Abstract
Generalized social anxiety disorder (GSAD) is characterized by excessive fears of scrutiny and negative evaluation, but neural circuitry related to scrutiny in GSAD has been little-studied. In this study, 16 unmedicated adults with GSAD and 16 matched healthy comparison (HC) participants underwent functional MRI to assess neural response to viewed images of faces simulating movement into eye contact versus away from eye contact. GSAD patients were then treated for eight weeks with paroxetine and 15 patients were re-imaged. At baseline, GSAD patients had elevated neural response to eye contact in parahippocampal cortex, inferior parietal lobule, supramarginal gyrus, posterior cingulate and middle occipital cortex. During paroxetine treatment, symptomatic improvement was associated with decreased neural response to eye contact in regions including inferior and middle frontal gyri, anterior cingulate, posterior cingulate, precuneus and inferior parietal lobule. Magnitude of GSAD symptom reduction with paroxetine treatment, and GSAD diagnosis in comparison to HCs at baseline, were both associated with neural processing of eye contact in distributed networks that included regions involved in self-referential processing. These findings demonstrate that eye contact in GSAD engages neurocircuitry consistent with the heightened self-conscious emotional states known to characterize GSAD patients during scrutiny.
Keywords: fMRI, social phobia, self-referential processing
1. Introduction
Social anxiety disorder (SAD) has a lifetime prevalence of 5 - 13% (Kessler et al., 1994, 2005) and is characterized by excessive fear of situations involving potential scrutiny by others, and by self-conscious emotions of embarrassment and humiliation. Generalized SAD (GSAD) is a subtype characterized by fear and avoidance of most social situations. It is associated with greater severity of symptoms, social and occupational impairment, depression, substance abuse and suicide (Schneier, 2006). Cognitive behavioral therapy and selective serotonin reuptake inhibitors (SSRIs) have established efficacy for SAD, but neural mechanisms of treatment response are not well-understood (Schneier, 2006).
Fears of making eye contact or being looked at, which evoke feelings of scrutiny and self-consciousness in persons with SAD, are associated with severity of SAD (Schneier et al., 2011). Leading explanatory models of SAD highlight the role of self-focused attention (Clark and Wells, 1995; Schultz and Heimberg, 2008) and biased attention to threat (Bögels and Mansell, 2004), and factor analyses of the Liebowitz Social Anxiety Scale (LSAS) (Liebowitz, 1987), which includes a “fear of eye contact” item, are consistent with this fear being a core feature of SAD (Safren et al., 1999; Baker et al., 2002; Stein et al., 2004). Eye contact functions more generally across primate species as an essential social signal, providing information on identity, status, interest, and intent (Emery, 2004).
Eye contact in SAD might be expected to engage brain regions involved in processing gaze direction, self-referential processing, and fear. Functional magnetic resonance imaging (fMRI) studies in monkeys and healthy subjects have most consistently identified the superior temporal sulcus to be involved in normal processing of others’ gaze direction (Nummenmaa and Calder, 2009). A meta-analysis of neuroimaging studies utilizing a variety of stimuli related to the self found that self-referential processing is mediated by cortical midline structures (Northoff et al., 2006). These include the ventromedial prefrontal cortex, dorsomedial prefrontal cortex, and posterior cingulate cortex/precuneus.
Neurocircuitry associated with eye contact or scrutiny fears has been little studied in SAD or other disorders. Most fMRI studies in SAD have used harsh facial expressions as threat stimuli, and a meta-analysis has documented increased activation of fear-related circuitry including amygdala, insula, hippocampus, and anterior cingulate (Etkin and Wager, 2007). These regional activations to facial expressions are also observed in other anxiety disorders, and during fear learning in healthy subjects (HCs) (Etkin and Wager, 2007). A limitation of prior imaging studies using threat stimuli has been lack of controls for individual differences in attention paid to stimuli, a known modulator of neural responses to facial expressions in anxiety disorder patients and HCs (Pessoa et al., 2002, 2005; Mitchell et al., 2007). Additionally, few studies have examined changes in neural activity in response to treatment of SAD, and none of these treatment studies have used fMRI, which offers advantages of high resolution, sensitivity to pharmacodynamic effects, and ability to assess neural function during performance of an ecologically valid task (Furmark et al., 2002; Kilts et al., 2006; Evans et al., 2008).
This study of GSAD patients and HCs used fMRI to contrast neural responses to viewing direct gaze from another person (i.e. involuntary eye contact, known to be feared by many GSAD patients, but inherently neutral in emotional valence) versus viewing averted gaze. Eye position of participants was monitored to assess visual attention to gaze stimuli in the scanner. Goals of this study were to compare neural response to direct vs. averted gaze stimuli in GSAD patients and HCs, and within the GSAD group to assess the relationship of changes in activations to changes in symptom severity during 8 weeks of treatment with the SSRI paroxetine.
2. Methods
2.1 Participants
Eighteen adults with a primary diagnosis of GSAD (age 20-52) and 17 HCs were recruited through media notices and clinical referrals. Diagnoses were based on psychiatric interview and confirmed by the Structured Clinical Interview for DSM-IV Axis I disorders (First et al., 1995). Exclusion criteria for GSAD participants included having a current Axis I disorder (other than secondary diagnoses of generalized anxiety disorder, dysthymia, or specific phobia), major depressive episode in the past year, substance abuse in the past six months, and clinically significant general medical conditions. HCs did not meet criteria for any lifetime Axis I disorder. Health status was confirmed by a physical examination including drug toxicology screen. All subjects were free of psychotropic medications for at least four weeks prior to study entry.
Data from two GSAD patients were excluded from analyses (one subsequently revealed a recent history of major depression, and one failed to follow imaging task instructions), yielding 16 evaluable GSAD patients. HCs were matched to patients by age, sex, and race. One HC failed to follow task instructions and was replaced, yielding 16 evaluable HCs.
Secondary comorbid diagnoses in participants with GSAD consisted of current generalized anxiety disorder (N=3), past major depression (N=6), and past alcohol abuse (N=1). Six GSAD subjects had taken medication for anxiety or depression prior to the past four weeks.
All subjects provided written informed consent after discussion of study procedures. This study was approved by the Institutional Review Board of New York State Psychiatric Institute.
2.2 Experimental Design
All participants underwent fMRI imaging at baseline, and GSAD patients were asked to return for a repeat imaging session after 8 weeks of treatment with paroxetine. Prior to each imaging session, participants were familiarized with study stimuli and tasks outside the scanner.
GSAD patients started paroxetine treatment after the first imaging session. The treating psychiatrist saw patients weekly for the first 2 weeks, then biweekly. Paroxetine dose was adjusted as clinically indicated within the range of 10-60 mg/day, and participants did not receive other psychoactive medications or any psychotherapy.
Clinical assessments were performed before each imaging session by a study clinician. Primary clinical assessment measures were the Liebowitz Social Anxiety Scale (LSAS), widely used in clinical trials to assess severity of SAD, and the Clinical Global Impression - Improvement scale (CGI-I) (Guy, 1976), which provides 7-point ratings of change from baseline, adapted for SAD with specific anchors (Zaider et al., 2003). The 17-item Hamilton Rating Scale for Depression (HRSD-17) (Hamilton, 1967) was administered to confirm the absence of clinically significant depression. Participants also completed the self-rated Gaze Anxiety Rating Scale (GARS), which assesses fear and avoidance of eye contact in 17 interpersonal situations (Schneier et al., 2011).
Stimuli were produced from photographs of faces of 12 male and 12 female adults with neutral expressions and three directions of eye gaze (neutral, direct, and averted) for each individual, modified from Schneier et al. (2009). Each face was displayed against a black background, with the chin aligned 30 degrees from the frontal plane (to the subject’s right). Each trial consisted of a sequence of two photographs of the same individual, beginning with a 1000 msec image showing neutral direction of eye gaze, aligned with the viewed individual’s face (i.e., gazing to the subject’s right). In the “averted gaze” trial the first image was immediately followed by a 1000 msec image of the same face identically aligned, but with eyes gazing upward. The “direct gaze” trial differed in that eyes in the second image align directly toward the subject, giving the illusion that gaze moves into eye contact. Thus direct and averted gaze stimuli varied only in path of apparent movement of gaze.
The run consisted of 16 blocks, eight of direct gaze and eight of averted gaze, presented in random order, with 10 sec intervals of viewing crosshair between blocks. Each block consisted of three face trials. Individual faces were presented in random order, and each individual face was presented in one direct gaze trial and one averted gaze trial. Subjects used a keypad to report for each face trial whether gaze was directed toward the subject or away.
2.3 Eye tracking data acquisition and processing
Subjects viewed the stimuli through goggles (Avotec Silent Vision™ SV-4021 Fiber Optic Visual System, Avotec, Inc., Stuart, FL) mounted to the head coil. Goggles were equipped with an eye-tracking device (Avotec Real Eye™ RE-4501 Fiber Optic Eye Imaging System) combined with iViewX Tracking System (SensoMotoric Instruments, Inc., Boston, MA), which continually recorded gaze position at a sample rate of 60 Hz through simultaneous pupil and corneal reflex tracking. The eye-tracking device was triggered simultaneously with the scanner, and to minimize distortion in gaze-position measurement, the built-in 9-point calibration procedure of the iViewX system was augmented by a 4-by-4 point calibration prior to each experimental run.
Eye tracking data analysis utilized the iView X bundled analysis package for fixation analysis. Data from the additional calibration procedure were processed by an artificial neural network interface using a Parameterized Self-Organizing Map, variants of which have previously been shown to considerably reduce distortions in gaze-position measurement (Pomplun et al., 1994; Essig et al., 2006). Pictorial analysis was performed with data for each stimulus block overlaid onto a sample stimulus image, giving scanpaths and eye fixations displayed with raindrop analysis (fixation duration directly proportional to diameter of circle). During post-processing, a rectangular “object” was created to encompass the eye region, which was previously aligned for all stimulus images during the stimulus development phase. Fixation analyses were further processed using Matlab (The MathWorks, Inc., Natick, MA) to give fixation duration and position for each stimulus condition. This analysis revealed the number and length of the subject’s gaze fixations within the defined object area for each condition. Eye fixations were defined as eye position maintained within a 30-pixel diameter region for at least 83 ms (five consecutive gaze-position samples). Percent of fixations in the eye region was calculated by dividing fixations within the eye region by total number of fixations. Scanpath length was computed as the sum (in pixels) of the Euclidean distances between all consecutive fixations within a trial.
2.4 Image acquisition and analysis
Images were obtained on a 1.5 T GE Signa Twin Speed MRI scanner. Functional images were acquired parallel to the anterior-posterior commissure line with a T2*-weighted sequence of twenty-seven contiguous axial slices (time repetition [TR] = 2000 ms, time echo [TE] = 40 ms, flip angle = 60°, field of view [FoV] = 190 × 190 mm, array size 64 × 64) of 4.5 mm thickness and 3.13 × 3.13 × 4.5 mm. Structural images were acquired with a T1-weighted SPGR sequence (TR = 19 ms, TE = 5 ms, flip angle = 20°, FoV = 220 × 220 mm) recording slices at a thickness of 1.5 mm with in-plane resolution of 0.86 × 0.86 mm.
Preprocessing and statistical analyses were carried out using Statistical Parametric Mapping 5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5). The first three volumes of each functional run were discarded to eliminate onset transients. The remaining volumes were then spatially realigned to the first volume of the first run. The mean image of the realigned volumes was normalized to a standardized EPI template and resampled at a voxel size of 2 mm3. Finally, the normalized images were spatially smoothed with a Gaussian kernel of 8-mm3. In order to remove low-frequency confounds, data were high-pass filtered (128 s).
Using the general linear model within SPM5, voxelwise statistical parametric maps (SPMs) convolved with the hemodynamic response function were generated for direct and averted gaze stimuli. The parameters from each subject were entered into group level analyses contrasting the two conditions. Group-level comparisons consisted of independent and paired t tests at a voxel threshold of P < 0.01 corrected by a cluster extent threshold of 10 voxels (Forman et al., 1995). Whole brain analyses and statistical levels appropriate for such low-power approaches were selected as a first step because the novelty of the gaze task did not offer sufficient basis to prespecify regions of interest and sample sizes were not large, we focused on whole brain analyses as a first step.
3. Results
3.1 Sample
Demographic and clinical features of the evaluable sample are presented in Table 1. GSAD patients evidenced elevated levels of anxiety and avoidance related to eye contact compared to HCs, moderate to severe social anxiety, and mild, subsyndromal levels of depressive symptoms. One GSAD patient completed the first scan and was dispensed paroxetine but did not return for any subsequent appointments. Fifteen GSAD patients completed the study.
Table 1.
Demographic, Clinical, and Gaze Behavior Characteristics at Baseline
| GSAD participants (n=16) No. (%) or Mean (SD) |
HC participants (n=16) No. (%) or Mean (SD) |
t or X2 |
df | P | |
|---|---|---|---|---|---|
| Age, y | 29.8 (9.0) | 30.3 (9.7) | 0.2 | 29.8 | 0.88 |
| Sex | |||||
| F | 10 (62.5%) | 10 (62.5%) | 0.0 | 1.00 | |
| M | 6 (37.5%) | 6 (37.5%) | |||
| Race | |||||
| White non-Hispanic | 11 (68.8%) | 13 (81.3%) | |||
| Asian | 2 (12.5%) | 1 ( 6.3%) | 0.731 | ||
| Hispanic | 3 (18.8%) | 2 (12.5%) | |||
| Education, y | 16.2 (1.9) | 16.5 (2.1) | 0.5 | 29 | 0.64 |
| LSAS | 81.4 (15.6) | 8.2 (5.4) | 17.7 | 18.5 | <0.001 |
| GARS | 49.8 (16.2) | 6.9 (6.9) | 9.8 | 20.3 | <0.001 |
| HRSD-17 | 6.4 (3.3) | 0.3 (0.7) | 7.4 | 16.4 | <0.001 |
| % fixations on eye region, direct stimuli |
60.4 (25.0) | 48.4 (22.2) | −1.4 | 28 | 0.18 |
| % fixations on eye region, averted stimuli |
60.3 (23.6) | 50.1 (26.6) | −1.1 | 28 | 0.28 |
| % fixations on eye region, direct – averted |
0.1 (7.2) | −1.8 (9.0) | −0.6 | 28 | 0.54 |
| Scanpath length (pixels), direct stimuli |
3038.8 (998.3) | 2578.4 (1216.4) | −1.1 | 28 | 0.27 |
| Scanpath length (pixels), averted stimuli |
2836.2 (1041.7) | 2660.7 (1078.7) | −0.5 | 28 | 0.65 |
| Scanpath length (pixels), direct – averted |
202.6 (498.4) | −82.3 (430.3) | −1.7 | 28 | 0.11 |
LSAS = Liebowitz Social Anxiety Scale; GARS = Gaze Anxiety Rating Scale; HRSD-17 = Hamilton Rating Scale for Depression, 17-Item Version;
Fishers Exact Test
3.2 Treatment response and behavioral response
Patients evidenced statistically significant and clinically meaningful improvement in GSAD symptoms during paroxetine treatment, as evidenced by a 36-point decrease in the mean score of the LSAS, similar to change reported in randomized clinical trials of paroxetine (Stein et al., 1998). At week 8, among the 15 GSAD completers, 12 were rated responders to treatment (three were rated “very much improved” and nine were “much improved”), and 3 were classified as nonresponders (all rated “minimally improved”). Self-reported anxiety and avoidance related to eye contact (GARS) also improved significantly (Table 2). Mean paroxetine dosage at week 8 was 34.0 +/− 8.3 mg/day (range 20 – 40 mg/day).
Table 2.
Symptom and Gaze Behavior Measures Pre- vs. Post-treatment in GSAD Group Completers (n=15)1
| Week 0 Mean (SD) |
Week 8 Mean (SD) |
T | df | P | |
|---|---|---|---|---|---|
| LSAS | 82.6 (15.5) | 45.9 (25.6) | 5.9 | 14 | <0.001 |
| GARS | 51.2 (15.7) | 29.6 (14.5) | 5.3 | 14 | <0.001 |
| HRSD-17 | 6.3 (3.3) | 3.9 (3.5) | 1.9 | 14 | 0.076 |
| % fixations on eye region, direct stimuli |
60.0 (23.4) | 39.2 (27.4) | 4.8 | 11 | <0.001 |
| % fixations on eye region, averted stimuli |
60.8 (20.9) | 40.9 (25.2) | 4.0 | 11 | 0.002 |
| % fixations on eye region, direct – averted |
−0.8 (7.7) | −1.6 (8.4) | 0.3 | 11 | 0.76 |
| Scanpath length (pixels), direct stimuli |
3154.0 (1077.1) | 4063.3 (1747.1) | 1.3 | 11 | 0.22 |
| Scanpath length (pixels), averted stimuli |
2842.2 (1167.8) | 4191.1 (2050.0) | 1.7 | 11 | 0.11 |
| Scanpath length (pixels), direct – averted |
311.8 (491.1) | −127.9 (945.2) | 1.5 | 11 | 0.17 |
LSAS = Liebowitz Social Anxiety Scale; GARS = Gaze Aversion Rating Scale; HRSD-17 = Hamilton Rating Scale for Depression, 17-Item Version
Eye gaze data for 3 patients were excluded from analysis due to technical problems with acquisition.
At baseline, there were no significant between-group differences in visual attention to stimuli (Table 1). From pre- to post-treatment (week 0 to week 8), GSAD patients evidenced no changes in the difference in percent of fixations on the eye region in response to direct vs. averted gaze (direct – averted, Table 2). However, percent of fixations on the eye region in response to direct gaze and percent of fixation on the eye region in response to averted gaze stimuli each decreased significantly pre- to post-treatment. These decreases were not significantly associated with improvement in GSAD symptoms (Rs = −0.02 for direct gaze and Rs = −0.04 for averted gaze).
3.3 Imaging results
To address the question of what neural regions are activated during the processing of direct gaze in persons with symptoms of GSAD, we report directional findings at week 0 for GSAD > HC, direct > averted gaze; and at weeks 0 and 8, within GSAD patients, for direct>averted gaze, week 0 > week 8.
3.3.1 Baseline Analyses
Group differences in BOLD at baseline (see Table 3 and Figure 1) were present in regions associated with emotion and place perception (parahippocampal cortex), self-referential processing (inferior parietal lobule, supramarginal gyrus, and posterior cingulate), and high level visual processing (middle occipital cortex) (Russ et al., 2003; Sugiura et al., 2005; Platek et al., 2006; Uddin et al., 2006). The 2 × 2 ANOVA for group vs. gaze direction was consistent with a differential response to gaze direction (P < 0.01) for ROIs including inferior parietal lobule and posterior cingulate.
Table 3.
Group Differences at Baseline: Areas of Significant Activation for Group (GSAD>HC) by Stimuli (Direct > Averted Gaze) at Week 0
| Region | Voxels | x | y | z | T |
|---|---|---|---|---|---|
| R parahippocampal gyrus | 21 | 22 | −40 | 2 | 2.97 |
| R inferior parietal lobule | 11 | 40 | −30 | 28 | 2.95 |
| R inferior parietal lobule, supramarginal gyrus |
160 | 52 | −58 | 44 | 3.22 |
| R posterior cingulate gyrus | 32 | 28 | −30 | 38 | 3.44 |
| R middle occipital gyrus | 13 | 36 | −72 | −2 | 3.15 |
All activations are effects observed in whole-brain analyses and are significant at P < 0. 01
Figure 1.
Group differences at baseline.
Participants with generalized social anxiety disorder had significantly greater blood oxygen-level dependent responses than healthy comparison participants for direct vs averted gaze in posterior cingulate cortex (PCC) and inferior parietal lobule (LPi). Numbers below each image refer to z-coordinates.
Secondary within-group analyses (direct > averted) for the HC group demonstrated BOLD responses to eye contact (P < 0.01) bilaterally in superior temporal sulci, the region most associated with response to gaze direction (Hooker et al., 2003; Pelphrey et al., 2004; Materna et al., 2008) (L temporal cortex, 400 voxels, x = −38, y = 0, z = −12, t = 4.44; and right temporal cortex, 578 voxels, x = 36, y = 6, z = −16, t = 4.09). These areas were not significantly activated within the GSAD group, which instead demonstrated significant BOLD responses in left globus pallidus, 23 voxels, x = −24, y = −16, z = 0, t = 4.02; L insula, 38 voxels, x = −36, y = −18, z = 14, t = 4.43; bilateral cingulate gyrus, 58 voxels, x = −2, y = −10, z = 28, t = 3.17 and 21 voxels, x = 8, y = −12, z = 40, t = 3.26; and right inferior parietal lobule, x = 54, y = −54, z = 48, t = 3.63.
Secondary within-group contrasts of fixation cross vs. direct and averted gaze stimuli conditions were used to evaluate response polarity in selected ROIs, identified with MarsBaR (Brett et al., 2002). These included regions previously shown to be activated by social threat in SAD (Stein et al., 2002) (amygdala and insula, defined anatomically) and regions activated by direct vs. averted gaze at baseline in this study (inferior parietal lobule and posterior cingulate cortex, defined functionally). BOLD signal in amygdala, insula, and inferior parietal lobule was significantly greater for both direct and averted gaze stimuli (relative to fixation cross) in both SAD and HCs, but for posterior cingulate cortex there were no significant differences.
3.3.2 Pre- vs. post-treatment
Within the GSAD group, regions showing a stimulus gaze direction by time interaction (week 0 > week 8, direct > averted, see Table 4) included regions associated with interoception and emotion (insula), high level visual processing (middle temporal gyrus, occipital cortex) and self-referential processing (posterior cingulate cortex and precuneus)(Vogt and Laureys, 2005; Paulus and Stein, 2006). The 2 × 2 ANOVA for stimulus gaze direction vs. time (pre-treatment and post-treatment) is consistent with a post-treatment reduction in the effect of direct eye gaze relative to averted gaze (P < 0.01) for ROIs including occipital cortex and middle temporal gyrus.
Table 4.
Pre- vs. Post-Treatment: Areas of Significant Activation for Treatment (Wk 0 > Wk 8) by Stimuli (Direct > Averted Gaze), Within GSAD Group
| Region | Voxels | x | y | z | T |
|---|---|---|---|---|---|
| L insula | 48 | −34 | −12 | 14 | 3.61 |
| R middle temporal gyrus | 18 | 40 | −52 | −2 | 3.46 |
| R precentral gyrus | 29 | 50 | −4 | 18 | 3.54 |
| R posterior cingulate gyrus, precuneus |
88 | 6 | −40 | 48 | 4.28 |
| L superior occipital gyrus | 29 | −26 | −72 | 28 | 3.90 |
All activations are effects observed in whole-brain analyses and are significant at P < 0.01
3.3.3 Correlates of Symptomatic Improvement
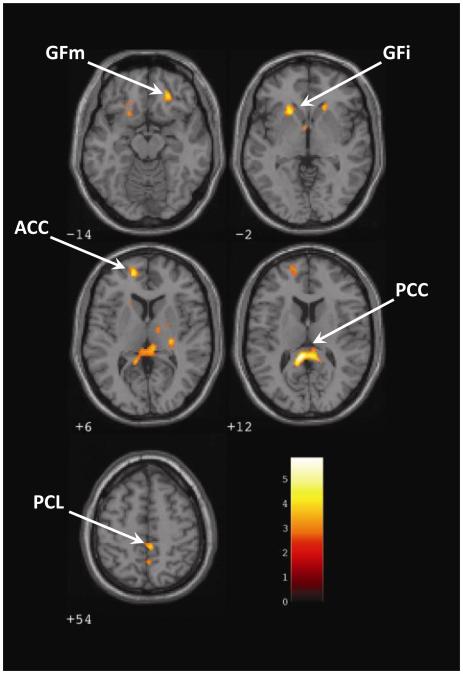
Within the GSAD group, regions in which changes in activations (direct > averted gaze) covaried with reductions in social anxiety during treatment (see Table 5 and Figure 1) included areas with functions that include emotion regulation and self -referential processing (subgenual anterior cingulate, ventromedial prefrontal cortex, inferior frontal gyrus, posterior cingulate cortex, precuneus, and inferior parietal lobule) (Buccino et al., 2001; Castelli et al., 2002, Vogt et al., 2006; Morita et al., 2008).
Table 5.
Correlates of Symptomatic Improvement: Areas of Change in Activation Significantly Associated with Change in LSAS During Treatment, Within GSAD Group
| Region | Voxels | x | y | z | T |
|---|---|---|---|---|---|
| L inferior frontal gyrus | 261 | −20 | 20 | −2 | 4.69 |
| L subgenual anterior cingulate | 100 | −14 | 54 | 6 | 4.62 |
| R middle frontal gyrus | 77 | 24 | 36 | −14 | 4.35 |
| L caudate | 53 | −2 | 2 | 0 | 3.26 |
| R anterior cingulate | 52 | 20 | 24 | −6 | 3.98 |
| R dorsal cingulate gyrus | 49 | 4 | −4 | 34 | 2.97 |
| L lentiform nucleus | 47 | −22 | −12 | −8 | 4.73 |
| R lentiform nucleus | 20 | 16 | −2 | 2 | 3.06 |
| R thalamus | 40 | 28 | −22 | 4 | 4.14 |
| R thalamus | 29 | 14 | −8 | 8 | 3.23 |
| R&L posterior cingulate | 493 | −6 | −38 | 12 | 5.87 |
| R paracentral lobule | 43 | 4 | −38 | 54 | 3.91 |
| R&L precuneus | 89 | 4 | −54 | 50 | 3.48 |
| L inferior parietal lobule | 97 | −30 | −52 | 62 | 3.89 |
All activations are effects observed in whole-brain analyses and are significant at P < 0. 01
4. Discussion
This study investigated responses to eye contact in patients with GSAD, a disorder known to be associated with fear of scrutiny. GSAD patients preferentially activated regions that have been associated with self-referential processing, including inferior parietal lobule and cortical midline structures of ventromedial prefrontal cortex and posterior cingulate cortex (Northoff et al., 2006), and regions associated with visual attention (occipital cortex, thalamus), and regions associated with emotion regulation and place perception (ventromedial prefrontal cortex, parahippocampal gyrus), compared to HCs. Of these regions, inferior parietal lobule and posterior cingulate also showed decreases in activation associated with improvement in SAD symptoms after paroxetine treatment. Decreases in activation associated with improvement in SAD symptoms also occurred in other regions that share an association with self-referential processing (inferior, medial and middle frontal gyri, precuneus, and subgenual anterior cingulate, as well as other regions with diverse functions (anterior and dorsal cingulate, lentiform nucleus, thalamus, paracentral lobule). The association of these regions with SAD symptoms is consistent with the centrality of excessive feelings of scrutiny and self-conscious emotions of embarrassment and humiliation to mechanisms of SAD, as well as the response to direct gaze as a threat. These regions are also associated with many other functions, including executive functions (inferior and middle frontal gyri)(Chikazoe, 2010), fear extinction (medial frontal gyri)(Kim et al., 2011), episodic memory (precuneus)(Cavanna and Trimble, 2006), depression (subgenual anterior cingulate)(Walter et al, 2009), reward processing (anterior and dorsal cingulate) (Liu et al., 2011), action selection (lentiform nucleus)(Stocco et al, 2010), and sensory processing (thalamus)(Wurtz et al., 2011).
Among the regions associated with SAD in this study, the inferior parietal lobule has been linked with own-body perception including self-face recognition (Sugiura et al., 2005; Platek et al., 2006; Uddin et al., 2006), and it is adjacent to the intraparietal sulcus, associated with spatial attention processes including response to gaze direction (Puce et al, 1998; Wicker et al., 1998; Hoffmann and Haxby, 2003). The supramarginal gyrus has been associated with the enactment effect, or facilitation of learning by performing actions (Russ et al., 2003). The predominance of right-sided differences are consistent with literature on right hemisphere role in self-related cognition (Decety and Chaminade, 2003; Platek et al., 2004), self-awareness (Andelman et al., 2004; Barnacz et al., 2004), and own-body perception (Blanke et al., 2002, 2005). These regional activations are consistent with heightened self-consciousness in GSAD patients, which may include elements of increased attention and visual processing, and the experience of viewing oneself from another’s perspective.
Within HCs, direct gaze stimuli (vs. averted) activated bilateral superior temporal sulci, the region most closely associated with processing of others’ eye movements in healthy subjects and in nonhuman primates (Heide et al., 2001; Hooker et al., 2003; Pelphrey et al., 2004; Nummenmaa and Calder, 2009). GSAD patients, in contrast, activated insula and cingulate, areas associated with monitoring and regulation of negative emotions (Paulus and Stein, 2006; Mohanty et al., 2007), and inferior parietal lobule. These differences in GSAD patients suggest interference in the normal processing of gaze direction stimuli.
Symptomatic improvement during paroxetine treatment correlated significantly with magnitude of decrease in BOLD response to direct (vs. averted) gaze in the inferior parietal lobule and other regions that share an association with self-referential processing (posterior cingulate, inferior frontal, medial prefrontal cortex). The posterior cingulate is involved in visuospatial orientation of the body in space (Vogt et al., 2004) and self-reflection (Johnson et al., 2002; Kelley et al., 2002). Both inferior parietal lobule and inferior frontal gyrus have been considered part of the “mirror system” that activates equally to the execution of or observation of a motor action (Buccino et al., 2001), and inferior frontal gyrus activation has been associated with embarrassment (Morita et al., 2008). Medial prefrontal cortex has been associated with inferring intentions of others (Castelli et al., 2002). Improvement also correlated with decreased BOLD response in subgenual anterior cingulate, which is connected to ventral posterior cingulate and involved in self-relevant information and self-reflection (Vogt et al., 2006), as well as processing of emotional stimuli (Gottlib et al., 2005).
Prior studies that have imaged SAD patients during task performance have rarely focused on regions associated with self-referential processing, possibly due to predominant use of paradigms comparing emotional expressions that are not designed to trigger self consciousness. A paradigm using self-referential (verbal) stimuli, however, did result in activation of medial prefrontal cortex and precuneus in one study (Blair et al., 2008), and persons with SAD have previously been noted to differ in activation of posterior cingulate/precuneus during viewing of faces or nonsense pictures (Gentili et al., 2009). Studies of public speaking or facial expression stimuli in SAD have sometimes reported activation of some of these regions, including medial and inferior prefrontal cortex (Tillfors et al., 2001; Stein et al., 2002; Straube et al., 2004; Blair et al., 2008), supramarginal gyrus (Evans et al., 2009), posterior cingulate (Evans et al., 2009), and precuneus (Warwick et al., 2008). Understanding of neural processes in SAD may benefit from study of both fear processing that may have dysfunction shared with other anxiety disorders, and self-referential processing that may be relatively specific to SAD.
A few imaging studies have examined changes in response to treatment of SAD. In O-15 PET studies, symptomatic improvement during treatment with the SSRI citalopram and cognitive-behavioral therapy was associated with reduced regional cerebral blood flow (rCBF) mainly in the medial temporal lobe, including the amygdala, hippocampus, and rhinal and parahippocampal cortices (Furmark et al., 2002), and improvement during treatment with citalopram and an NK1-antagonist reduced rCBF response to public speaking in the same regions, with citalopram also reducing activity in the posterior cingulate (Furmark et al., 2005). Citalopram also decreased resting-state neuronal activity in the left temporal cortex in a SPECT study (Van der Linden et al., 2000). Another O-15 PET study found that treatment with the antidepressant nefazodone was associated with reductions in rCBF in the precentral gyrus, insula, midbrain/hypothalamus, and middle frontal and anterior cingulate cortex, and increases in the middle occipital and lingual gyri, postcentral gyrus, gyrus rectus, and hippocampus (Kilts et al., 2006). Treatment with the GABA reuptake inhibitor tiagabine was associated with increased glucose metabolism in the ventromedial prefrontal cortex in a study using resting state 18-FDG PET (Evans et al., 2009). The heterogeneity of these imaging findings reflects marked differences in imaging methods, tasks, and treatments, but does overlap with our findings of decreased BOLD response in insula and posterior cingulate after paroxetine treatment, and the correlation of medial frontal cortex activation with symptomatic improvement during paroxetine treatment.
Absent from our findings, in both whole brain and ROI analyses, is evidence of differential activation to direct vs. averted gaze for amygdala, which has been shown to engage in response to threat in animals (LeDoux, 2000) and in healthy humans (Phelps and LeDoux, 2005), and to be hyperresponsive in persons with SAD and other anxiety disorders (Etkin and Wager, 2007). The absence of amygdala findings may be in part due to the nature of the stimuli and comparisons used in this study, where both the direct and averted stimuli activated the amygdala relative to fixation cross.
Eye tracking data showed that this study’s BOLD findings at baseline and during treatment were not attributable to differences in gaze behavior. Percentage of fixations to the eye region of direct vs. averted stimuli did not differ between groups, nor pre- vs. post-treatment in GSAD. Treatment was associated, however, with decreased fixations to the eyes for direct and averted stimuli, considered separately. In post hoc tests these decreases were not correlated with clinical improvement, suggesting that decreased fixations on the eyes may be a general effect of paroxetine treatment, unrelated to symptom reduction, but consistent with a report of subchronic paroxetine treatment reducing vigilance in healthy volunteers (Schmitt et al., 2002).
Our findings must be considered in the context of several other limitations. A limitation of the eye contact paradigm was that the nature of the “control” stimulus of averted gaze (eyes looking up) may have also contributed to differences in activation. Additionally, the attribution of neural differences to self-referential processing was inferred from the nature of the task, known features of SAD, and functions previously associated with these neural regions. Each of these regions is believed to have multiple functions, however, and self-referential mental processes were not directly measured, so the reported associations could have also been related to other mental processes.
The potential specificity to GSAD of the activation of self-referential processing regions by the direct gaze paradigm requires confirmation by further study. Gaze aversion may also be related to other disorders, non-pathological temperaments such as behavioral inhibition, or sociocultural influences. In the absence of a placebo-treated GSAD group and of re-test data on the HC group, it is unknown to what extent observed changes during paroxetine treatment may have been due to nonspecific effects, including expectancy, time, learning, and limits on test-retest reliability of the paradigm.
Future investigations of specific responses to the treatment and of prespecified ROIs can test for relationships between regional BOLD responses and treatment effects. The limited sample size (n=16 GSAD subjects) of this study constrains such analyses and the levels of statistical significance employed in this study, given the variability in a patient sample. These findings, however, may guide subsequent fMRI studies of treatment.
In summary, using a paradigm focused on the ecologically salient stimulus of direct eye gaze in patients with GSAD before and after paroxetine treatment, we found GSAD symptoms to be associated with increased activation of a distributed network, involving posterior midline structures, inferior parietal lobule, and inferior and medial frontal cortex that have been identified in animal and human studies as mediating self-referential processing. Other areas of activation reflected high level visual processing (occipital and parahippocampal cortex), and processing and regulation of negative affect (insula and subgenual cingulate cortex). Response to treatment with paroxetine was associated with decreased activation to direct gaze in many of the same regions, including inferior parietal lobule, posterior cingulate, inferior frontal, and medial prefrontal cortex, suggesting a possible normalization of activity in this system. Studies in other patient populations utilizing other treatments may help clarify the specificity of these findings to GSAD and its treatment.
Figure 2.
Among participants with generalized social anxiety disorder, magnitude of symptomatic improvement during paroxetine treatment was associated with decreases in blood oxygen-level dependent responses for direct vs averted gaze. GFm and GFi indicate middle and inferior frontal gyri, ACC and PCC anterior and posterior cingulate cortices, PCL paracentral lobule. Numbers below each image refer to z-coordinates.
Acknowledgments
Financial Support Supported in part by NIH grant MH077976 and the Sycamore Fund (Dr. Schneier).
Footnotes
Disclosure of Competing Interests Dr. Schneier reports support for research from Forest Laboratories. All other authors report no competing interests.
Dr. Schneier had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andelman F, Zuckerman-Feldhay E, Hoffien D, Fried I, Neufeld MY. Lateralization of deficit in self-awareness of memory in patients with intractable epilepsy. Epilepsia. 2004;45:826–833. doi: 10.1111/j.0013-9580.2004.51703.x. [DOI] [PubMed] [Google Scholar]
- Baker SL, Heinrichs N, Kim HJ, Hofmann SG. The Liebowitz social anxiety scale as a self-report instrument: a preliminary psychometric analysis. Behaviour Research and Therapy. 2002;40:701–715. doi: 10.1016/s0005-7967(01)00060-2. [DOI] [PubMed] [Google Scholar]
- Barnacz AL, Johnson A, Constantino P, Keenan JP. Schizotypal personality traits and deception: the role of self-awareness. Schizophrenia Research. 2004;70:115–116. doi: 10.1016/j.schres.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Blair K, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M, Ng P, Hollon N, Jones M, Blair RJ, Pine DS. Neural response to self- and other referential praise and criticism in generalized social phobia. Archives of General Psychiatry. 2008;65:1176–1184. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, McCaffrey D, Vythilingam M, Finger E, Mondillo K, Jacobs M, Charney DS, Blair RJ, Drevets WC, Pine DS. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. American Journal of Psychiatry. 2008;165:1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M. Stimulating illusory own-body perceptions. Nature. 2002;419:269–270. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- Blanke O, Mohr C, Michel CM, Pascual-Leone A, Brugger P, Seeck M, Landis T, Thut G. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. Journal of Neuroscience. 2005;25:550–557. doi: 10.1523/JNEUROSCI.2612-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clinical Psychology Review. 2004;24:827–856. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. June 2-6, 2002; Available on CD-ROM in NeuroImage. [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. European Journal of Neuroscience. 2001;13:400–404. [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chikazoe J. Localizing performance of go/no-go tasks to prefrontal cortical subregions. Current Opinion in Psychiatry. 2010;23:267–272. doi: 10.1097/YCO.0b013e3283387a9f. [DOI] [PubMed] [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social Phobia: Diagnosis, Assessment, and Treatment, 1995. Guilford Publications; N.Y: 1995. [Google Scholar]
- Decety J, Chaminade T. When the self represents the other: a new cognitive neuroscience view on psychological identification. Conscious Cognition. 2003;12:577–596. doi: 10.1016/s1053-8100(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neuroscience and Biobehavioral Reviews. 2004;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Essig K, Pomplun M, Ritter H. A neural network for 3D gaze recording with binocular eye trackers. International Journal of Parallel, Emergent, and Distributed Systems. 2006;21:79–95. [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Simon NM, Dougherty DD, Hoge EA, Worthington JJ, Chow C, Kaufman RE, Gold AL, Fischman AJ, Pollack MH, Rauch SL. A PET study of tiagabine treatment implicates ventral medial prefrontal cortex in generalized social anxiety disorder. Neuropsychopharmacology. 2009;34:390–398. doi: 10.1038/npp.2008.69. [DOI] [PubMed] [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depression and Anxiety. 2008;25:496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York State Psychiatric Institute, Biometrics Research; New York, NY: 1995. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Furmark T, Appel L, Henningsson S, Ahs F, Faria V, Linnman C, Pissiota A, Frans O, Bani M, Bettica P, Pich EM, Jacobsson E, Wahlstedt K, Oreland L, Langstrom B, Eriksson E, Fredrikson M. A link between serotonin-related gene polymorphisms, amygdala activity, and placebo-induced relief from social anxiety. Journal of Neuroscience. 2000;28:13066–13074. doi: 10.1523/JNEUROSCI.2534-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmark T, Appel L, Michelgård A, Wahlstedt K, Ahs F, Zancan S, Jacobsson E, Flyckt K, Grohp M, Bergström M, Pich EM, Nilsson LG, Bani M, Långström B, Fredrikson M. Cerebral blood flow changes after treatment of social phobia with the neurokinin-1 antagonist GR205171, citalopram, or placebo. Biological Psychiatry. 2005;15:132–142. doi: 10.1016/j.biopsych.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, Fredrikson M. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Archives of General Psychiatry. 2002;59:425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- Gentili C, Ricciardi E, Gobbini MI, Santarelli MF, Haxby JV, Pietrini P, Guazzelli M. Beyond amygdala: Default Mode Network activity differs between patients with Social Phobia and healthy controls. Brain Research Bulletin. 2009;79:409–413. doi: 10.1016/j.brainresbull.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JD, Whitfield-Gabrieli S, Goldin P, Minor KL, Canli T. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 16:1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- Guy W. Assessment Manual of Psychopharmacology -Revised (DHEW Publ No ADM 76-338) US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; Rockville, MD: 1976. [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Heide W, Binkofski F, Seitz RJ, Posse S, Nitschke MF, Freund HJ. Activation of frontoparietal cortices during memorized triple-step sequences of saccadic eye movements: an fMRI study. European Journal of Neuroscience. 2001;13:1177–1189. doi: 10.1046/j.0953-816x.2001.01472.x. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neuroscience. 2003;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Honey G, Bullmore E. Human pharmacological MRI. Trends in Pharmacological Sciences. 2004;25:366–374. doi: 10.1016/j.tips.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Paller KA, Gitelman DR, Parrish TB, Mesulam MM, Reber PJ. Brain networks for analyzing eye gaze. Cognitive Brain Research. 2003;17:406–418. doi: 10.1016/s0926-6410(03)00143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the national comorbidity survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Kelsey JE, Knight B, Ely TD, Bowman FD, Gross RE, Selvig A, Gordon A, Newport DJ, Nemeroff CB. The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology. 2006;31:2243–2253. doi: 10.1038/sj.npp.1301053. 2006. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behavioural Brain Research. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR. Social phobia. Modern Problems in Pharmacopsychiatry. 1987;22:14–17. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Liebowitz M, Hope DA, Schneier FR, editors. Social Phobia: Diagnosis, Assessment and Treatment. The Guilford Press; New York: pp. 69–93. [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosciences and Biobehavioral Reviews. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, Ballenger JC, Lydiard RB, Brodrick PS, Bohning DE, George MS. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15:2701–2705. [PubMed] [Google Scholar]
- Materna S, Dicke PW, Their P. Dissociable roles of the superior temporal sulcus and the intraparietal sulcus in joint attention: a functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2008;20:108–119. doi: 10.1162/jocn.2008.20.1.108. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Nakic M, Fridberg D, Kamel N, Pine DS, Blair RJ. The impact of processing load on emotion. Neuroimage. 2007;34:1299–1309. doi: 10.1016/j.neuroimage.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ho MH, Banich MT, Webb AG, Warren SL, Miller GA. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Morita T, Itakura S, Saito DN, Nakashita S, Harada T, Kochiyama T, Sadato N. The role of the right prefrontal cortex in self-evaluation of the face: a functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2008;20:342–355. doi: 10.1162/jocn.2008.20024. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Calder AJ. Neural mechanisms of social attention. Trends in Cognitive Science. 2009;13:135–143. doi: 10.1016/j.tics.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Viola RJ, McCarthy G. When strangers pass: processing of mutual and averted social gaze in the superior temporal sulcus. Psychological Science. 2004;15:598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences USA. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28:249–255. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Platek SM, Keenan JP, Gallup GG, Jr., Mohamed FB. Where am I? The neurological correlates of self and other. Cognitive Brain Research. 2004;19:114–122. doi: 10.1016/j.cogbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Platek SM, Loughead JW, Gur RC, Busch S, Ruparel K, Phend N, Panyavin IS, Langleben DD. Neural substrates for functionally discriminating self-face from personally familiar faces. Human Brain Mapping. 2006;27:91–98. doi: 10.1002/hbm.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomplun M, Velichkovsky BM, Ritter H. An artificial neural network for high precision eye movement tracking. In: Nebel B, Dreschler-Fischer L, editors. Lecture Notes in Artificial Intelligence: AI-94 Proceedings. Springer Verlag; Berlin: 1994. pp. 63–69. [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. Journal of Neuroscience. 1998;18:2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ MO, Mack W, Grama CR, Lanfermann H, Knopf M. Enactment effect in memory: evidence concerning the function of the supramarginal gyrus. Experimental Brain Research. 2003;149:497–504. doi: 10.1007/s00221-003-1398-4. [DOI] [PubMed] [Google Scholar]
- Safren SA, Heimberg RG, Horner KJ, Juster HR, Schneier FR, Liebowitz MR. Factor structure of social fears: The Liebowitz social anxiety scale. Journal of Anxiety Disorders. 1999;13:253–270. doi: 10.1016/s0887-6185(99)00003-1. [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Ramaekers JG, Kruizinga MJ, van Boxtel MP, Vuurman EF, Riedel WJ. Additional dopamine reuptake inhibition attenuates vigilance impairment induced by serotonin reuptake inhibition in man. Journal of Psychopharmacology. 2002;16:207–214. doi: 10.1177/026988110201600303. [DOI] [PubMed] [Google Scholar]
- Schneier FR. Clinical Practice: Social anxiety disorder. New England Journal of Medicine. 2006;355:1029–1036. doi: 10.1056/NEJMcp060145. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Kent J, Star A, Hirsch J. Neural circuitry of submissive behavior in social anxiety disorder: a preliminary study of response to direct eye gaze. Psychiatry Research: Neuroimaging. 2009;173:248–250. doi: 10.1016/j.pscychresns.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR, Rodebaugh TL, Blanco C, Lewin H, Liebowitz MR. Fear and avoidance of eye contact in social anxiety disorder. Comprehensive Psychiatry. 2011;52:81–87. doi: 10.1016/j.comppsych.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz LT, Heimberg RG. Attentional focus in social anxiety disorder: potential for interactive processes. Clinical Psychology Review. 2008;28:1206–1221. doi: 10.1016/j.cpr.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Kasper S, Andersen EW, Nil R, Lader M. Escitalopram in the treatment of social anxiety disorder: analysis of efficacy for different clinical subgroups and symptom dimensions. Depression and Anxiety. 2004;20:175–181. doi: 10.1002/da.20043. 2004. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LTE, Brown GC. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Stein MB, Liebowitz MR, Lydiard RB, Pitts CD, Bushnell W, Gergel I. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. Journal of the American Medical Association. 1998;280:708–713. doi: 10.1001/jama.280.8.708. [DOI] [PubMed] [Google Scholar]
- Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biological Psychiatry. 2004;56:921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Stocco A, Lebiere C, Anderson, John R. Conditional routing of information to the cortex: A model of the basal ganglia’s role in cognitive coordination. Psychological Review. 2010;117:541–574. doi: 10.1037/a0019077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R. Cortical mechanisms of visual self-recognition. NeuroImage. 2005;24:143–149. doi: 10.1016/j.neuroimage.2004.07.063. [DOI] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, Fredrikson M. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. American Journal of Psychiatry. 2001;158:1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, Fredrikson M. Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biological Psychiatry. 2002;52:1113–1119. doi: 10.1016/s0006-3223(02)01396-3. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Molnar-Szakacs I, Zaidel E, Iacoboni M. rTMS to the right inferior parietal lobule disrupts self-other discrimination. Social Cognition and Affective Neuroscience. 2006;1:65–71. doi: 10.1093/scan/nsl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linden G, van Heerden B, Warwick J, Wessels C, van Kradenburg J, Zungu-Dirwayi N, Stein DJ. Functional brain imaging and pharmacotherapy in social phobia: single photon emission computed tomography before and after treatment with the selective serotonin reuptake inhibitor citalopram. Progress in Neuropsychopharmacology and Biological Psychiatry. 2000;24:419–438. doi: 10.1016/s0278-5846(99)00109-8. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Progress in Brain Research. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Farber NB. Cingulate cortex and disease models. In: Paxinos G, editor. The Rat Nervous System. 3. Elsevier; San Diego, CA: 2004. pp. 705–727. [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, Schnepf B, Boeker H, Boesiger P, Northoff G. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Archives of General Psychiatry. 2009;66:478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- Warwick JM, Carey P, Jordaan GP, Dupont P, Stein DJ. Resting brain perfusion in social anxiety disorder: a voxel-wise whole brain comparison with healthy control subjects. Progress in Neuropsychopharmacology and Biological Psychiatry. 2008;32:1251–1256. doi: 10.1016/j.pnpbp.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Wicker B, Michel F, Henaff MA, Decety J. Brain regions involved in the perception of gaze: a PET study. Neuroimage. 1998;8:221–227. doi: 10.1006/nimg.1998.0357. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, McAlonan K, Cavanaugh J, Berman RA. Thalamic pathways for active vision. Trends in Cognitive Science. 2011;15:177–184. doi: 10.1016/j.tics.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaider TI, Heimberg G, Fresco DM, Schneier FR, Liebowitz MR. Evaluation of the Clinical Global Impression Scale among individuals with social anxiety disorder. Psychological Medicine. 2003;33:611–622. doi: 10.1017/s0033291703007414. [DOI] [PubMed] [Google Scholar]