Abstract
Historically, the use of external beam radiotherapy for hepatocellular carcinoma (HCC) has been limited by toxicity to the uninvolved liver and surrounding structures. Advances in photon radiotherapy have improved dose conformality to the tumor and facilitated dose escalation, a key contributor to improved HCC radiation treatment outcomes. However, despite these advances in photon radiotherapy, significant volumes of liver still receive low doses of radiation that can preclude dose escalation, particularly in patients with limited functional liver reserves. By capitalizing on the lack of exit dose along the beam path beyond the tumor and higher biological effectiveness, charged particle therapy offers the promise of maximizing tumor control via dose escalation without excessive liver toxicity. In this review we discuss the distinctive biophysical attributes of both proton and carbon ion radiotherapy, particularly as they pertain to treatment of HCC. We also review the available literature regarding clinical outcomes and toxicity of using charged particles for the treatment of HCC.
Introduction
Hepatocellular carcinoma (HCC) is becoming an increasingly important cause of cancer mortality globally1, and the majority of patients present with large, multifocal tumors, often with major vessel invasion, and advanced cirrhosis. Thus there is a pressing need for improved local therapies in these challenging patients.
As noted in the accompanying articles in this issue, external beam radiotherapy was historically considered ineffective for treatment of unresectable HCC because the doses of radiation necessary to cure HCC far exceeded the tolerance of the entire liver to radiation. The risk of potentially fatal radiation-induced liver disease (RILD), observed when whole-liver radiation doses exceeded 30 Gy over 3 weeks, resulted in the near abandonment of radiation therapy as a treatment modality for HCC. Indeed, what makes treatment of HCC especially challenging is the fact that therapy must be guided not only by the characteristics of the tumor but also by the function of the cirrhotic liver that harbors the tumor and often other premalignant foci of HCC.
It is has been recognized that with three-dimensional conformal RT, tumoricidal doses of radiation can be safely delivered to the tumor while sparing the uninvolved surrounding liver parenchyma, thus, largely avoiding RILD in selected patients. Furthermore, there is accumulating evidence that dose escalation can improve both objective responses as well as survival in these patients2–4. With the goal of escalating radiation dose to the tumor while sparing critical normal structures there have been unprecedented efforts to develop and use sophisticated, conformal photon techniques. One promising avenue for increasing dose without a concurrent increase in toxicity in HCC is via the use of heavy charged-particle therapy such as proton and carbon ion therapy.
Characteristics of charged particle therapy
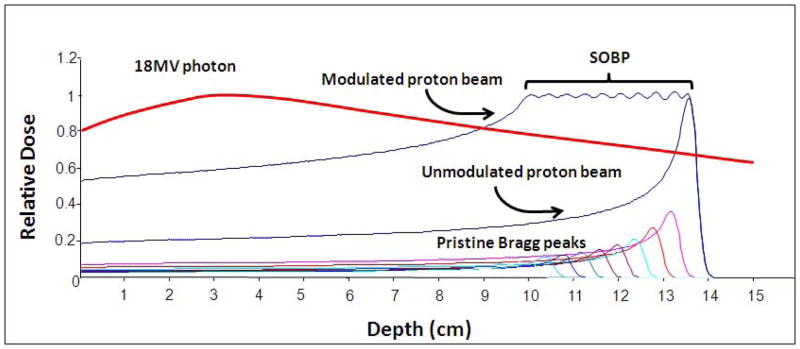
Charged particle therapy has distinct dosimetric advantages over photon radiotherapy. In regards to proton radiotherapy, much of the dosimetric advantage is due to the physical laws that determine the absorption of energy in tissues exposed to either photon or proton beams. In a specific tissue, photons are absorbed exponentially whereas protons have a finite range dependent upon the initial proton energy. Therefore, the depth dose characteristics of the two beams are qualitatively different (see Figure 1). Protons lose their energy in tissue mostly by coulombic interactions with electrons in the constituent atoms; however, a small fraction of energy is transferred through nuclear collisions. The energy loss per unit path length is relatively small and constant as the proton traverses the tissue until near the end of the proton range where the residual energy is lost over a short distance (approximately 0.7 cm in width at 80% of the maximum dose) and the proton comes to rest. This results in a distinctive sharp rise in the tissue absorbed dose (energy absorbed per unit mass) - known as the Bragg peak (see the curve labeled “unmodulated proton beam” in Figure 1). In physical terms, the magnitude of the transfer of energy to tissue per unit path length traversed by the protons is inversely proportional to the square of the proton velocity. The low dose region between the entrance and the Bragg peak is known as the “plateau” of the dose distribution and the dose at that point is 30–40% of the maximum dose.
Figure 1.
Proton (Bragg peak and modulated Bragg peak) and 18 MV photon depth dose curves.
The Bragg peak is too narrow in extent to irradiate any but the smallest of targets. To irradiate larger targets the beam energy needs to be modulated. Specifically, several beams of closely spaced energies (ranges) are superimposed to create a region of uniform dose over the depth of the target. These extended regions of uniform dose are called the “spread-out Bragg peaks” (SOBP, Figure 1).
Similar to protons in many beam characteristics, carbon ion therapy also exhibits a narrow Bragg peak at a defined depth for a defined energy. This Bragg peak is then modulated to create a SOBP, which can encompass the entirety of the target volume. Again, similar to a proton beam, the dose falloff distal to the Bragg peak is dramatic, with little if any dose deposited in this region. The main difference in beam characteristics between proton and carbon ion beam radiation is in regards to the higher linear energy transfer (LET) found in carbon ion beams, which will be discussed later.
The rationale for charged particle therapy for HCC
The fundamental characteristics of the SOBP noted above predictably confer significant dosimetric advantages for proton radiotherapy compared to conventional photon radiotherapy. As illustrated by the depth-dose curve for a 18 MV photon beam in Figure 1, dose delivered in tissues rises to a maximum value at relatively shallow depths, then falls off exponentially to lower doses at greater depths. Thus, the entry dose is far greater than the dose at depth. Similarly, the extent of scattering that accounts for the lateral penumbra of the beam is less for proton beams when compared to photon beams. Inmost clinical scenarios, more than one radiation beam is used in both photon and proton treatments. However, the dosimetricadvantage illustrated for protons using single beams is present for each beam used. One cannot overcome the physical disadvantage of photons by the use of multiple beams or complex beam arrangements. As demonstrated by a dosimetric comparison study, in addition to better sparing of uninvolved liver via a reduction in the fractional volume of liver receiving dose greater than or equal to 30 Gy (V30) and the mean liver dose, proton radiotherapy significantly reduces the V45 for the stomach and duodenum, V40 and V50 for the heart, and maximal dose to the spinal cord5. Proton radiotherapy is alsoable to completely spare one kidney more often and deliver less dose to one (generally the left) kidney than photon radiotherapy. Furthermore, in modern proton therapy facilities, which have isocentric gantries and sophisticated beam delivery and control systems, proton therapy capabilities are equivalent to those for state-of-the-art, conformal therapy using photons with respect to numbers of beams, beam directions and complex delivery techniques such as intensity modulated radiation therapy (IMRT). These techniques are likely to be used increasingly for the treatment of liver tumors.
Beyond conformality, the use of proton radiotherapy has a distinct advantage in regards to integral dose compared to the use of IMRT. The sensitivity of the liver to radiation as well as the low functional hepatic reserve in many patients with HCC necessitates that the scatter dose to the uninvolved (often cirrhotic) liver parenchyma be kept as low as possible. As shown in Figure 2, although IMRT may achieve conformality of the prescription dose similar to that of proton radiotherapy, the scatter dose to the normal liver will almost certainly be higher. This level of conformality is not only important in limiting dose to the uninvolved liver, but it also leaves the door open for repetition of radiotherapy in the future if the tumor recurs outside the radiation field. Lastly, this reduction in integral dose has the potential to reduce the risk of second malignancies, which may have relevance in patients treated with liver transplant who are immunosuppressed6.
Figure 2.
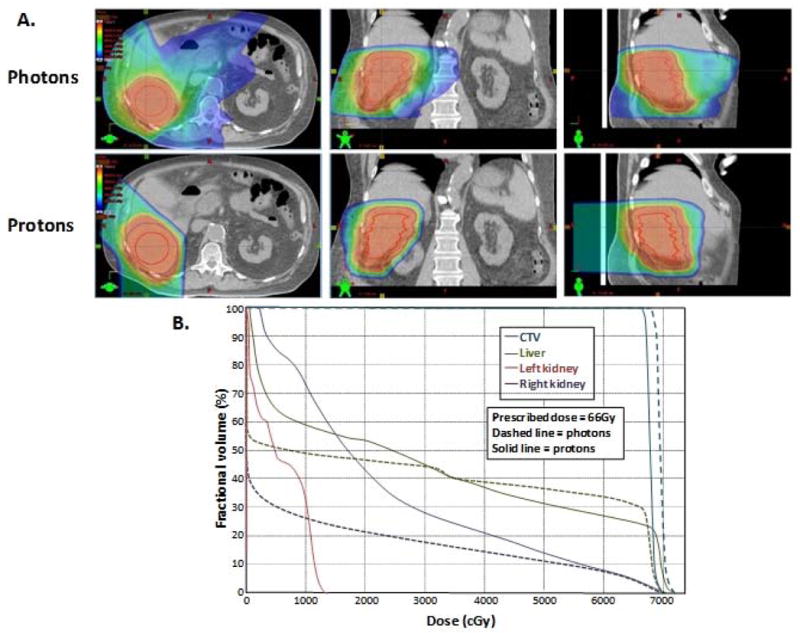
Comparison between intensity modulated radiation therapy (photons) and proton radiotherapy (protons) for an unresectable hepatocellular carcinoma in a patient with multiple medical co-morbidities. A. Representative computed tomography (CT) axial, coronal and sagittal slices. Isodose lines are for a delivered proton dose of 66Gy in 20 fractions. B. Dose volume histogram comparison of the photon and proton plans for this patient. Note relative sparing of more liver in the low-dose region (below 30Gy). Mean liver dose was 30Gy for photons and 28Gy for protons.
Charged Particle Radiation Planning
The treatment planning for proton radiotherapy involves some challenges not encountered in photon radiotherapy planning. First, one must define the proximal as well as the distal edge of the beam accurately. When using the more common “passively scattered” proton beam, one creates a distal edge of the beam through the use of compensators, which are unique for each beam used for treatment. These compensators must account for target motion, daily set-up error, and the range uncertainty of protons; this is achieved via the use of a “smearing” algorithm. Although smearing ensures coverage of the distal extent of the tumor along the path of the beam regardless of daily variation, this leads to a significant volume of normal tissue beyond the distal extent of the target being treated unnecessarily.
While these challenges with target motion and daily set-up error are present even in photon radiotherapy planning, range uncertainty is a unique problem encountered with proton radiotherapy planning. Uncertainties in the range of the proton beam are a result of greater attenuation of the beam by higher density structures in its path and the consequent, greater sensitivity of proton beams to tissue inhomogeneity than traditional photon beams. In practice, these range uncertainties originate from computed tomography (CT) artifacts, errors in converting CT Hounsfield units to proton stopping power, changes in patient geometry during treatment, and changes in tissue density along the beam path, especially when the beam traverses a loop of bowel with variable amount of air, a high-density rib that moves during breathing, or a sliver of lung tissue that expands and contracts with respiration. In turn, this range uncertainty may result in unexpected areas of under- or over-dosage within the treatment volume and also in adjacent normal structures if the actual proton range is different from that assumed during treatment plan optimization. This range uncertainty presents much less of an issue for liver tumors than other disease sites because beams can be chosen such that they traverse through uniform density of liver tissue to reach the tumor and because there is little opportunity for large changes in the structures in the beam path after the time of simulation, at least from the standpoint of their relative density. By the same token, though, restricting beam angles to those that traverse entirely through liver tissue and those that do not stop directlyin front of a critical normal structure (see below), does pose some limits on the degree of conformality achievable with proton therapy. Lastly, when using passive scatter technology, there is currently no method to conform the dose to the proximal extent of the target.
Many of these difficulties can be at least partially surmounted via the use of “spot scanning” proton radiotherapy. In this technique, magnetic beam scanning technology is used to place individual Bragg peaks within small areas of the target, similar to a pixelated image. Taken together, these “spots” encompass the whole of the target volume and, thus, allows for conformality, especially to the distal extent of the target. This technique also permits modulation of the delivered dose within a target, in essence, achieving intensity modulated proton radiotherapy.
With both passively scattered and spot scanned proton beams, the relative biological effectiveness (RBE) of protons as compared to photons is, by convention and consensus, assigned a value of 1.1 at most institutions. This means that a physical dose of 1 Gy delivered using proton radiotherapy is deemed biologically equivalent to 1.1 Gy delivered using photon radiotherapy, otherwise described as 1.1 CGE or Cobalt Gray Equivalent. This assignment of proton RBE of a value of 1.1 is controversial since it depends on the tissue in question, the choice of biological endpoints, the timing of assessment of these endpoints, and the clinical relevance of these endpoints7. However, above and beyond this debate, there is some question of unpredictable variation of the RBE of the proton beam, particularly at the Bragg peak7, 8. Due to this unpredictability and the issue of range uncertainty noted above, beams are often chosen such that they do not stop directlyin front of a critical normal structure such as the duodenum or the spinal cord.
The theoretical advantages of carbon ion therapy include all of those previously discussed with proton therapy with the added potential benefit of increased RBE. The increased RBE is generally attributed to the greater energy transferred per unit length of tissue along the ionization track (linear energy transfer, LET) track of the carbon ion beam. Generally, an RBE of 3 is used for planning with carbon ion therapy9. However, in a recent review of calculated RBE values for carbon ion radiotherapy, the mean RBE was noted to be 2.2, with significant variability of this value based upon the model system used10. Furthermore, it is thought that the RBE for carbon ion radiotherapy may vary considerably with the fractionation schedule used11. Although, in theory, a high RBE could offer an advantage in regards to tumor kill, similar concerns regarding normal tissue toxicity remain. In addition to the benefits of a particle with finite particle range and higher RBE, another potential clinical advantage of carbon ions is the lower oxygen enhancement ratio (OER) due to their higher linear energy transfer (LET). Much of the work examining OER for carbon ion therapy has been performed in cultured cells, with estimates of an OER of around 2 compared to an OER of 3 for photon irradiation12. In a pre-clinical tumor model, an OER for carbon ion therapy of 1.6 was seen, compared to 3.4 for photons13. This decreased OER could lead to improved responses in hypoxic areas of HCC, which are more resistant to photon irradiation.
While not true in previous decades, proton radiotherapy is increasingly available to patients across the United States and globally. Since the initial conception of therapeutic proton radiotherapy in the 1940s and its clinical deployment for cancer treatment by investigators in Sweden, the Berkeley Radiation Laboratory and the Harvard Cyclotron laboratory in the following decade, nearly thirty proton radiotherapy facilities have been established worldwide with over 60,000 patients treated for a variety of malignancies14, 15. Conversely, the facilities offering carbon ion therapy for HCC are extraordinarily limited, with the largest clinical treatment experience coming from the Heavy Ion Medical Accelerator in Chiba (HIMAC), Japan and more recent treatment facilities in Darmstadt and Heidelberg, Germany, and Hyogo, Japan.
Practical considerations in proton therapy for HCC
In many respects, the practical considerations for proton radiotherapy in HCC are very similar to photon radiotherapy. However, a few notable differences are worth stressing. Some of these pertain to treatment plan optimization and have been addressed previously. Largely, these are related to the use of smearing margins and minimizing the use of beams that traverse through lung or bowel loops and those that terminate in a critical normal structure like the spinal cord or duodenum. In addition, wherever the beam traverses a structure that only temporarily has increased contrast, that high-density material is arbitrarily assigned a soft tissue density value (i.e., overriding the Hounsfield units so as to calculate proton range in the body accurately). For instance, density of recent oral contrast from a diagnostic CT evaluation is converted to tissue equivalent density but lipiodol from a recent transarterial chemoembolization or implanted metal fiducials are not. One caveat when contemplating fiducial placement for use with proton radiotherapy is the dose scatter from traditional metallic fiducials, which can lead to unwanted cold spots behind these fiducials (this shadow is most prominent with single beams but less of an issue when multiple beams are employed). An alternative approach when implanting fiducials for use with proton therapy is to use carbon coated or stainless steel fiducials which are specifically designed for this purpose16.
When comparing different potential treatment plans, it is always prudent to evaluate the chestwall and skin dose since these can be higher than what one expects with photon IMRT, especially when only 2–3 beams are used to deliver the entire dose to a superficial tumor. With superficial tumors, spot scanning may also be problematic due to the lack of availability of proton energies that have such short beam ranges. On the flip side, in larger patients the treatment unit’s upper energy range may limit the beam range and depth of treatment.
As with photon radiotherapy, the treatment of HCCs with radiation involves addressing issues related to inter- and, to a lesser extent, intra-fraction tumor motion17, 18. Management of tumor motion for treating HCCs as addressed in this issue by Brock largely focuses on the use of implantable fiducial markers19, 20, 4D simulation21, 22, daily cone-beam CT23 or ultrasound24, respiratory gating25, 26, abdominal compression27, or a combination of these28, 29 . Although not the primary focus of this article, management of motion is at least as important in proton radiotherapy as in photon radiotherapy, particularly when using hypofractionated proton therapy. The special considerations include those alluded to earlier, i.e., shadow effect behind implanted metallic fiducials and variability of delivered dose when organs with different Hounsfield units move differentially compared to the liver during breathing. At our institutions, we simulate patients with their arms stretched above their heads in an immobilization device that does not extend down past the lower chest (minimizing range uncertainty from extraneous sources). For patients who are treated during free breathing, we perform a contrast-aided 4D CT simulation where the intravenous contrast infusion is synchronized with CT acquisition such that the tumor is visualized optimally in the appropriate phase of contrast enhancement; no oral contrast is used22. In our experience, optimal discrimination between tumor and normal liver parenchyma tumor is achieved when the 4D sequence is obtained in the portal venous phase rather than the early arterial enhancement phase, because the 4D sequence takes more time to accrue than the typical duration of arterial enhancement. In patients who are treated during a breath-hold, the coached breath-hold images are obtained at a similar time frame following contrast infusion. While all of this holds true for photon radiotherapy planning, one caveat for proton radiotherapy simulations is that a non-contrast scan (without extrinsic sources of alteration of tissue density or Hounsfield units) is always obtained prior to the contrast scan and this non-contrast scan is used for treatment planning purposes.
Dose constraints and models for proton based planning
The normal tissue complication probability (NTCP) model developed at the University of Michigan has served as a guiding beacon for many of the dose volume constraints used in current clinical practice and has been discussed by Kavanagh and Guhain this issue of the journal. Since this model is based on classic radiation induced liver disease (RILD) that occurred in 19 or 203 patients treated with hyperfractionated photon conformal radiation30,care is warranted when considering these NTCP models in the context of hypofractionatedproton planning for HCCs which is associated with very different dose distributions and non-classic RILD, not well predicted by this model. The proton therapy group at the Massachusetts General Hospital developed a biological model based on the equivalent uniform dose, or EUD. This formalism takes the 2D information from the dose volume histogram (DVH) of an inhomogeneously irradiated liver and expresses it as a single dose value (the EUD) for a homogeneously irradiated organ that are assumed to yield the same radiobiological effect. The value of the EUD, as opposed to mean dose, is that it adds a biological volume dependence that is not accounted for by physical dose. In the first practical application of this formalism in a phase I study conducted by one of us (TSH), dose escalation to the tumor was not curtailed by the risk of liver toxicity but rather by the dose escalation constraints posed by non-liver normal tissues.
On-going studies at both MGH and MD Anderson Cancer Center employ a two-pronged prescription, where peripheral tumors are treated with 67.5 CGE and central tumors receive 58.05 CGE in 15 fractions. The dichotomy in radiation dose prescribed to peripheral vs. central tumors is due to the early data from the University of Tsukuba that suggested that the risk of biliary stenoses was more common after hypofractionated high-dose proton therapy, as described in the subsequent section31.In this study, the normal liver constraints are set at an EUD below 20 CGE and/or a mean liver dose ≥ 24 CGE. The EUD threshold was based on this being the maximum dose used in a phase I study. Non-liver normal tissue constraints are as follows: spinal cord maximum dose of 30 GyE, stomach maximum point dose of 42 GyE, bowel maximum point dose of 45 GyE, kidney V14<30%, chest wall V60< 2 cc, and heart maximum point dose of 45 GyE and V40<10%.
Clinical outcomes with proton therapy
The use of protons for liver tumors has been studied by several groups, mainly in the Far East. One of the first large retrospective series was presented by Chiba and colleagues31. In this series of 162 patients (192 HCCs) treated with proton radiotherapy, all treatments were delivered using hypo-fractionated regimens (3.5–5 CGE) and total doses ranged between 50 CGE (10 fractions) and 84 CGE (24 fractions), with the median dose being 72 CGE in 16 fractions over 29 days. Two ports (lateral and anterior/posterior) were typically used but other angles were used when necessary to avoid over-dosing the gastrointestinal tract. Median tumor size was 3.8 cm (ranging from 1.5 to 14.5 cm), 25 patients (15%) had a portal vein tumor thrombus, and 72 patients (44%) had Child-Pugh Class B and C cirrhosis (see Table 1).
Table 1.
Clinical outcomes following proton radiotherapy for hepatocellular carcinoma
| Study | N | Study type | Inclusion | Median dose | Child-Pugh Score | Outcome | Toxicity (≥ Gr 2) | Comments |
|---|---|---|---|---|---|---|---|---|
| Chiba et al. (2005) | 162 | Retrospective | Local HCC, <3 lesions. | 72 CGE (50–88) 4.5 CGE/fx (2.9–6) |
A: 50.6% B: 38.3% C: 6.2% |
5 yr OS: 23.5% 5 yr LC: 86.9% Class A and solitary lesion: 5 yr OS 53.5% |
Acute: 16.6% Late: 2.7% |
HCV in 80%; Prior and concurrent treatment NS. |
| Nakayama et al. (2009) | 318 | Retrospective | Local HCC, <3 lesions, PS 0-2. | 72.6 CGE (55/10fx-79.2/22fx) | A: 73.6% B: 24.2% C: 2.2% |
5 yr OS: 44.6% 5 yr LC: NS |
Acute:12.2% Late: 4% |
HCV in 78%; Dose altered based upon tumor location; 56.6% treated with other local modality prior to proton. |
| Bush et al. (2004) | 34 | Prospective | Local HCC, Child score ≤ 10. | 63 CGE/15 fx | Score: 5–6: 14 7–8: 7 (?) 9–10: 7 (?) |
2 yr OS: 55% 2 yr LC: 75% |
Acute (not graded): 60%, no hospitalizations Late: Post-treatment ascites in 17% |
HCV NS; 6 patients received liver transplant post-treatment with 2 CR. |
| Kawashima et al. (2005) | 30 | Prospective | Local HCC, ≥ 20 years old, all tumors within target volume and not invasive of GI tract, no previous abdominal radiation, no recent HCC treatment, bilirubin ≤ 3.0 mg/dL, AST and ALT < 5.0 X ULN, PS 0-2. | 76 CGE/20 fx | A: 67% B: 33% |
3 yr OS: 62% 3 yr LC: ~95% |
Acute: NS Late: HI within 6 months of treatment in 27% |
HCV in 90%; ICG R15 used to determine functional liver reserve. |
| Fukumitsu et al. (2009) | 51 | Prospective | Local HCC , ≤3 tumors ≤10 cm in diameter and ≥2 cm from the porta hepatis or GI tract, PS 0-2. | 66 CGE /10 fx | A: 80.4% B: 19.6% |
5 yr OS: 38.7% 5 yr LC: 87.8%; CR in 57% |
Acute: NS Late: 8% |
HCV in 66.6%; 65% had prior local therapy. |
Abbreviations: HCC – Hepatocellular carcinoma, PS –performance status, Fx – fraction, OS – overall survival, LC – local control, NS – not specified, CR – complete response, HCV – hepatitis C virus & ICG R15 - indocyanine green clearance and retention rate at 15 minutes, HI – hepatic insufficiency.
At a median follow-up interval of 31.7 months (range 3.1–133.2 months), the five-year local control rate was 86.9% and corresponding overall survival rate was 23.5%. Survival appeared to be impacted by the level of liver dysfunction with over 50% dying without tumor progression (usually complications of liver cirrhosis). The acute side effects were limited primarily to elevation in liver enzymes (9.7%) and only 5 patients (3%) had grade 2 or higher late toxicity. Although there was no difference in local control rates among the four radiation schedules of 79.2 CGE in 16 fractions, 85.8 CGE in 20 fractions, 92.4 CGE in 24 fractions, and 72.6 CGE in 22 fractions, the three patients who experienced treatment-related bile duct stenosis received 79.2 CGE in 16 fractions (2 patients) and91.3 CGE in 23 fractions (1 patient). Based on these results, a lower dose fractionation schedule delivering 72.6 CGE in 22 fractions was subsequently chosen for tumors involving the portahepatis and a more recent analysis of 53 patients treated in this manner revealed no evidence of biliary stenosis32. Among a subset of 50 patients with solitary tumors and Child Class A cirrhosis, the observed 5-year survival rate of 53.5% rivals that of surgery31.Similarlyfavorable outcomes have been observed in patients with unfavorable features and limited treatment options32–36. In several more recent studies in a similar patient population, similar overall survival and local control rates were seen37, 38.
Patients with portal venous thrombosis might benefit especially from proton radiotherapy because they tend to require that a larger volume of liver be irradiated and most of these patients have few treatment options. Often, because of the size, extent and location of the HCC and portal vein thrombosis, conventional photon radiotherapy at higher doses may not be feasible. In a series of 35 of these patients, Sugahara and colleagues found that treatment to 50–72 CGE led to local control rates of more than 45% at two years, with only 3 patients developing severe acute toxicity39. As noted previously, the excellent conformality of proton radiotherapy offers the possibility of retreatment if new HCCs arise within the liver further away from the originally treated HCC. The Tsukuba proton radiotherapy group has reported on the safety, feasibility, and efficacy of repeated courses of proton radiotherapy in a series of 27 patients with 68 lesions40. With a median interval of 24 months between the first and second courses, and a median dose of 66 CGE in 16 fractions for the re-treatment, they report a 5-year overall survival of 56% and a five-year local control rate of 87.8%.
Similarly, in a phase II trial, Kawashima and colleagues reported overall survival rates of 66% at 2 years in cirrhotic patients after proton radiotherapy to 76 CGE for in 3.8 CGE daily fractions41. Median tumor size was 4.5 cm (ranging from 2.5 to 8.2 cm), 40% of patients had vascular invasion, and one-third of all patients had Child-Pugh Class B cirrhosis. In this study, 27% of patients developed hepatic insufficiency, with a rate of grade 3 toxicity of about 40%.
Data regarding outcomes and toxicity with the use of protons in populations where Hepatitis B virus is non-endemic is much more limited. In a phase II trial of proton radiotherapy for HCC, Bush and colleagues treated 34 cases of unresectable HCC to 63 CGE in 15 fractions42. In this study two year overall survival was 55%, with local control rates of 75%. No RILD was seen in this study; however 60% of patients were noted to have mild acute toxicity due to radiotherapy. Furthermore, less than 10% of these patients experienced a gastrointestinal bleed, which was due, in large part, to the proximity of gross disease to the colon or duodenum. Finally, of these patients, 6 went to liver transplant and of these 2 patients had a complete response after pathological review.
A Phase I study evaluating the use of high dose conformal proton beam irradiation for HCC, cholangiocarcinoma and hepatic metastases was recently completed by Hong and colleagues (unpublished). A total of 22 lesions were treated with gated proton beam therapy using 4D treatment planning techniques. Generally, patients were treated to a dose of 45–75 CGE in 15 fractions, with dose escalation based on the liver EUD as well as predefined normal tissue constraints, especially for bowel and chestwall. In this trial, 1-year local control was 100%, with a median overall survival of 12 months. Acute toxicity in this trial was moderate, with 14% of patients developing grade 3 thrombocytopenia. Following treatment, 2 patients (both Child-Pugh Class B), developed grade 3 hyperbilirubinemia and 1 patient developed a grade 3 gastrointestinal bleed that resolved with conservative management. One patient with long standing portal hypertension and a congenital single ventricle died secondary to a stomach perforation adjacent to his liver tumor. One patient had documented worsening of Child-Pugh score by worsening encephalopathy. A maximum tolerated dose based on liver-related toxicity could not be established because dose-escalation was limited more by bowel and chest wall constraints, which vary considerably based on relative proximity of normal tissue to the tumor.
Overall, some of the best outcomes in HCC have been reported following charged particle therapy. Due to the variable potential for toxicity, based on location of HCC within the liver, ongoing studies use a schedule modeled on the Tsukuba experience where peripheral tumors receive a higher dose than central tumors.
Clinical outcomes with carbon ion therapy for HCCs
As carbon ion therapy is not widely available, outcomes and toxicity data are somewhat limited. Kato and colleagues treated 24 patients with HCC using carbon ion radiotherapy in a feasibility and dose-escalation study9. Median tumor size was 5 cm (ranging from 2.1 to 8.5 cm), 12.5% of patients had vascular invasion, and one-third of all patients had Child-Pugh Class B cirrhosis. In this series, patients were treated with step-wise dose escalation to between 49.5 to 79.5 CGE, with mild liver toxicity. Five-year local control rates were greater than 80%, with a 5-year overall survival of 25%. Only one patient developed a serious acute toxicity (radiation associated dermatitis) with no long term complications reported.
Conclusions
The distinctive biophysical attributes of charged particles, namely the lack of exit dose along the beam path beyond the tumor and higher biological effectiveness, confer unique advantages to charged particle therapy over photon radiotherapy in the treatment of HCC. These theoretical advantages have been borne out in clinical practice where multiple emerging and mature clinical experiences report consistent and durable in-field local control rates exceeding 80%. Taken together with the gratifying 5-year overall survival rates of nearly 25%, these results make a compelling argument for the use of proton radiotherapy as a viable liver-directed treatment option for patients with localized HCCs who are unable to undergo surgical resection or transplantation. In selected favorable clinical scenarios such as solitary tumors arising in the setting of preserved liver function, the remarkable 5-year overall survival rate of nearly 50% reported with proton radiotherapy makes this an even more appealing choice as a curative-intent treatment.
Defining a definitive role for charged particle therapy in the treatment of HCC is, however, hampered by the relative scarcity of treatment facilities and the lack of randomized trials demonstrating the clinical benefit of charged particle therapy over other modalities for treating HCC. Nevertheless, the accumulating evidence for the safety and efficacy of proton radiotherapy needs to be placed in the context of other liver-directed therapies that also largely face a similar hurdle of lacking randomized clinical data. Integration of charged particle therapy into clinical algorithms for HCC treatment will, therefore, require some individualization and multidisciplinary collaboration. In general, patients with nonmetastatic large (>5 cm) HCCs, either solitary or closely interspaced multifocal lesions, with or without satellitosis or portal vein invasion are suitable candidates for consideration of charged particle therapy. This is especially true for centrally located lesions or lesions in locations less optimal for radiofrequency ablation (close to the diaphragm or a major blood vessel). In scenarios such as major portal venous thrombosis, where treatment with other liver-directed therapies is a relative contraindication, proton radiotherapy is likely to offer a unique definitive treatment option that results in resolution of thrombosis and reconsideration of previously nonviable therapeutic options. In principle, it is likely that greater sparing of uninvolved liver using charged particle therapy may be safer in patients with Child-Pugh Class B and C cirrhosis. The rational use of carbon ion therapy for treating HCCs is based on extrapolations from proton radiotherapy and theoretical advantages of carbon ions over photons or protons. In coming years, the improved understanding of radiation tolerance of the cirrhotic liver, technological advances in imaging, tracking and targeting tumors during treatment, and improvements in charged particle therapy techniques (such as scanning beam technology for intensity modulated proton therapy) are likely to converge to further improve treatment outcomes following charged particle therapy for HCC.
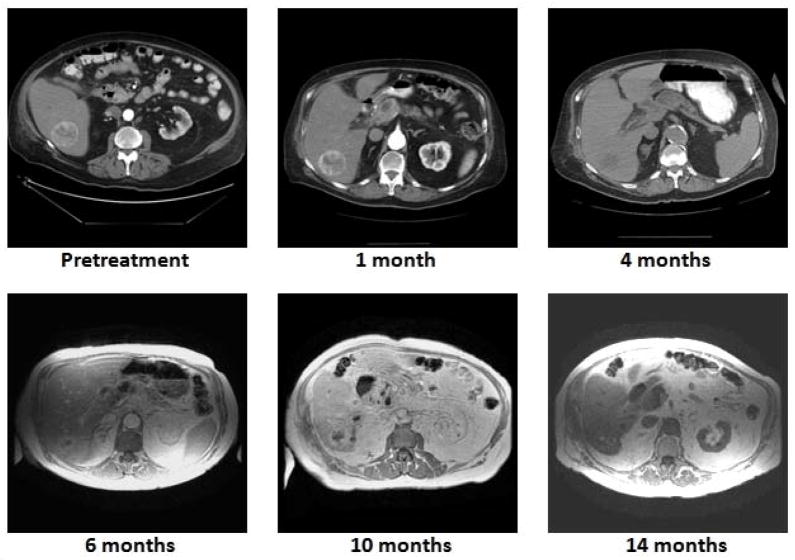
Figure 3.
Radiographic assessment of treatment response. Worsening renal function due to underlying coronary artery disease and peripheral vascular disease prompted switching to non-contrast CT (4 months) and magnetic resonance imaging (beyond 4 months). Note durable local control with progressive reduction in tumor volume and vascularity with evolution of central tumor necrosis.
Acknowledgments
The authors thank Matthew B. Palmer, MBA, CMD for valuable assistance with generating the figures. This work was supported in part by a program project grant from the National Institutes of Health (NIH CA021239) to TSH.
Footnotes
No financial disclosures pertinent to the work described herein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Dawson LA, McGinn CJ, Normolle D, Ten Haken RK, Walker S, Ensminger W, Lawrence TS. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000;18:2210–2218. doi: 10.1200/JCO.2000.18.11.2210. [DOI] [PubMed] [Google Scholar]
- 3.Seong J, Park HC, Han KH, Chon CY. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys. 2003;55:329–336. doi: 10.1016/s0360-3016(02)03929-9. [DOI] [PubMed] [Google Scholar]
- 4.Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:150–155. doi: 10.1016/s0360-3016(02)02864-x. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Krishnan S, Zhang X, Dong L, Briere T, Crane CH, Martel M, Gillin M, Mohan R, Beddar S. Proton radiotherapy for liver tumors: dosimetric advantages over photon plans. Med Dosim. 2008;33:259–267. doi: 10.1016/j.meddos.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Taddei PJ, Howell RM, Krishnan S, Scarboro SB, Mirkovic D, Newhauser WD. Risk of second malignant neoplasm following proton versus intensity-modulated photon radiotherapies for hepatocellular carcinoma. Phys Med Biol. 2010;55:7055–7065. doi: 10.1088/0031-9155/55/23/S07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerweck LE, Kozin SV. Relative biological effectiveness of proton beams in clinical therapy. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology. 1999;50:135–142. doi: 10.1016/s0167-8140(98)00092-9. [DOI] [PubMed] [Google Scholar]
- 8.Dale RG, Jones B, Cárabe-Fernández A. Why more needs to be known about RBE effects in modern radiotherapy. Applied Radiation and Isotopes: Including Data, Instrumentation and Methods for Use in Agriculture, Industry and Medicine. 2009;67:387–392. doi: 10.1016/j.apradiso.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Kato H, Tsujii H, Miyamoto T, Mizoe J, Kamada T, Tsuji H, Yamada S, Kandatsu S, Yoshikawa K, Obata T. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis1, 1. International Journal of Radiation OncologyBiologyPhysics. 2004;59:1468–1476. doi: 10.1016/j.ijrobp.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Ando K, Kase Y. Biological characteristics of carbon-ion therapy. International Journal of Radiation Biology. 2009;85:715–728. doi: 10.1080/09553000903072470. [DOI] [PubMed] [Google Scholar]
- 11.Carabe-Fernandez A, Dale RG, Hopewell JW, Jones B, Paganetti H. Fractionation effects in particle radiotherapy: implications for hypo-fractionation regimes. Physics in Medicine and Biology. 2010;55:5685–5700. doi: 10.1088/0031-9155/55/19/005. [DOI] [PubMed] [Google Scholar]
- 12.Raju MR. Heavy-particle studies with silicon detectors. Radiat Res Suppl. 1967;7:43–52. [PubMed] [Google Scholar]
- 13.Ando K, Koike S, Ohira C, Chen YJ, Nojima K, Ando S, Ohbuchi T, Kobayashi N, Shimizu W, Urano M. Accelerated reoxygenation of a murine fibrosarcoma after carbon-ion radiation. Int J Radiat Biol. 1999;75:505–512. doi: 10.1080/095530099140438. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RR. Radiological use of fast protons. Radiology. 1946;47:487–491. doi: 10.1148/47.5.487. [DOI] [PubMed] [Google Scholar]
- 15.Miller DW. A review of proton beam radiation therapy. Medical Physics. 1995;22:1943–1954. doi: 10.1118/1.597435. [DOI] [PubMed] [Google Scholar]
- 16.Cheung J, Kudchadker RJ, Zhu XR, Lee AK, Newhauser WD. Dose perturbations and image artifacts caused by carbon-coated ceramic and stainless steel fiducials used in proton therapy for prostate cancer. Phys Med Biol. 2010;55:7135–7147. doi: 10.1088/0031-9155/55/23/S13. [DOI] [PubMed] [Google Scholar]
- 17.Case RB, Sonke JJ, Moseley DJ, Kim J, Brock KK, Dawson LA. Inter- and intrafraction variability in liver position in non-breath-hold stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:302–308. doi: 10.1016/j.ijrobp.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 18.Casse BD, Lu WT, Banyal RK, Huang YJ, Selvarasah S, Dokmeci MR, Perry CH, Sridhar S. Imaging with subwavelength resolution by a generalized superlens at infrared wavelengths. Opt Lett. 2009;34:1994–1996. doi: 10.1364/ol.34.001994. [DOI] [PubMed] [Google Scholar]
- 19.Wunderink W, Mendez Romero A, Seppenwoolde Y, de Boer H, Levendag P, Heijmen B. Potentials and limitations of guiding liver stereotactic body radiation therapy set-up on liver-implanted fiducial markers. Int J Radiat Oncol Biol Phys. 2010;77:1573–1583. doi: 10.1016/j.ijrobp.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 20.Kothary N, Heit JJ, Louie JD, Kuo WT, Loo BW, Koong A, Chang DT, Hovsepian D, Sze DY, Hofmann LV. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. Journal of Vascular and Interventional Radiology: JVIR. 2009;20:235–239. doi: 10.1016/j.jvir.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Xi M, Liu M-Z, Deng X-W, Zhang L, Huang X-Y, Liu H, Li Q-Q, Hu Y-H, Cai L, Cui N-J. Defining internal target volume (ITV) for hepatocellular carcinoma using four-dimensional CT. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology. 2007;84:272–278. doi: 10.1016/j.radonc.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Beddar AS, Briere TM, Balter P, Pan T, Tolani N, Ng C, Szklaruk J, Krishnan S. 4D-CT imaging with synchronized intravenous contrast injection to improve delineation of liver tumors for treatment planning. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology. 2008;87:445–448. doi: 10.1016/j.radonc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins MA, Brock KK, Eccles C, Moseley D, Jaffray D, Dawson LA. Assessment of residual error in liver position using kV cone-beam computed tomography for liver cancer high-precision radiation therapy. Int J Radiat Oncol Biol Phys. 2006;66:610–619. doi: 10.1016/j.ijrobp.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Fuss M, Salter BJ, Cavanaugh SX, Fuss C, Sadeghi A, Fuller CD, Ameduri A, Hevezi JM, Herman TS, Thomas CR. Daily ultrasound-based image-guided targeting for radiotherapy of upper abdominal malignancies. International Journal of Radiation Oncology, Biology, Physics. 2004;59:1245–1256. doi: 10.1016/j.ijrobp.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Dawson LA, Brock KK, Kazanjian S, Fitch D, McGinn CJ, Lawrence TS, Ten Haken RK, Balter J. The reproducibility of organ position using active breathing control (ABC) during liver radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51:1410–1421. doi: 10.1016/s0360-3016(01)02653-0. [DOI] [PubMed] [Google Scholar]
- 26.Dawson LA, Eccles C, Bissonnette JP, Brock KK. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Radiat Oncol Biol Phys. 2005;62:1247–1252. doi: 10.1016/j.ijrobp.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 27.Eccles CL, Patel R, Simeonov AK, Lockwood G, Haider M, Dawson LA. Comparison of liver tumor motion with and without abdominal compression using cine-magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2011;79:602–608. doi: 10.1016/j.ijrobp.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 28.Briere TM, Beddar S, Balter P, Murthy R, Gupta S, Nelson C, Starkschall G, Gillin MT, Krishnan S. Respiratory gating with EPID-based verification: the MDACC experience. Physics in Medicine and Biology. 2009;54:3379–3391. doi: 10.1088/0031-9155/54/11/007. [DOI] [PubMed] [Google Scholar]
- 29.Guckenberger M, Sweeney RA, Wilbert J, Krieger T, Richter A, Baier K, Mueller G, Sauer O, Flentje M. Image-guided radiotherapy for liver cancer using respiratory-correlated computed tomography and cone-beam computed tomography. International Journal of Radiation Oncology, Biology, Physics. 2008;71:297–304. doi: 10.1016/j.ijrobp.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 31.Chiba T, Tokuuye K, Matsuzaki Y, Sugahara S, Chuganji Y, Kagei K, Shoda J, Hata M, Abei M, Igaki H, Tanaka N, Akine Y. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin Cancer Res. 2005;11:3799–3805. doi: 10.1158/1078-0432.CCR-04-1350. [DOI] [PubMed] [Google Scholar]
- 32.Mizumoto M, Tokuuye K, Sugahara S, Nakayama H, Fukumitsu N, Ohara K, Abei M, Shoda J, Tohno E, Minami M. Proton beam therapy for hepatocellular carcinoma adjacent to the porta hepatis. Int J Radiat Oncol Biol Phys. 2008;71:462–467. doi: 10.1016/j.ijrobp.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 33.Hata M, Tokuuye K, Sugahara S, Fukumitsu N, Hashimoto T, Ohnishi K, Nemoto K, Ohara K, Matsuzaki Y, Akine Y. Proton beam therapy for hepatocellular carcinoma with limited treatment options. Cancer. 2006;107:591–598. doi: 10.1002/cncr.22039. [DOI] [PubMed] [Google Scholar]
- 34.Hata M, Tokuuye K, Sugahara S, Kagei K, Igaki H, Hashimoto T, Ohara K, Matsuzaki Y, Tanaka N, Akine Y. Proton beam therapy for hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2005;104:794–801. doi: 10.1002/cncr.21237. [DOI] [PubMed] [Google Scholar]
- 35.Hata M, Tokuuye K, Sugahara S, Tohno E, Nakayama H, Fukumitsu N, Mizumoto M, Abei M, Shoda J, Minami M, Akine Y. Proton beam therapy for aged patients with hepatocellular carcinoma. International Journal of Radiation Oncology, Biology, Physics. 2007;69:805–812. doi: 10.1016/j.ijrobp.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Sugahara S, Oshiro Y, Nakayama H, Fukuda K, Mizumoto M, Abei M, Shoda J, Matsuzaki Y, Thono E, Tokita M, Tsuboi K, Tokuuye K. Proton beam therapy for large hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2010;76:460–466. doi: 10.1016/j.ijrobp.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 37.Nakayama H, Sugahara S, Tokita M, Fukuda K, Mizumoto M, Abei M, Shoda J, Sakurai H, Tsuboi K, Tokuuye K. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer. 2009;115:5499–5506. doi: 10.1002/cncr.24619. [DOI] [PubMed] [Google Scholar]
- 38.Fukumitsu N, Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Abei M, Shoda J, Thono E, Tsuboi K, Tokuuye K. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. International Journal of Radiation Oncology, Biology, Physics. 2009;74:831–836. doi: 10.1016/j.ijrobp.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 39.Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Tokita M, Abei M, Shoda J, Matsuzaki Y, Thono E, Tsuboi K, Tokuuye K. Proton-Beam Therapy for Hepatocellular Carcinoma Associated with Portal Vein Tumor Thrombosis*. Strahlentherapie und Onkologie. 2009;185:782–788. doi: 10.1007/s00066-009-2020-x. [DOI] [PubMed] [Google Scholar]
- 40.Hashimoto T, Tokuuye K, Fukumitsu N, Igaki H, Hata M, Kagei K, Sugahara S, Ohara K, Matsuzaki Y, Akine Y. Repeated proton beam therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:196–202. doi: 10.1016/j.ijrobp.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 41.Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, Nagase M, Nihei K, Ogino T. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005;23:1839–1846. doi: 10.1200/JCO.2005.00.620. [DOI] [PubMed] [Google Scholar]
- 42.Bush DA, Hillebrand DJ, Slater JM, Slater JD. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology. 2004;127:S189–193. doi: 10.1053/j.gastro.2004.09.033. [DOI] [PubMed] [Google Scholar]