinomas originating from ovary, breast and colon. Gynecol Obstet Invest. 1996;42:58–62. doi: 10.1159/000291890. [DOI] [PubMed] [Google Scholar]
- 53.Ceriani RL, Peterson JA, Blank EW, Lamport DT. Epitope expression on the breast epithelial mucin. Breast Cancer Res Treat. 1992;24:103–113. doi: 10.1007/BF01961243. [DOI] [PubMed] [Google Scholar]
- 54.Pichon MF, Brun GL, Hacene K, Basuyau JP, Riedinger JM, Eche N, Fulla Y, Charlier-Bret N. Comparison of fifteen immunoassays for the measurement of serum MUC-1/CA 15-3 in breast cancer patients. Clin Chem Lab Med. 2009;47:985–992. doi: 10.1515/CCLM.2009.213. [DOI] [PubMed] [Google Scholar]
- 55.O'Brien DP, Horgan PG, Gough DB, Skehill R, Grimes H, Given HF. CA15-3: a reliable indicator of metastatic bone disease in breast cancer patients. Ann R Coll Surg Engl. 1992;74:9–11. [PMC free article] [PubMed] [Google Scholar]
- 56.Agrawal AK, Jelen M, Rudnicki J, Grzebieniak Z, Zysko D, Kielan W, Slonina J, Marek G. The importance of preoperative elevated serum levels of CEA and CA15-3 in patients with breast cancer in predicting its histological type. Folia Histochem Cytobiol. 2010;48:26–29. doi: 10.2478/v10042-010-0030-2. [DOI] [PubMed] [Google Scholar]
- 57.Dixon AR, Price MR, Hand CW, Sibley PE, Selby C, Blamey RW. Epithelial mucin core antigen (EMCA) in assessing therapeutic response in advanced breast cancer--a comparison with CA15.3. Br J Cancer. 1993;68:947–949. doi: 10.1038/bjc.1993.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laessig D, Nagel D, Heinemann V, Untch M, Kahlert S, Bauerfeind I, Stieber P. Importance of CEA and CA 15-3 during disease progression in metastatic breast cancer patients. Anticancer Res. 2007;27:1963–1968. [PubMed] [Google Scholar]
- 59.Kim HS, Park YH, Park MJ, Chang MH, Jun HJ, Kim KH, Ahn JS, Kang WK, Park K, Im YH. Clinical significance of a serum CA15-3 surge and the usefulness of CA15-3 kinetics in monitoring chemotherapy response in patients with metastatic breast cancer. Breast Cancer Res Treat. 2009;118:89–97. doi: 10.1007/s10549-009-0377-2. [DOI] [PubMed] [Google Scholar]
- 60.Geraghty JG, Coveney EC, Sherry F, O'Higgins NJ, Duffy MJ. CA 15-3 in patients with locoregional and metastatic breast carcinoma. Cancer. 1992;70:2831–2834. doi: 10.1002/1097-0142(19921215)70:12<2831::aid-cncr2820701218>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 61.Keshaviah A, Dellapasqua S, Rotmensz N, Lindtner J, Crivellari D, Collins J, Colleoni M, Thurlimann B, Mendiola C, Aebi S, Price KN, Pagani O, Simoncini E, Castiglione GM, Gelber RD, Coates AS, Goldhirsch A. CA15-3 and alkaline phosphatase as predictors for breast cancer recurrence: a combined analysis of seven International Breast Cancer Study Group trials. Ann Oncol. 2007;18:701–708. doi: 10.1093/annonc/mdl492. [DOI] [PubMed] [Google Scholar]
- 62.Bensouda Y, Andre F, Boulet T, Al-Ghuzlan A, Conforti R, Troalen F, Bourgier C, Errihani H, Spielmann M, Delaloge S. Prevalence of elevated serum CA 15-3 at time of metastatic relapse of breast cancer and correlation with hormone receptor status. Bull Cancer. 2009;96:923–928. doi: 10.1684/bdc.2009.0919. [DOI] [PubMed] [Google Scholar]
- 63.McGuckin MA, Walsh MD, Hohn BG, Ward BG, Wright RG. Prognostic significance of MUC1 epithelial mucin expression in breast cancer. Hum Pathol. 1995;26:432–439. doi: 10.1016/0046-8177(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 64.Bieglmayer C, Szepesi T, Neunteufel W. Follow-up of metastatic breast cancer patients with a mucin-like carcinoma-associated antigen: comparison to CA 15.3 and carcinoembryonic antigen. Cancer Lett. 1988;42:199–206. doi: 10.1016/0304-3835(88)90305-9. [DOI] [PubMed] [Google Scholar]
- 65.Nicolini A, Colombini C, Luciani L, Carpi A, Giuliani L. Evaluation of serum CA15-3 determination with CEA and TPA in the post-operative follow-up of breast cancer patients. Br J Cancer. 1991;64:154–158. doi: 10.1038/bjc.1991.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen AD, Gopas J, Karplus G, Cohen Y. CA 15-3 mucin-like carcinoma-associated antigen and tissue polypeptide-specific antigen: correlation to disease state and prognosis in breast cancer patients. Isr J Med Sci. 1995;31:155–159. [PubMed] [Google Scholar]
- 67.Kruit A, Tilanus-Linthorst MM, Boonstra JG, van Schaik RH, Grutters JC, van den Bosch JM, Ruven HJ. MUC1 568 A/G genotype-dependent cancer antigen 15-3 levels in breast cancer patients. Clin Biochem. 2009;42:662–665. doi: 10.1016/j.clinbiochem.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 68.Duffy MJ, Evoy D, McDermott EW. CA 15-3: uses and limitation as a biomarker for breast cancer. Clin Chim Acta. 2010;411:1869–1874. doi: 10.1016/j.cca.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 69.Ross JS, Slodkowska EA. Circulating and disseminated tumor cells in the management of breast cancer. Am J Clin Pathol. 2009;132:237–245. doi: 10.1309/AJCPJI7DEOLKCS6F. [DOI] [PubMed] [Google Scholar]
- 70.Berois N, Varangot M, Sonora C, Zarantonelli L, Pressa C, Lavina R, Rodriguez JL, Delgado F, Porchet N, Aubert JP, Osinaga E. Detection of bone marrow-disseminated breast cancer cells using an RT-PCR assay of MUC5B mRNA. Int J Cancer. 2003;103:550–555. doi: 10.1002/ijc.10853. [DOI] [PubMed] [Google Scholar]
- 71.Zieglschmid V, Hollmann C, Gutierrez B, Krehan A, Kaul S, Bocher O. Heterogeneous expression of tumor-associated genes in disseminated breast cancer cells. Anticancer Res. 2007;27:1769–1776. [PubMed] [Google Scholar]
- 72.Bolke E, Orth K, Gerber PA, Lammering G, Mota R, Peiper M, Matuschek C, Budach W, Rusnak E, Shaikh S, Dogan B, Prisack HB, Bojar H. Gene expression of circulating tumour cells and its correlation with tumour stage in breast cancer patients. Eur J Med Res. 2009;14:359–363. doi: 10.1186/2047-783X-14-8-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ren J, Agata N, Chen D, Li Y, Yu WH, Huang L, Raina D, Chen W, Kharbanda S, Kufe D. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell. 2004;5:163–175. doi: 10.1016/s1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yin L, Kharbanda S, Kufe D. Mucin 1 oncoprotein blocks hypoxia-inducible factor 1alpha activation in a survival response to hypoxia. J Biol Chem. 2007;282:257–266. doi: 10.1074/jbc.M610156200. [DOI] [PubMed] [Google Scholar]
- 75.Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein blocks glycogen synthase kinase 3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 2005;65:10413–10422. doi: 10.1158/0008-5472.CAN-05-2474. [DOI] [PubMed] [Google Scholar]
- 76.Li Y, Liu D, Chen D, Kharbanda S, Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22:6107–6110. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rahn JJ, Shen Q, Mah BK, Hugh JC. MUC1 initiates a calcium signal after ligation by intercellular adhesion molecule-1. J Biol Chem. 2004;279:29386–29390. doi: 10.1074/jbc.C400010200. [DOI] [PubMed] [Google Scholar]
- 78.Kam JL, Regimbald LH, Hilgers JH, Hoffman P, Krantz MJ, Longenecker BM, Hugh JC. MUC1 synthetic peptide inhibition of intercellular adhesion molecule-1 and MUC1 binding requires six tandem repeats. Cancer Res. 1998;58:5577–5581. [PubMed] [Google Scholar]
- 79.Regimbald LH, Pilarski LM, Longenecker BM, Reddish MA, Zimmermann G, Hugh JC. The breast mucin MUCI as a novel adhesion ligand for endothelial intercellular adhesion molecule 1 in breast cancer. Cancer Res. 1996;56:4244–4249. [PubMed] [Google Scholar]
- 80.Ren J, Raina D, Chen W, Li G, Huang L, Kufe D. MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol Cancer Res. 2006;4:873–883. doi: 10.1158/1541-7786.MCR-06-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pochampalli MR, el Bejjani RM, Schroeder JA. MUC1 is a novel regulator of ErbB1 receptor trafficking. Oncogene. 2007;26:1693–1701. doi: 10.1038/sj.onc.1209976. [DOI] [PubMed] [Google Scholar]
- 82.Ramasamy S, Duraisamy S, Barbashov S, Kawano T, Kharbanda S, Kufe D. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol Cell. 2007;27:992–1004. doi: 10.1016/j.molcel.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bitler BG, Goverdhan A, Schroeder JA. MUC1 regulates nuclear localization and function of the epidermal growth factor receptor. J Cell Sci. 2010;123:1716–1723. doi: 10.1242/jcs.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ligtenberg MJ, Kruijshaar L, Buijs F, van MM, Litvinov SV, Hilkens J. Cell-associated episialin is a complex containing two proteins derived from a common precursor. J Biol Chem. 1992;267:6171–6177. [PubMed] [Google Scholar]
- 85.Macao B, Johansson DG, Hansson GC, Hard T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat Struct Mol Biol. 2006;13:71–76. doi: 10.1038/nsmb1035. [DOI] [PubMed] [Google Scholar]
- 86.Ahmad R, Raina D, Trivedi V, Ren J, Rajabi H, Kharbanda S, Kufe D. MUC1 oncoprotein activates the IkappaB kinase beta complex and constitutive NF-kappaB signalling. Nat Cell Biol. 2007;9:1419–1427. doi: 10.1038/ncb1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leng Y, Cao C, Ren J, Huang L, Chen D, Ito M, Kufe D. Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. J Biol Chem. 2007;282:19321–19330. doi: 10.1074/jbc.M703222200. [DOI] [PubMed] [Google Scholar]
- 88.Wei X, Xu H, Kufe D. MUC1 oncoprotein stabilizes and activates estrogen receptor alpha. Mol Cell. 2006;21:295–305. doi: 10.1016/j.molcel.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 89.Pitroda SP, Khodarev NN, Beckett MA, Kufe DW, Weichselbaum RR. MUC1-induced alterations in a lipid metabolic gene network predict response of human breast cancers to tamoxifen treatment. Proc Natl Acad Sci U S A. 2009;106:5837–5841. doi: 10.1073/pnas.0812029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khodarev NN, Pitroda SP, Beckett MA, MacDermed DM, Huang L, Kufe DW, Weichselbaum RR. MUC1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer Res. 2009;69:2833–2837. doi: 10.1158/0008-5472.CAN-08-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y, Yu WH, Ren J, Chen W, Huang L, Kharbanda S, Loda M, Kufe D. Heregulin targets gamma-catenin to the nucleolus by a mechanism dependent on the DF3/MUC1 oncoprotein. Mol Cancer Res. 2003;1:765–775. [PubMed] [Google Scholar]
- 92.Khodarev N, Ahmad R, Rajabi H, Pitroda S, Kufe T, McClary C, Joshi MD, MacDermed D, Weichselbaum R, Kufe D. Cooperativity of the MUC1 oncoprotein and STAT1 pathway in poor prognosis human breast cancer. Oncogene. 2010;29:920–929. doi: 10.1038/onc.2009.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schroeder JA, Adriance MC, Thompson MC, Camenisch TD, Gendler SJ. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene. 2003;22:1324–1332. doi: 10.1038/sj.onc.1206291. [DOI] [PubMed] [Google Scholar]
- 94.Wei X, Xu H, Kufe D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell. 2005;7:167–178. doi: 10.1016/j.ccr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 95.Daniel JM, Reynolds AB. The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin. Mol Cell Biol. 1995;15:4819–4824. doi: 10.1128/mcb.15.9.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001;10:721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- 98.Sarrio D, Moreno-Bueno G, Hardisson D, Sanchez-Estevez C, Guo M, Herman JG, Gamallo C, Esteller M, Palacios J. Epigenetic and genetic alterations of APC and CDH1 genes in lobular breast cancer: relationships with abnormal E-cadherin and catenin expression and microsatellite instability. Int J Cancer. 2003;106:208–215. doi: 10.1002/ijc.11197. [DOI] [PubMed] [Google Scholar]
- 99.Virmani AK, Rathi A, Sathyanarayana UG, Padar A, Huang CX, Cunnigham HT, Farinas AJ, Milchgrub S, Euhus DM, Gilcrease M, Herman J, Minna JD, Gazdar AF. Aberrant methylation of the adenomatous polyposis coli (APC) gene promoter 1A in breast and lung carcinomas. Clin Cancer Res. 2001;7:1998–2004. [PubMed] [Google Scholar]
- 100.Moll UM, Riou G, Levine AJ. Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc Natl Acad Sci U S A. 1992;89:7262–7266. doi: 10.1073/pnas.89.15.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carraway KL, Price-Schiavi SA, Komatsu M, Jepson S, Perez A, Carraway CA. Muc4/sialomucin complex in the mammary gland and breast cancer. J Mammary Gland Biol Neoplasia. 2001;6:323–337. doi: 10.1023/a:1011327708973. [DOI] [PubMed] [Google Scholar]
- 102.Komatsu M, Yee L, Carraway KL. Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res. 1999;59:2229–2236. [PubMed] [Google Scholar]
- 103.Chaturvedi P, Singh AP, Chakraborty S, Chauhan SC, Bafna S, Meza JL, Singh PK, Hollingsworth MA, Mehta PP, Batra SK. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68:2065–2070. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Funes M, Miller JK, Lai C, Carraway KL, III, Sweeney C. The mucin Muc4 potentiates neuregulin signaling by increasing the cell-surface populations of ErbB2 and ErbB3. J Biol Chem. 2006;281:19310–19319. doi: 10.1074/jbc.M603225200. [DOI] [PubMed] [Google Scholar]
- 105.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, III, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Workman HC, Sweeney C, Carraway KL., III The membrane mucin Muc4 inhibits apoptosis induced by multiple insults via ErbB2-dependent and ErbB2-independent mechanisms. Cancer Res. 2009;69:2845–2852. doi: 10.1158/0008-5472.CAN-08-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gilewski T, Adluri S, Ragupathi G, Zhang S, Yao TJ, Panageas K, Moynahan M, Houghton A, Norton L, Livingston PO. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000;6:1693–1701. [PubMed] [Google Scholar]
- 108.Gulley JL, Arlen PM, Tsang KY, Yokokawa J, Palena C, Poole DJ, Remondo C, Cereda V, Jones JL, Pazdur MP, Higgins JP, Hodge JW, Steinberg SM, Kotz H, Dahut WL, Schlom J. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14:3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Braun DP, Crist KA, Shaheen F, Staren ED, Andrews S, Parker J. Aromatase inhibitors increase the sensitivity of human tumor cells to monocyte-mediated, antibody-dependent cellular cytotoxicity. Am J Surg. 2005;190:570–571. doi: 10.1016/j.amjsurg.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 110.Yuan S, Shi C, Ling R, Wang T, Wang H, Han W. Immunization with two recombinant Bacillus Calmette-Guerin vaccines that combine the expression of multiple tandem repeats of mucin-1 and colony stimulating-factor suppress breast tumor growth in mice. J Cancer Res Clin Oncol. 2010;136:1359–1367. doi: 10.1007/s00432-010-0787-x. [DOI] [PubMed] [Google Scholar]
- 111.Carr-Brendel V, Markovic D, Ferrer K, Smith M, Taylor-Papadimitriou J, Cohen EP. Immunity to murine breast cancer cells modified to express MUC-1, a human breast cancer antigen, in transgenic mice tolerant to human MUC-1. Cancer Res. 2000;60:2435–2443. [PubMed] [Google Scholar]
- 112.Chen L, Chen D, Manome Y, Dong Y, Fine HA, Kufe DW. Breast cancer selective gene expression and therapy mediated by recombinant adenoviruses containing the DF3/MUC1 promoter. J Clin Invest. 1995;96:2775–2782. doi: 10.1172/JCI118347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Akewanlop C, Watanabe M, Singh B, Walker M, Kufe DW, Hayes DF. Phagocytosis of breast cancer cells mediated by anti-MUC-1 monoclonal antibody, DF3, and its bispecific antibody. Cancer Res. 2001;61:4061–4065. [PubMed] [Google Scholar]
- 114.Buckman R, De AC, Shaw P, Covens A, Osborne R, Kerr I, Reed R, Michaels H, Woo M, Reilly R. Intraperitoneal therapy of malignant ascites associated with carcinoma of ovary and breast using radioiodinated monoclonal antibody 2G3. Gynecol Oncol. 1992;47:102–109. doi: 10.1016/0090-8258(92)90084-v. [DOI] [PubMed] [Google Scholar]
- 115.Engebraaten O, Sivam G, Juell S, Fodstad O. Systemic immunotoxin treatment inhibits formation of human breast cancer metastasis and tumor growth in nude rats. Int J Cancer. 2000;88:970–976. doi: 10.1002/1097-0215(20001215)88:6<970::aid-ijc21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 116.Persson J, Backstrom M, Johansson H, Jirstrom K, Hansson GC, Ohlin M. Molecular evolution of specific human antibody against MUC1 mucin results in improved recognition of the antigen on tumor cells. Tumour Biol. 2009;30:221–231. doi: 10.1159/000240634. [DOI] [PubMed] [Google Scholar]
- 117.Moniaux N, Varshney GC, Chauhan SC, Copin MC, Jain M, Wittel UA, Andrianifahanana M, Aubert JP, Batra SK. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem. 2004;52:253–261. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- 118.Dharma RT, Park KJ, Smith-Jones P, Iasonos A, Linkov I, Soslow RA, Spriggs DR. Novel monoclonal antibodies against the proximal (carboxy-terminal) portions of MUC16. Appl Immunohistochem Mol Morphol. 2010;18:462–472. doi: 10.1097/PAI.0b013e3181dbfcd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Block A, Milasinovic D, Mueller J, Schaefer P, Schaefer H, Greten H. Amplified Muc1-specific gene expression in colon cancer cells utilizing a binary system in adenoviral vectors. Anticancer Res. 2002;22:3285–3292. [PubMed] [Google Scholar]
- 120.Nagy P, Friedlander E, Tanner M, Kapanen AI, Carraway KL, Isola J, Jovin TM. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473–482. [PubMed] [Google Scholar]
- 121.Price-Schiavi SA, Jepson S, Li P, Arango M, Rudland PS, Yee L, Carraway KL. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer. 2002;99:783–791. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- 122.Fessler SP, Wotkowicz MT, Mahanta SK, Bamdad C. MUC1* is a determinant of trastuzumab (Herceptin) resistance in breast cancer cells. Breast Cancer Res Treat. 2009;118:113–124. doi: 10.1007/s10549-009-0412-3. [DOI] [PubMed] [Google Scholar]
- 123.Bafna S, Kaur S, Momi N, Batra SK. Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br J Cancer. 2009;101:1155–1161. doi: 10.1038/sj.bjc.6605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Domenech N, Henderson RA, Finn OJ. Identification of an HLA-A11-restricted epitope from the tandem repeat domain of the epithelial tumor antigen mucin. J Immunol. 1995;155:4766–4774. [PubMed] [Google Scholar]
- 125.Wang HY, Fu T, Wang G, Zeng G, Perry-Lalley DM, Yang JC, Restifo NP, Hwu P, Wang RF. Induction of CD4(+) T cell-dependent antitumor immunity by TAT-mediated tumor antigen delivery into dendritic cells. J Clin Invest. 2002;109:1463–1470. doi: 10.1172/JCI15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Viehl CT, Tanaka Y, Chen T, Frey DM, Tran A, Fleming TP, Eberlein TJ, Goedegebuure PS. Tat mammaglobin fusion protein transduced dendritic cells stimulate mammaglobin-specific CD4 and CD8 T cells. Breast Cancer Res Treat. 2005;91:271–278. doi: 10.1007/s10549-005-0450-4. [DOI] [PubMed] [Google Scholar]
- 127.Yang H, Cho NH, Seong SY. The Tat-conjugated N-terminal region of mucin antigen 1 (MUC1) induces protective immunity against MUC1-expressing tumours. Clin Exp Immunol. 2009;158:174–185. doi: 10.1111/j.1365-2249.2009.03997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huyn ST, Burton JB, Sato M, Carey M, Gambhir SS, Wu L. A potent, imaging adenoviral vector driven by the cancer-selective mucin-1 promoter that targets breast cancer metastasis. Clin Cancer Res. 2009;15:3126–3134. doi: 10.1158/1078-0432.CCR-08-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raina D, Ahmad R, Joshi MD, Yin L, Wu Z, Kawano T, Vasir B, Avigan D, Kharbanda S, Kufe D. Direct targeting of the mucin 1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009;69:5133–5141. doi: 10.1158/0008-5472.CAN-09-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cordon-Cardo C. Cancer stem cells. Ann Oncol. 2010;21 7:vii93–vii94. doi: 10.1093/annonc/mdq540. [DOI] [PubMed] [Google Scholar]
- 131.Engelmann K, Shen H, Finn OJ. MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Res. 2008;68:2419–2426. doi: 10.1158/0008-5472.CAN-07-2249. [DOI] [PubMed] [Google Scholar]
- 132.Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, Jacob S, Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Iorio MV, Casalini P, Tagliabue E, Menard S, Croce CM. MicroRNA profiling as a tool to understand prognosis, therapy response and resistance in breast cancer. Eur J Cancer. 2008;44:2753–2759. doi: 10.1016/j.ejca.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 134.Jin C, Rajabi H, Kufe D. miR-1226 targets expression of the mucin 1 oncoprotein and induces cell death. Int J Oncol. 2010;37:61–69. doi: 10.3892/ijo_00000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sachdeva M, Mo YY. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010;70:378–387. doi: 10.1158/0008-5472.CAN-09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sachdeva M, Mo YY. miR-145-mediated suppression of cell growth, invasion and metastasis. Am J Transl Res. 2010;2:170–180. [PMC free article] [PubMed] [Google Scholar]
- 137.Rajabi H, Jin C, Ahmad R, McClary C, Joshi MD, Kufe D. Mucin 1 oncoprotein expression is suppressed by the miR-125b ONCOMIR. Genes Cancer. 2010;1:62–68. doi: 10.1177/1947601909357933. [DOI] [PMC free article] [PubMed] [Google Scholar]


