Abstract
BACKGROUND
Increasing the activity of defective cystic fibrosis transmembrane conductance regulator (CFTR) protein is a potential treatment for cystic fibrosis.
METHODS
We conducted a randomized, double-blind, placebo-controlled trial to evaluate ivacaftor (VX-770), a CFTR potentiator, in subjects 12 years of age or older with cystic fibrosis and at least one G551D-CFTR mutation. Subjects were randomly assigned to receive 150 mg of ivacaftor every 12 hours (84 subjects, of whom 83 received at least one dose) or placebo (83, of whom 78 received at least one dose) for 48 weeks. The primary end point was the estimated mean change from baseline through week 24 in the percent of predicted forced expiratory volume in 1 second (FEV1).
RESULTS
The change from baseline through week 24 in the percent of predicted FEV1 was greater by 10.6 percentage points in the ivacaftor group than in the placebo group (P<0.001). Effects on pulmonary function were noted by 2 weeks, and a significant treatment effect was maintained through week 48. Subjects receiving ivacaftor were 55% less likely to have a pulmonary exacerbation than were patients receiving placebo, through week 48 (P<0.001). In addition, through week 48, subjects in the ivacaftor group scored 8.6 points higher than did subjects in the placebo group on the respiratory-symptoms domain of the Cystic Fibrosis Questionnaire–revised instrument (a 100-point scale, with higher numbers indicating a lower effect of symptoms on the patient’s quality of life) (P<0.001). By 48 weeks, patients treated with ivacaftor had gained, on average, 2.7 kg more weight than had patients receiving placebo (P<0.001). The change from baseline through week 48 in the concentration of sweat chloride, a measure of CFTR activity, with ivacaftor as compared with placebo was −48.1 mmol per liter (P<0.001). The incidence of adverse events was similar with ivacaftor and placebo, with a lower proportion of serious adverse events with ivacaftor than with placebo (24% vs. 42%).
CONCLUSIONS
Ivacaftor was associated with improvements in lung function at 2 weeks that were sustained through 48 weeks. Substantial improvements were also observed in the risk of pulmonary exacerbations, patient-reported respiratory symptoms, weight, and concentration of sweat chloride.
Cystic fibrosis, the most common lethal genetic disease in whites, affects approximately 70,000 people worldwide.1–3 There is no cure for this disease, and the progressive lung disease associated with it is the leading cause of death. Current treatments for cystic fibrosis target the secondary effects of dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) protein.
The CFTR protein is an epithelial ion channel contributing to the regulation of absorption and secretion of salt and water in various tissues, including the lung, sweat glands, pancreas, and gastrointestinal tract.4,5 Cystic fibrosis is caused by mutations in CFTR that affect the quantity of the protein that reaches the cell surface or that affect the function of CFTR channels at the cell surface.6,7 The missense mutation G551D is the most prevalent example of the latter.8 Approximately 4 to 5% of patients with cystic fibrosis have the G551D mutation on at least one allele.1,9
Ivacaftor (VX-770) is an investigational, orally bioavailable agent that is designed to increase the time that activated CFTR channels at the cell surface remain open (a “potentiator”). Ivacaftor was shown to augment the chloride-transport activity of G551D-CFTR protein in vitro.10 A small, randomized, controlled study of subjects with cystic fibrosis and at least one G551D-CFTR allele evaluated the safety profile of ivacaftor over the course of 14 to 28 days of treatment.11 In that study, ivacaftor led to significant changes from baseline in forced expiratory volume in 1 second (FEV1) and in two biomarkers of CFTR activity — sweat chloride and nasal potential difference — at several dose levels. The trial reported here was designed to evaluate the efficacy and safety of ivacaftor treatment for up to 48 weeks in subjects with cystic fibrosis who had a G551D-CFTR mutation.
METHODS
STUDY OVERSIGHT
We conducted a phase 3, randomized, double-blind, placebo-controlled, international study of orally administered ivacaftor (VX-770, Vertex Pharmaceuticals). The protocol, available with the full text of this article at NEJM.org, was reviewed and approved by the institutional review board at each participating center, and each subject provided written informed consent or written or oral assent. The protocol was designed by the sponsor (Vertex Pharmaceuticals) in collaboration with the academic authors. Site investigators collected the data, which were analyzed by the sponsor. All the authors had full access to the data. The lead author wrote the first draft of the manuscript and all the authors participated in subsequent revisions. The first author, after consultation with coauthors, made the decision to submit the manuscript for publication. All the authors vouch for the accuracy and completeness of the reported data and for the fidelity of the study, as reported, to the protocol.
STUDY SUBJECTS
Subjects were eligible for inclusion if they were 12 years of age or older, had received a diagnosis of cystic fibrosis,12 had the G551D mutation on at least one CFTR allele, and had an FEV1 of 40 to 90% of the predicted value for persons of their age, sex, and height.13 Subjects were randomly assigned, in a 1:1 ratio, to receive ivacaftor, at a dose of 150 mg every 12 hours, or placebo, for 48 weeks. Throughout the study, all subjects continued to take their prestudy medications with the exception of hypertonic saline, which was not permitted, since it does not have regulatory approval in the United States as a therapy for cystic fibrosis. Randomization was stratified according to age (<18 years vs. ≥18 years) and pulmonary function (<70% vs. ≥70% of the predicted FEV1).
END POINTS
The primary efficacy end point was the absolute change from baseline through week 24 in the percent of predicted FEV1. Secondary end points included the change from baseline through week 48 in the percent of predicted FEV1; the time to the first pulmonary exacerbation14 through week 24 and week 48; subject-reported respiratory symptoms through week 24 and week 48, as assessed with the use of the respiratory domain of the Cystic Fibrosis Questionnaire–revised (CFQ-R)15 (which is scored on a 100-point scale, with higher numbers indicating a lower effect of symptoms on the patient’s quality of life and 4 points considered to be a minimal clinically important difference); the change in weight from baseline to week 24 and week 48; and the change from baseline in the concentration of sweat chloride, a measure of CFTR channel function, through week 24 and week 48. Tertiary efficacy end points included the number and duration of pulmonary exacerbations, the total number of days of hospitalization for pulmonary exacerbations, and the need for antibiotic therapy for sinopulmonary signs or symptoms. Safety was also evaluated.
STATISTICAL ANALYSIS
On the basis of previous data on ivacaftor,11 we estimated that with a sample of at least 80 subjects, the study would have 80% power to detect a change of 4.5 percentage points in the percent of predicted FEV1. All subjects who received at least one dose of a study drug were included in the analyses. The primary analysis was based on a mixed-effects model for repeated measures. The primary end point and key secondary end points (absolute change from baseline through week 24 in the score on the CFQ-R respiratory domain, with pooled data from the children’s version and the adolescent–adult version of the instrument; absolute change from baseline through week 24 in the concentration of sweat chloride; time to first pulmonary exacerbation through week 48; and absolute change in weight from baseline at week 48) were analyzed with the use of a multistage gatekeeping procedure. The change in FEV1 through day 15 was analyzed with the use of linear comparisons between the treatment groups at the day 15 visit. Further details of the methods are provided in the statistical analysis plan included with the protocol and in the Supplementary Appendix, both of which are available at NEJM.org.
RESULTS
SUBJECTS
The study was conducted from June 2009 through January 2011. The screening, randomization, and follow-up of the subjects are shown in Figure 1 in the Supplementary Appendix. The study population consisted of 161 subjects who underwent randomization and received at least one dose of ivacaftor (83 subjects) or placebo (78). The mean age of the subjects was 25.5 years, and the mean percent of predicted FEV1 was 63.6; a total of 52% of the subjects were women or girls (Table 1). The mean concentrations of sweat chloride and the mean weights were similar in the two groups. At the time of study entry, 12 subjects in the placebo group (15%) and 8 in the ivacaftor group (10%) were using inhaled hypertonic saline, which they discontinued before receiving the first dose of the study drug. Confirmatory genotyping identified 1 subject in the placebo group who was homozygous for F508del-CFTR despite a previous test indicating a G551D allele. Data from this subject were included in the analyses.
Table 1.
Baseline Characteristics of the Subjects.*
| Characteristic | Placebo (N = 78) | Ivacaftor (N = 83) | Total (N = 161) |
|---|---|---|---|
| Sex — no. (%) | |||
| Male | 38 (49) | 39 (47) | 77 (48) |
| Female | 40 (51) | 44 (53) | 84 (52) |
| Non-Hispanic or white — no. (%)† | 77 (99) | 81 (98) | 158 (98) |
| Geographic distribution — no. (%) | |||
| North America | 50 (64) | 50 (60) | 100 (62) |
| Europe | 19 (24) | 23 (28) | 42 (26) |
| Australia | 9 (12) | 10 (12) | 19 (12) |
| Age — yr | |||
| Mean | 24.7 | 26.2 | 25.5 |
| Range | 12–53 | 12–53 | 12–53 |
| Age distribution — no. (%) | |||
| <18 yr | 17 (22) | 19 (23) | 36 (22) |
| ≥18 yr | 61 (78) | 64 (77) | 125 (78) |
| Height — cm | |||
| Mean | 166.5 | 167.7 | 167.1 |
| Range | 142.2–189.8 | 142.8–185.0 | 142.2–189.8 |
| Weight — kg | |||
| Mean | 61.2 | 61.7 | 61.5 |
| Range | 31.9–109.9 | 30.2–107.2 | 30.2–109.9 |
| Body-mass index‡ | |||
| Mean | 21.9 | 21.7 | 21.8 |
| Range | 15.2–38.6 | 14.8–38.9 | 14.8–38.9 |
| Positive for Pseudomonas aeruginosa — no. (%) | 57 (73) | 64 (77) | 122 (76) |
| Non–G551D-CFTR allele — no. (%)§ | |||
| Class I | 11 (14) | 10 (12) | 21 (13) |
| Class II¶ | 62 (79) | 70 (84) | 132 (82) |
| Class III|| | 1 (1) | 0 | 1 (1) |
| Class IV | 2 (3) | 2 (2) | 4 (2) |
| Class V | 1 (1) | 0 | 1 (1) |
| Unknown | 1 (1) | 1 (1) | 2 (1) |
| FEV1 | |||
| % of predicted value | |||
| Mean | 63.7 | 63.5 | 63.6 |
| Range | 31.6–97.1 | 37.3–98.2 | 31.6–98.2 |
| Distribution — no. (%) | |||
| <70% of predicted value | 45 (58) | 49 (59) | 94 (58) |
| ≥70% of predicted value | 33 (42) | 34 (41) | 67 (42) |
| Sweat chloride — mmol/liter | |||
| Mean | 100.1 | 100.4 | 100.2 |
| Range | 58.0–121.5 | 74.5–128.0 | 58.0–128.0 |
None of the characteristics differed significantly between the groups (P>0.05 for all comparisons). FEV1 denotes forced expiratory volume in 1 second.
Race or ethnic group was self-reported.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The classes of CFTR mutations are based on their molecular mechanisms in the cell, as classified by Welsh et al.16
One subject in the placebo group who was homozygous for G551D-CFTR is included here.
One subject in the placebo group who was homozygous for F508del-CFTR is included here.
A total of 77 subjects in the ivacaftor group (93%) and 68 in the placebo group (87%) completed 48 weeks of treatment. The mean rate of adherence to the study drug was 91% in the ivacaftor group and 89% in the placebo group. Of the 145 subjects who completed 48 weeks of treatment, only 1 subject (in the placebo group) declined to enter the open-label extension study (VX08-770-105).
CLINICAL EFFICACY
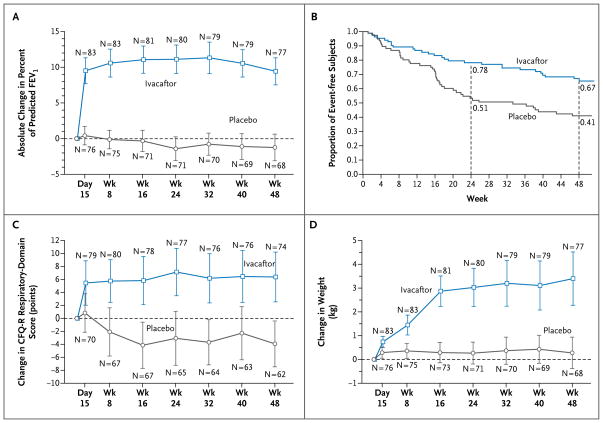
Through week 24, there was an increase from baseline of 10.4 percentage points in the percent of predicted FEV1 in the ivacaftor group, as compared with a decrease of 0.2 percentage points in the placebo group — a treatment effect of 10.6 percentage points (P<0.001) (Fig. 1A). The improvement in the ivacaftor group reflected a mean increase in FEV1 of 0.367 liters, as compared with an increase of 0.006 liters in the placebo group (treatment effect, 0.361 liters; P<0.001) through week 24, which corresponded to a relative change from baseline of 17.2% in the ivacaftor group as compared with 0.1% in the placebo group. An effect of ivacaftor was noted by day 15 of treatment (treatment effect, 9.3 percentage points; P<0.001). A significant treatment effect was maintained throughout the study, with a change in the percent of predicted FEV1 from baseline through week 48 that was 10.5 percentage points greater with ivacaftor than with placebo (P<0.001) (Table 1 in the Supplementary Appendix). The distribution of individual changes from baseline through week 24 showed that nearly 75% of the subjects who were treated with ivacaftor had a mean improvement of 5 percentage points or more (Fig. 2 in the Supplementary Appendix). The change in the percent of predicted FEV1 was also analyzed within predefined subgroups, including subgroups defined according to baseline FEV1, age, and sex. The effect of ivacaftor as compared with placebo was significant in each subgroup that was analyzed (Table 2).
Figure 1. Changes from Baseline in Percent of Predicted FEV1, Respiratory Symptoms, and Weight, and Time to the First Pulmonary Exacerbation, According to Study Group.
Panel A shows the absolute mean change from baseline in the percent of predicted forced expiratory volume in 1 second (FEV1), through week 48. Panel B shows the time to the first pulmonary exacerbation, expressed as estimates of the proportion of subjects free from events. Panel C shows the absolute mean change from baseline in the score on the respiratory domain of the Cystic Fibrosis Questionnaire–revised (CFQ-R), a quality-of-life questionnaire that is scored on a 100-point scale, with higher numbers indicating a lower effect of symptoms on the patient’s quality of life. The established minimum clinically important difference for the CFQ-R respiratory domain is 4 points. Panel D shows the absolute mean change from baseline in weight, through week 48. The values and the 95% confidence intervals (indicated by I bars) in Panels A, C, and D are unadjusted. The first data points in Panels A, C, and D are baseline data.
Table 2.
Treatment Effect of Ivacaftor with Respect to the Change from Baseline through Week 48 in the Percent of Predicted FEV1, According to Subgroups.*
| Subgroup | Treatment Effect | P Value |
|---|---|---|
| Baseline % of predicted FEV1 | ||
| <70% | 10.6 | <0.001 |
| ≥70% | 10.3 | <0.001 |
| Geographic region | ||
| North America | 9.0 | <0.001 |
| Europe | 9.9 | <0.001 |
| Australia | 11.9 | 0.008 |
| Sex | ||
| Male | 11.0 | <0.001 |
| Female | 11.6 | <0.001 |
| Age | ||
| <18 yr | 11.4 | 0.005 |
| ≥18 yr | 9.9 | <0.001 |
The treatment effect represents the difference between the ivacaftor group and the placebo group with respect to the absolute change from baseline through week 48 in the percent of predicted FEV1.
At week 48, a total of 67% of subjects in the ivacaftor group, as compared with 41% in the placebo group, were free from pulmonary exacerbations, corresponding to a hazard ratio with ivacaftor of 0.455 (P = 0.001), or a 55% reduction in the risk of pulmonary exacerbation (Fig. 1B). There were 99 exacerbations (in 44 subjects) in the placebo group, as compared with 47 exacerbations (in 28 subjects) in the ivacaftor group. A total of 31 events (in 23 subjects) in the placebo group, as compared with 21 events (in 11 subjects) in the ivacaftor group, led to hospitalization. The mean (±SD) total number of days of hospitalization for pulmonary exacerbations per subject (normalized to a 48-week period) was 3.9±13.6 in the ivacaftor group, as compared with 4.2±8.7 in the placebo group (P=0.03) (Table 2 in the Supplementary Appendix).
Subjects treated with ivacaftor, as compared with those receiving placebo, had an improvement in scores on the CFQ-R respiratory domain (indicating a reduction in respiratory symptoms). From baseline to week 48, the scores increased by 5.9 points in the ivacaftor group, as compared with a decrease of 2.7 points in the placebo group (treatment effect, 8.6 points; P<0.001) (Fig. 1C). By week 48, subjects in the ivacaftor group had gained 3.1 kg, as compared with a gain of 0.4 kg in the placebo group (treatment effect, 2.7 kg; P<0.001) (Fig. 1D).
CFTR FUNCTION
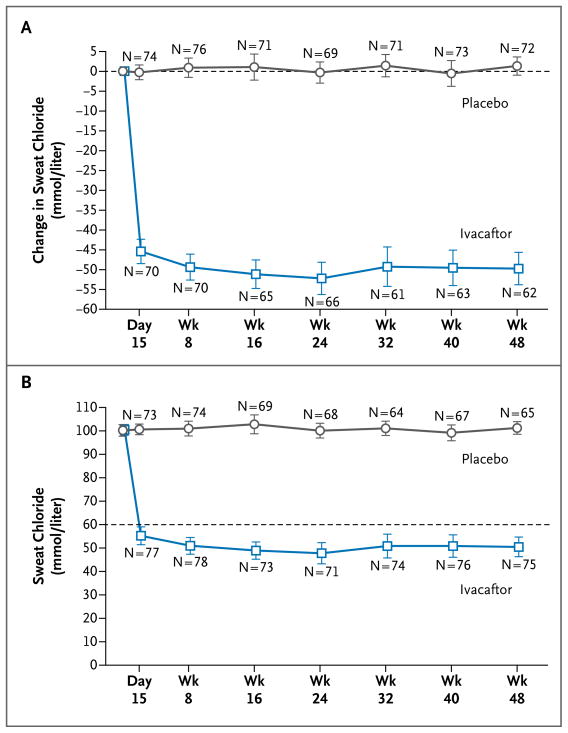
Through week 24, the change from baseline in sweat chloride was −48.7 mmol per liter in the ivacaftor group and −0.8 mmol per liter in the placebo group (treatment effect, −47.9 mmol per liter; P<0.001) (Fig. 2A). The mean values for sweat chloride were 47.8 and 100.0 mmol per liter in the ivacaftor and placebo groups, respectively, at week 24 (Fig. 2B). A treatment effect was initially seen at day 15 and was maintained through week 48 (treatment effect, −48.1; P<0.001).
Figure 2. Changes from Baseline through Week 48 in Sweat Chloride, According to Study Group.
Panel A shows the mean change from baseline in the concentration of sweat chloride. Panel B shows the actual mean concentrations of sweat chloride over time; the dashed line at 60 mmol per liter represents the cutoff point for the diagnosis of cystic fibrosis. The values and 95% confidence intervals (indicated by I bars) in both panels are unadjusted. The first data points in both panels are baseline data.
SAFETY AND ADVERSE-EVENT PROFILE
The incidence of adverse events through week 48 was similar in the two groups (Table 3). As compared with the placebo group, the ivacaftor group had a higher incidence of adverse events leading to interruption of the study drug (13% vs. 6%) (Table 3 in the Supplementary Appendix). All the subjects who interrupted treatment were able to resume taking the study drug and to complete the trial, with the exception of one subject in the placebo group who subsequently withdrew from the study owing to severe respiratory distress. Subjects in the ivacaftor group had a lower incidence of adverse events leading to discontinuation of the study drug (1% vs. 5%). Overall, five adverse events led to discontinuation: four in the placebo group (increased hepatic enzyme levels, atrioventricular block, panic attack, and respiratory failure) and one in the ivacaftor group (increased hepatic enzyme levels). Adverse events occurring in at least 10% of the subjects in either group are shown in Table 4 in the Supplementary Appendix. Pulmonary exacerbation, cough, hemoptysis, and decreased pulmonary function occurred less frequently in the ivacaftor group than in the placebo group (≥5 percentage-point difference between the groups in incidence; minimum 10% incidence in either group). Adverse events that occurred more frequently in the ivacaftor group were headache, upper respiratory tract infection, nasal congestion, rash, and dizziness; none of these events were considered to be serious or led to discontinuation of the study drug.
Table 3.
Adverse Events.
| Adverse Event | Placebo (N = 78) | Ivacaftor (N = 83) |
|---|---|---|
| no. of subjects (%) | ||
| Any adverse event | 78 (100) | 82 (99) |
|
| ||
| Serious adverse event* | 33 (42) | 20 (24) |
|
| ||
| Pulmonary exacerbation | 26 (33) | 11 (13) |
|
| ||
| Hemoptysis | 4 (5) | 1 (1) |
|
| ||
| Hypoglycemia | 0 | 2 (2) |
|
| ||
| Adverse event leading to study-drug interruption | 5 (6) | 11 (13) |
|
| ||
| Adverse event leading to study-drug discontinuation | 4 (5) | 1 (1) |
Included are serious adverse events that occurred in more than one subject per group.
A total of 53 serious adverse events were reported over the course of the treatment period. There was a lower rate of serious adverse events in the ivacaftor group than in the placebo group (24% vs. 42%). Pulmonary exacerbation and hemoptysis occurred more frequently in the placebo group than in the ivacaftor group. There were no cases of hypoglycemia in the placebo group, whereas there were two cases in the ivacaftor group: one of the subjects had diabetes related to the cystic fibrosis and was receiving insulin, and the other had had previous episodes of symptoms suggestive of hypoglycemia. No deaths occurred during the study. No clinically important trends attributable to ivacaftor were identified in clinical laboratory tests (serum chemical, hematologic, and coagulation tests and urinalysis), vital signs, digital or ambulatory electrocardiograms, or physical examinations.
DISCUSSION
In this randomized, placebo-controlled trial, administration of ivacaftor, an oral CFTR potentiator, was associated with significant improvements in primary and secondary end points in persons with cystic fibrosis who had at least one copy of the G551D-CFTR mutation. Progressive loss of lung function is a major source of illness in patients with cystic fibrosis, and decreased FEV1 is associated with an increased risk of death.5 Consequently, FEV1 has been a key end point for the evaluation of new therapies for cystic fibrosis. Inhaled tobramycin, as compared with placebo, was associated with a 12% increase in the improvement from baseline in FEV1 at 20 weeks17; dornase alfa, as compared with placebo, was associated with a 5.8% improvement in FEV1 after 24 weeks14; and hypertonic saline was associated with a 3.2% improvement in FEV1 after 48 weeks.18 The standards for managing cystic fibrosis have changed considerably over the past two decades.19 The subjects in the current study received the standard of care, with the exception of hypertonic saline therapy. In the year before the study and during the course of the study, subjects received dornase alfa (69%), oral azithromycin (63%), and inhaled tobramycin (39%). When added to these therapies, ivacaftor, as compared with placebo, was associated with a relative improvement of 17.2% in FEV1 over baseline values at 24 weeks, a change that was sustained to 48 weeks. Nearly 75% of the subjects who were treated with ivacaftor had a mean improvement through week 24 of 5 percentage points or more in the percent of predicted FEV1. The fact that lung function in some subjects did not appear to have a response to ivacaftor may indicate that other factors, such as pulmonary exacerbation during the course of the study, might have occurred in these subjects. The subjects in the ivacaftor trial were slightly older than were participants in studies of other therapies,14,16,17 but the severity of disease, as measured by baseline FEV1, was similar. Subgroup analyses were conducted to ascertain whether the clinical response was affected by age, sex, or severity of lung disease. Although the number of subjects included in some of these subgroups was small, and caution is therefore advised in drawing conclusions, the analyses revealed consistent responses across subgroups.
Pulmonary exacerbations are another clinically important end point, since they frequently lead to hospitalization, and 25% of hospitalized patients have permanent loss of lung function.20 At 24 weeks, dornase alfa, as compared with placebo, reduced the risk of exacerbations by 22%.14 At 48 weeks, hypertonic saline, as compared with placebo, reduced the risk by 66%.18 With the use of a similar definition of exacerbation, ivacaftor, as compared with placebo, in addition to the standard of care, resulted in a relative reduction in the risk of exacerbation of 60% at 24 weeks and 55% at 48 weeks. Treatment with ivacaftor also reduced the number of days of hospitalization, the total number and duration of exacerbations, and the number of pulmonary exacerbations requiring intravenous antibiotics. The within-group change in the score on the CFQ-R respiratory domain through 48 weeks exceeded the minimal clinically important difference of 4 points established for this domain in patients with stable disease.21
Patients with cystic fibrosis typically have difficulty gaining and maintaining weight.1 Weight gain was measured in two studies of oral azithromycin.22,23 In those trials, the mean treatment effect with azithromycin as compared with placebo during the 24-week treatment period was a weight gain of 0.7 kg in subjects with endobronchial colonization with Pseudomonas aeruginosa22 and 0.58 kg in subjects without P. aeruginosa endobronchial colonization,23 and these treatment effects were associated with improved pulmonary status in the subjects receiving azithromycin. In the current study, the weight gain at 48 weeks was 3.1 kg in subjects receiving ivacaftor, as compared with 0.4 kg in subjects receiving placebo, with a similar between-group difference at 24 weeks. In patients with cystic fibrosis, weight is affected by multiple factors, including pancreatic insufficiency with maldigestion, increased caloric needs, diabetes, and anorexia.5,24 A systemic CFTR modulator, such as ivacaftor, may also affect CFTR function in gastrointestinal epithelia, which may contribute to improved absorption of nutrients in patients with cystic fibrosis; however, no causal relationship was studied or proven in this trial.
CFTR plays an important role in the reabsorption of chloride in the sweat duct.25 We observed a large correction of elevated levels of sweat chloride in the ivacaftor group, as compared with the placebo group, as early as 2 weeks after the initiation of the study drug. Ivacaftor is the first agent to show a reduction in the sweat chloride level to values below the diagnostic threshold for cystic fibrosis (60 mmol per liter). This finding confirms the observation from an early-phase trial that ivacaftor improved CFTR-mediated ion-transport function.11
The mechanisms by which changes in CFTR function may lead to pulmonary and weight changes are incompletely understood and probably multifactorial. In vitro studies of air–liquid interface cultures of bronchial epithelial cells from the lungs of patients with cystic fibrosis have shown that correction of abnormal CFTR-mediated ion transport increases the air–surface fluid level and ciliary beat frequency.10 Thus, the improvement in FEV1 observed after 2 weeks in the current study may reflect improved airway clearance. The limited additional improvements in FEV1 through 48 weeks suggest that continued longitudinal data will be required to assess whether CFTR modulation can effect further physiological changes in the airways of patients with cystic fibrosis. Weight gain appeared to plateau after 16 weeks. This may indicate either that subjects reached their ideal body weight or that other physiological factors prevented them from gaining additional weight. The longer-term effects of modulation of CFTR on lung function will be monitored in the participants receiving ivacaftor in the ongoing open-label follow-up study. Although the complete chain of events from CFTR dysfunction to ion-transport imbalance to progressive obstructive and destructive airway disease is still unknown, this study suggests that a drug targeting CFTR dysfunction can affect lung function and symptoms, thus confirming that CFTR is a valid therapeutic target and providing an important tool for further study of the pathophysiology of cystic fibrosis.
Daily oral administration of ivacaftor for 48 weeks was not associated with a greater safety risk than that observed with placebo. Serious adverse events were less common in the ivacaftor group, primarily owing to a reduced incidence of pulmonary exacerbations and hemoptysis. The frequency of liver enzyme levels that were more than 2 times the upper limit of the normal range for age was similar in the ivacaftor and placebo groups (Table 5 in the Supplementary Appendix) and was also similar to the frequency in adult and adolescent populations with cystic fibrosis.26
In summary, these findings represent an important milestone in the development of treatments designed to improve CFTR protein function as a means of addressing the underlying cause of cystic fibrosis and begin to fulfill the promise ushered in with the discovery of the CFTR gene.
Supplementary Material
Acknowledgments
Supported by Vertex Pharmaceuticals; grants from the National Institute for Health Research Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield National Health Service Foundation Trust and Imperial College London; grants from the Cystic Fibrosis Foundation Therapeutic Development Center; the Institute for Translational Health Sciences; the National Center for Research Resources of the National Institutes of Health (NIH) (UL1 RR025014 to the University of Washington, UL1 RR024153 to Children’s Hospital of Pittsburgh of the University of Pittsburgh Medical Center, and UL1 RR 025005 to Johns Hopkins University); the Cystic Fibrosis Foundation Therapeutics Development Network Coordinating Center (to Seattle Children’s Hospital); Clinical Translational Research Center (UL1-RR-024134 to the University of Pennsylvania/Children’s Hospital of Philadelphia); and the NIH (UL1 RR024989 and P30 DK27651 to Case Western Reserve University, UL1 RR 025758 to Children’s Hospital Boston, K23 DK075788 and 5UL1 RR025777 to the University of Alabama at Birmingham, and 1UL1 RR025744 to Stanford University); and an infrastructure grant from Northern Ireland Clinical Research Network (Respiratory Medicine).
We thank Nicole Mayer-Hamblett, Ph.D., of Seattle Children’s Hospital; all the patients involved in the study; the study coordinators (see the Supplementary Appendix for a complete list); and the following employees of Vertex Pharmaceuticals: Adrienne Aiello, Pharm.D., and Barry Lubarsky, Ph.D., for providing medical-writing, editorial, and coordination support; Lily Lee, Ph.D., for preparing the study document; Robert Kauffman, M.D., Ph.D., for providing advice on the study design and data interpretation; Christopher Simard, M.D., for performing the patient-safety analysis; Sorana Ailinca, Nikki Shannon, R.N., B.H.Sc.A., and Jennifer Webster, M.B.A., for providing clinical-operations support; and Jiuhong Zha, Ph.D., for performing the pharmacokinetic analysis.
(Funded by Vertex Pharmaceuticals and others; VX08-770-102 ClinicalTrials.gov number, NCT00909532.)
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Patient registry: 2008 annual data report to the Center directors. Bethesda, MD: Cystic Fibrosis Foundation; 2009. [Google Scholar]
- 2.Farrell PM. The prevalence of cystic fibrosis in the European Union. J Cyst Fibros. 2008;7:450–3. doi: 10.1016/j.jcf.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–51. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 4.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 5.Welsh MJ, Ramsey BW, Accurso FJ, Cutting GR. Cystic fibrosis. In: Scriver CR, Beaudet AR, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease. 8. New York: McGraw-Hill; 2001. pp. 521–88. [Google Scholar]
- 6.Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–80. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 7.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [Erratum, Science 1989;245:1437.] [DOI] [PubMed] [Google Scholar]
- 8.LeGrys VA. Sweat analysis proficiency testing for cystic fibrosis. Pediatr Pulmonol. 2000;30:476–80. doi: 10.1002/1099-0496(200012)30:6<476::aid-ppul7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361:1671–6. doi: 10.1016/S0140-6736(03)13368-5. [DOI] [PubMed] [Google Scholar]
- 10.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106:18825–30. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrell PM, Rosenstein BJ, White TB, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–34. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med. 1994;331:637–42. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 15.Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128:2347–54. doi: 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]
- 16.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73:1251–4. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- 17.Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 18.Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–40. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 19.Flume PA, O’Sullivan BP, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957–69. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 20.Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182:627–32. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest. 2009;135:1610–8. doi: 10.1378/chest.08-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290:1749–56. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 23.Saiman L, Anstead M, Mayer-Hamblett N, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2010;303:1707–15. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 24.Durie PR, Pencharz PB. A rational approach to the nutritional care of patients with cystic fibrosis. J R Soc Med. 1989;82(Suppl 16):11–20. [PMC free article] [PubMed] [Google Scholar]
- 25.Rich DP, Anderson MP, Gregory RJ. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990;347:358–63. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- 26.Goss CH, Mayer-Hamblett N, Kronmal RA, Williams J, Ramsey BW. Laboratory parameter profiles among patients with cystic fibrosis. J Cyst Fibros. 2007;6:117–23. doi: 10.1016/j.jcf.2006.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.