Abstract
It has been known for a long time that the interaction between cancer cells and tissue microenvironment plays a major role in cancer development, progression and metastasis. The biochemical aspect of cancer-stromal interactions, however, is less appreciated. This short review article first provides a brief summary of the communications between cancer cells and the tissue microenvironment by direct cell-cell interactions and by soluble factors, and then describes several biochemical pathways that are important for the interaction between stromal and cancer cells with respect to energy metabolism, redox balance, cell survival and drug resistance. The potential therapeutic implications of abolishing stromal protective mechanisms to overcome drug resistance are also discussed.
Key words: cancer-stromal interaction, microenvironment, cancer metabolism, drug resistance, glycolysis, glutathione, reactive oxygen species
Introduction
While the “seed and soil” hypothesis was proposed by Stephen Paget more than 100 years ago,1 it is only recently that the biochemical and molecular mechanisms underlying the dynamic interactions between tumor cells and their surrounding micro-environment began to be elucidated. Importantly, the crosstalk of “seed” and “soil” provides a mechanism to protect a certain subpopulation of cancer cells from the cytotoxicity of chemotherapeutic agents, leaving surviving cancer cells that constitute minimal residual disease and contribute to treatment failure.2 To better understand the interactions of tumor cells and the tissue microenvironment and to design effective therapeutic strategies, recent research efforts have increasingly focused on the molecular determinants and the important survival pathways involved in tumor cell communication with the microenvironment.3 The development of tumor metabolomics has enabled the discovery of the potential roles of low-molecular-weight metabolites in cancer development.4–6 Furthermore, since fast growing cancer cells have a high demand for nutrients,7 the tumor micro-environment seems to play an important role in meeting the metabolic needs of cancer cells.8–11 The microecosystem formed between cancer cells and the microenvironment may promote cancer cell survival and drug resistance. The crosstalk between a tumor and the surrounding microenvironment occurs at various levels. Herein, we review the molecular and biochemical communications between cancer cells and microenvironment with a focus on their contributions to drug resistance. Additionally, we discuss potential opportunities for the development of drugs that may block the crosstalk between cancer cells and the tissue microenvironment and thus overcome stromal-mediated drug resistance.
The tumor microenvironment consists of a network of various accessory cells and extracellular components surrounding the cancer cells. In solid tumors, the surrounding stroma forms the connective tissue microenvironment which includes the extracellular matrix, cancer-associated fibroblasts, immune and inflammatory cells and blood vessel cells.12 In hematologic malignancies, bone marrow and peripheral lymphoid organs are the major refuge sites for malignant cells. These sites protect the cancer cells from the cytotoxic effect of anticancer agents.13,14 The bone marrow microenvironment consists of various cell components including bone marrow stromal cells, bone marrow endothelial cells, osteoclasts, osteoblasts, macrophages, etc. In the peripheral lymphoid microenvironment, the accessory cells include T cells, follicular dendritic and follicular stromal cells. How the tumor microenvironment supports cancer cells to evade apoptosis and to facilitate metastasis is a fundamental question that still remains to be answered. Recent evidence suggests that the tumor microenvironment may activate important molecular pathways in cancer cells to promote drug resistance either through direct cell-cell contact or via secretion of soluble factors essential for cell survival.
Cell Contact-Mediated Molecular Interactions Between Tumor Cells and Microenvironment
Adhesion of cancer cells to the extracellular matrix and accessory cells in the tumor microenvironment is mediated in part through integrin molecules. Integrin expression patterns are often altered in tumor cells and certain integrins seem to promote tumor progression.15 Increasing evidence suggests that integrins may be associated with receptor tyrosine kinases that are important for tumor metastasis, cell survival and drug resistance. For example, chronic lymphocytic leukemia (CLL) cells have variable expression of lymphocyte function-associated antigen 1 (LFA-1), very late antigen-4 (VLA-4), inter-cellular adhesion molecule 1 (ICAM-1/CD54), ICAM-2 (CD102), ICAM-3 (CD50) and L-selectin (CD62L).16,17 CD44 is also detected in certain aggressive CLL cell populations.4,5 Besides mediating migration of CLL cells to their niche in the bone marrow and secondary lymphoid tissues,2,6 some of these adhesion molecules also protect CLL cells and confer drug resistance by binding to their receptors on bone marrow stromal cells. For instance, the β1 and β2 integrins on CLL cells seem to act simultaneously to mediate binding to ICAM-1 (CD54) and VCAM-1 (CD106) on bone marrow stromal cells and prevent apoptosis of CLL cells, in part by preventing the loss of Bcl-2 protein expression. In contrast, normal B cells may not be protected by stromal cells through this mechanism due to the lack of this pattern of molecular adhesion molecules.18,19 Another adhesion molecule that might be involved in promoting CLL survival is CD44, whose high expression was found in aggressive CLL cells.20 In multiple myeloma, cell adhesion-mediated drug resistance is also often observed.21–23 Adhesion of multiple myeloma cells to bone marrow stromal cells via CD44 leads to upregulation of p27 and activation of the NFκB survival pathway.24
Another important pathway is the Notch/Jagged axis. Notch1 receptor expression on multiple myeloma cells is stimulated by their adhesion to the bone marrow stromal cells, which express the Notch ligand, Jagged. The signaling pathway induced by this interaction upregulates p21Cip1/WAF1, leading to growth arrest and suppression of drug-induced apoptosis.17 This cell contact-mediated molecular pathway is also found in non-Hodgkins B-cell lymphoma.18
In solid tumors, the expression of integrins is important for epithelial tumor cells to metastasize to the bone marrow. For instance, αvβ3 and α4β1, whose ligands are highly expressed in the bone marrow, are upregulated in epithelial cancers including prostate, breast, lung, cervix and glioblastoma.15,25–27 Moreover, as in hematologic malignancies, integrin expression in solid tumors is also associated with increased cancer cell survival and drug resistance.28 The pathways activated by integrins, including PI3K/AKT, ERK/MAPK, NFκB, are critical to induce cell migration and to promote cell survival.15
Soluble Factor-Mediated Molecular Interactions Between Tumor Cells and the Microenvironment
The cytokines and growth factors secreted by stromal cells are known to have profound effect on cancer cells. The accessory cells in the tumor microenvironment secrete various cytokines and growth factors including stromal derived factor-1 (SDF-1), IL-6, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), insulin-like growth factor 1 (IGF1), hepatocyte growth factor (HGF), etc.12,29 The receptors of these ligands are usually highly expressed in a variety of cancer cells.30 Through signal transmission, the ligand-receptor interaction contributes to the aberrant growth, survival, angiogenesis, metastasis and drug resistance of the cancer cells. For example, SDF-1α, which interacts with its receptor, CXCR4, on malignant hematopoietic cells and certain solid tumor cells induces the homing and metastasis of malignant cells to the frequent sites of metastasis including bone marrow, lymph nodes, lungs and liver.30 In addition to its role in chemoattraction, SDF-1α also stimulates cancer cell survival. The protective effect of SDF-1α on cancer cells has been shown in experiments using exogenous cytokines and blocking antibodies. It is known that SDF-1α interacts with CXCR4 on chronic lymphocytic leukemia (CLL) cells and activates MAPK/ERK1/2,31 and PI3K/AKT pathway,32 leading to Bcl-2 upregulation33 and thus promoting CLL cells survival.
Another example is IGF-1, one of the critical growth factors required for survival and growth of tumor cells. Melanoma cells seem to rely on IGF-1 secreted by fibroblasts, since the malignant cells do not produce this growth factor.34 IGF-1 enhances the survival, migration and growth of melanoma cells through activating phosphorylation of Erk1/2 in the MAP kinase pathway and the Akt pathway.35
Moreover, stromal cells in the tumor microenvironment alter the tumor cell secretome including those required for tumor growth and dissemination. Recent data showed that co-culture of a murine K-ras mutant lung adenocarcinoma cell line (LKR-13) with murine lung stromal cells (macrophages, endothelial cells or fibroblasts) regulated the secretion of proteins including CXCL1 and IL-18 involved in angiogenesis, inflammation, cell proliferation and the epithelial-to-mesenchymal transition.36 Another study showed that fibroblast-derived IGF-1 can induce IL-8 expression in melanoma cells through the MAP kinase/JNK/c-Jun/AP-1 pathway.37
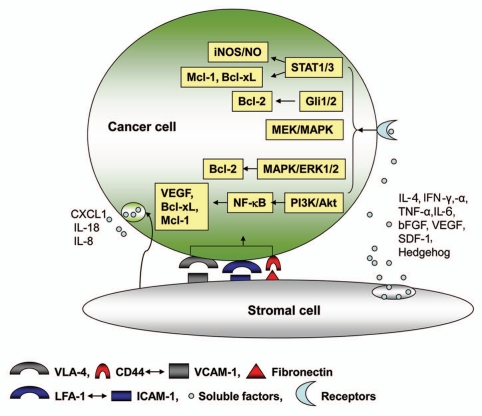
Soluble factor-mediated molecular interactions between the tumor cells and microenvironment have been extensively studied in hematopoietic cancers. The bone marrow microenvironment is rich in cytokines and growth factors that can be hijacked by malignant cells homing to the bone marrow. Some of the critical molecular signaling events have been identified. The migration of multiple myeloma (MM) cells induced by VEFG seems to be mediated through a PKC-dependent pathway.38 The proliferation of MM cells triggered by IL-6, IGF-1, SDF-1α, TNF-α, VEGF is mediated through the MEK/p42/p44/MAPK signaling cascade.39–43 In CLL, IL-4, INF-α, IFN-γ and bFGF prevent apoptosis through a Bcl-2-dependent pathway.44–49 In addition, IL-4 and INF-γ can also upregulate the expression of inducible nitric oxide synthase (iNOS) in CLL cells and cause an endogenous release of NO, which seems to suppress apoptosis through S-nitrosylation of caspase-3, thereby suppressing its pro-apoptotic activity.50,51 Moreover, VEGF activates STAT-1 and STAT-3 in CLL cells, and thus upregulates the expressions of Mcl-1 and XIAP that promote resistance to apoptotic induction.52 Besides cytokines and growth factors, the stromal-secreted hedgehog protein has also been shown to promote the survival of lymphoma, MM and CLL cells in vitro in co-culture systems with bone marrow stromal cells.53–55 The hedgehog protein activates its receptor, PTC, on malignant cells leading to transcriptional activation of Gli1 and Gli2 and upregulation of Bcl-2 expression.53–55 Some of the molecular events mediated by direct cell contact and soluble factors are illustrated in Figure 1.
Figure 1.
Molecular interaction between cancer cells and stromal cells leading to increased cancer cell survival and drug resistance. The dynamic interaction between cancer and stromal cells can be mediated through adhesion molecules and soluble factors, leading to activation of major signaling pathways involving JAK/STAT, Gli1/2, MAPK, PI3K/Akt, NFκB and increased expression of the downstream effectors including iNOS/NO, VEGF and anti-apoptotic proteins (Bcl-2, Bcl-xL, Mcl-1) in cancer cells. These survival pathways and anti-apoptotic molecules promote cell viability and drug resistance. Furthermore, stromal cells may also alter the tumor cell secretome including those required for tumor growth and dissemination, such as IL-18, CXCL1 and IL-8.
Biochemical Interactions between Tumor Cells and the Microenvironment
The rapid growth and abnormal proliferation of cancer cells are often associated with substantial alterations in energy metabolism and skewed redox homeostasis. The aberrant cancer metabolism seems to be associated with complex interactions between tumor cells and their tissue microenvironment to meet the high demand for nutrients to support tumor growth. Furthermore, tumor cells also release certain metabolic products into the stromal environment to be further processed. As such, the tumor microenvironment functions as a special niche to take up the “unneeded” metabolites from cancer cells and to provide the critical nutrients and metabolites for the cancer cells. The interaction between cancer cells and their microenvironment at the biochemical level is an emerging area of research. Recent reports in this area have provided significant new insights into the biochemical mechanisms of tumor-microenvironment interactions and how such biochemical events might affect cancer cells survival and drug resistance.
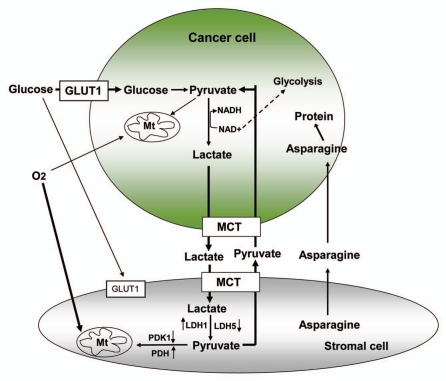
Glucose is a primary energy source for most normal cells and tumor cells. Glycolysis, the metabolic pathway that converts glucose to pyruvate, is the key biochemical process for generating ATP without the consumption of oxygen. When glucose is metabolized in normal cells in the presence of adequate oxygen, this metabolic process produces pyruvate, which is then imported into the mitochondria for further metabolism leading to oxidative phosphorylation and the generation of ATP. When an adequate oxygen supply is not available, cells may switch to anaerobic glycolysis and convert pyruvate to lactate. Interestingly, many cancer cells seem to heavily use the glycolytic pathway even in the presence of plenty of oxygen, a phenomenon first described by Otto Warburg more than 70 years ago.56 Anaerobic glycolysis generates only two ATP per glucose. To meet the energy need and provide metabolic intermediates, the glycolytic rate in tumor cells is often significantly higher than that in normal cells.7 Such a high rate cannot be sustained unless NAD+ can be regenerated to support the reaction catalyzed by GAPDH. Tumor cells regenerate NAD+ by converting pyruvate into lactate at a high rate. However, the increased generation of lactate causes cellular acidification. Recent studies suggest that the tumor-associated stroma express complementary metabolic molecules, which play an important role in buffering and recycling the products of anaerobic metabolism to sustain cancer cell survival.8,9,11 Analysis of the expression pattern of proteins involved in cellular metabolism shows that the tumor-associated stromal cells, by having low LDH5/PDK1 expression and high PDH/LDH1 reactivity, favor aerobic glycolysis.8 High concentrations of lactate in cancer cells are rapidly extruded to the extracellular matrix through the highly active MCT1 membrane monocarboxylate pump. Stromal cells also have high levels of MCT1 that take up lactate, which is eventually used as a fuel to generate ATP through aerobic metabolism after its oxidation back to pyruvate.8 The regenerated pyruvate in stromal cells can also be exported through MCT1 and reused subsequently by the cancer cells. Interestingly, cancer-associated stromal cells seem to have a low expression of the glucose transporter GLUT1, suggesting the possibility of low glucose uptake and thus sparing the glucose use by tumor cells.8 Using a unbiased proteomic analysis, Lisanti et al. found that epithelial cancer cells induced the expression of metabolic enzymes in the neighboring stromal fibroblasts and turned them into a factory for the production of energy-rich metabolites.9,11 As such, the stromal cells directly feed the epithelial cancer cells in a “host-parasite” interaction. The nutrients derived from tumor-associated stromal cells effectively reduce the dependence of cancer cells on blood supply, and thus provide an escape mechanism to resist anti-angiogenic therapy.11
Cancer cells have a unique need for amino acids as the building blocks for the synthesis of macromolecules. Importantly, recent studies have shown that the role of certain amino acids in cancer cells seems go beyond the synthesis of proteins. For example, cancer cells have an increased need for glutamine not only because of its role in protein synthesis and as a nitrogen source for nucleotides. Glutamine metabolism via glutaminolysis also generates ATP and NADPH for cancer cells through metabolic channeling into the TCA cycle.57 Asparagine is a non-essential amino acid that can be synthesized through the catalysis of aspartic acid and ammonia (from glutamine) by the enzyme, asparagine synthetase. However, asparagine becomes an essential amino acid for leukemic lymphoblasts owing to their low expression of asparagine synthetase, rendering the leukemia cells highly dependent on extracellular sources of asparagine for their protein synthesis. This seems to be particular important in acute lymphoblastic leukemia (ALL) and constitutes the biochemical basis for treating ALL with asparaginase, which rapidly converts circulating asparagine and glutamine to aspartate and glutamtate, respectively.58–60 Drug resistance to asparaginase treatment is associated with the upregulation of aspragine synthetase in the leukemia cells, enabling them to generate sufficient asparagine for protein synthesis.61,62 However, this seems not the case in vivo since upregulated asparagine synthetase expression is not correlated with a poor clinical response to asparaginase.63 Interestingly, a recent study showed that the bone marrow mesenchymal cells regulate the response of ALL cells to asparaginase through generating and secreting asparagine to feed ALL cells.10 The levels of asparagine synthetase in bone marrow mesenchymal cells was found to be 20 times higher than those in ALL cells and protected the leukemia cells from the drug treatment. Conversely, reduced expression of asparagine synthetase by the mesenchymal cell leads to enhanced sensitivity of ALL cells. Thus, a detailed understanding of the leukemia cell niche in the bone marrow and the biochemical aspect of the interactions between leukemia cells and the bone marrow microenvironment is important in designing effective therapeutic strategies to overcome drug resistance.64
Cysteine is essential for the synthesis of glutathione (GSH). In cancer cells, GSH plays an important role in cell survival and drug resistance due in part to its ability to neutralize the cytotoxic effect of reactive oxygen species (ROS) and to conjugate with many cytotoxic agents for export from the cells.65 Recent reports also suggest that GSH inhibits apoptosis through other mechanisms. For example, post-translational modifications of proteins through glutathionylation are critical for regulation of apoptosis in cancer cells.66–68 GSH may promote lymphoid cell survival through maintaining intracellular ionic homeostasis.69 Among the three precursor amino acids (glutamate, cysteine, glycine) for GSH synthesis, cysteine is chemically unstable and exists at a lower concentration than glutamate and glycine,70 and thus is a rate-limiting substrate for GSH synthesis. Similar to asparagine, cysteine is a conditionally essential amino acid that can be synthesized from methionine only in certain tissues via the transsulfuration pathway.71 Therefore, the uptake of cysteine by the ubiquitously expressed ASC transporter is an important determinant of GSH status in cells.72 Since cysteine is present in blood predominantly in the oxidized form, cystine, uptake of cysteine in most cells occurs in the form of cystine by a transporter known as Xc-.73 However, comparison of radioactive cysteine and cystine uptake shows that lymphocytes preferentially utilize cysteine compared to cystine.74 Interestingly, antigen-presenting cells could take up cystine and secrete cysteine into the microenvironment where it is available to nearby T cells for GSH synthesis, through which the antigen-presenting cells may affect T-cell proliferation and activation.75,76 Human fibroblasts also secrete cysteine in the culture medium.77 Because GSH turns over quickly in cancer cells to retain the redox balance and promote cell viability, cancer cells have high demand for the limiting substrate, cysteine. Whether stromal cells in the bone marrow microenvironment could serve as cysteine providers to feed the malignant cells for GSH synthesis remains to be investigated further.
Recent technological developments such as isotope dilution combined with liquid/gas chromatography and mass spectrometry have allowed the quantitative analyses of biochemical alterations in cancer, and enabled the discovery of essential oncometabolites in cancer. Analysis of global metabolomic alterations during prostate cancer progression has identified the potential role of sarcosine, owing to its elevated levels during cancer progression as well as its possible links to the disease mechanisms.4 Addition of exogenous sarcosine or knockdown of the enzyme that causes sarcosine degradation induced an invasive phenotype in benign prostate epithelial cells.
The observations of somatic mutations in cytosolic isocitrate dehydrogenase 1 (IDH1) in gliomas as well as IDH1 and 2 mutations in leukemia cells lead to the discovery of 2-hydroxyglutarate (2HG) as a potential oncometabolite in tumorigenesis.5,6 Although the exact oncogenic function of 2HG is still unknown, it has been postulated that this compound might function through inhibiting hypoxia-inducible factor prolyl hydroxylases (PHDs) thus leading to activation of hypoxia-inducible factor (HIF).78,79 It is also speculated that 2HG may affect the tumor microenvironment since cells could secrete 2HG.6,80 In future studies, it would be interesting to evaluate whether the tumor microenvironment could regulate the metabolism of these oncometabolites to support tumor cell growth. Some of the key biochemical events critical for the crosstalk between cancer cells and stromal cells are illustrated in Figure 2.
Figure 2.
Biochemical crosstalk between cancer cells and stromal cells. Glucose uptake by cancer cells is highly active, due in part to high expression of GLUT1. in cancer cells, glucose is converted to pyruvate and then to lactate through glycolysis. The high concentration of lactate in cancer cells is exported out of the cells through the highly expressed MCT1 to avoid excessive cellular acidification. High expression of MCT1 on stromal cells enables the uptake of lactate, which can be used as a fuel to produce energy after its oxidation back to pyruvate for aerobic metabolism in the mitochondria of the stromal cells. The increased expression of LDH1 and PDH and reduced expression of LDH5 and PDK1 in stromal cells seem to facilitate this metabolism. The regenerated pyruvate in stromal cells can also be exported through MCT1 and reused subsequently by cancer cells to convert back to lactate, with a generation of NAD+ to support glycolysis. Stromal cells also produce asparagine to feed leukemia cells. Depletion of asparagine using asparaginase is an effective way to abrogate this feeding mechanism and thus can be used to treat certain leukemia such as ALL.
Conclusion
In the recent years, it has become clear that the interactions between cancer cells and the microenvironment play critical roles in tumorigenesis and drug resistance. Based on these new findings, strategies have been developed to exploit the involved molecular and biochemical pathways for anticancer therapies. The potential approaches that have been tested include targeting the tumor-microenvironment interactions using integrin and receptor antagonists81,82 and targeting the downstream pathways in tumor cells as well as stromal cells in the tumor microenvironment using specific receptor tyrosine kinase inhibitors and proteasome inhibitors.83–85 Preclinical studies showed that these strategies can increase the efficacy of primary therapies in combinatorial treatment strategies to some extent.2 Advances in biochemistry have also expanded our knowledge of the roles of metabolites in cancer development and drug resistance. Due to the increased need for ATP and certain metabolic intermediates for cancer cells to synthesize macromolecules to support rapid cell proliferation, the tumor microenvironment plays a major role in providing the needed nutrients and in handling certain metabolic products (e.g., lactate) from the cancer cells. As such, the biochemical interactions between cancer cells and their tissue microenvironment significantly affect cancer cell survival and drug sensitivity. This also suggests that it may be possible to target the biochemical fluxes between cancer cells and their microenvironment for therapeutic purposes. It is important to develop specific inhibitors that block the generation of “oncometabolites” or their transport. A combination of conventional therapeutic agents with strategies to abolish the interactions between cancer cells and the microenvironment at molecular and biochemical levels may effectively overcome stromal-mediated drug resistance and improve the in vivo therapeutic activity.
Abbreviations
- VLA-4
very-late antigen 4
- VCAM-1
vascular cell adhesion molecule 1
- LFA-1
lymphocyte function-associated antigen 1
- ICAM-1
intercellular adhesion molecule 1
- IL-4, 6, 18
interleukin 4, 6, 18
- INF-α, γ
interferon α, γ
- bFGF
basic fibroblast growth factor
- VEGF
vascular endothelial growth factor
- SDF-1
stromal cell-derived factor 1
- CXCL1
chemokine (C-X-C motif) ligand 1
- IGF-1
insulin-like growth factor 1
- HGF
hepatocyte growth factor
- TNF-α
tumor necrosis factor α
- NO
nitrogen oxide
- PDH
pyruvate dehydrogenase
- LDH
lactate dehydrogenase
- PDK
pyruvate dehydrogenase kinase
- MCT1
monocarboxylate transporter
- GLUT1
glucose transporter 1
- TCA cycle
citric acid cycle
- NADPH
nicotinamide adenine dinucleotide phosphate
- NAD+
nicotinamide adenine dinucleotide
- GSH
glutathione
- ROS
reactive oxygen species
- IDH1
cytosolic isocitrate dehydrogenase 1
- 2HG
2-hydroxyglutarate
- PHDs
hypoxia-inducible factor prolyl hydroxylases
- HIF
hypoxia-inducible factor
- CLL
chronic lymphocytic leukemia
- ALL
acute lymphoblastic leukemia
- MM
multiple myeloma
References
- 1.Paget S. The distribution of secondary growths in cancer of the breast 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 2.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 3.Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nature reviews. 2002;2:927–937. doi: 10.1038/nrc952. [DOI] [PubMed] [Google Scholar]
- 4.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 5.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66:632–637. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 9.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 10.Iwamoto S, Mihara K, Downing JR, Pui CH, Campana D. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. The Journal of clinical investigation. 2007;117:1049–1057. doi: 10.1172/JCI30235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migneco G, Whitaker-Menezes D, Chiavarina B, Castello-Cros R, Pavlides S, Pestell RG, et al. Glycolytic cancer associated fibroblasts promote breast cancer tumor growth, without a measurable increase in angiogenesis: Evidence for stromal-epithelial metabolic coupling. Cell Cycle. 2010;9:2412–2422. doi: 10.4161/cc.9.12.11989. [DOI] [PubMed] [Google Scholar]
- 12.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nature reviews. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 13.Ramakrishnan A, Deeg HJ. A novel role for the marrow microenvironment in initiating and sustaining hematopoietic disease. Expert Opin Biol Ther. 2009;9:21–28. doi: 10.1517/14712590802603093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–3375. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 16.Vincent AM, Cawley JC, Burthem J. Integrin function in chronic lymphocytic leukemia. Blood. 1996;87:4780–4788. [PubMed] [Google Scholar]
- 17.Caligaris-Cappio F, Hamblin TJ. B-cell chronic lymphocytic leukemia: a bird of a different feather. J Clin Oncol. 1999;17:399–408. doi: 10.1200/JCO.1999.17.1.399. [DOI] [PubMed] [Google Scholar]
- 18.Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91:2387–2396. [PubMed] [Google Scholar]
- 19.Lagneaux L, Delforge A, De Bruyn C, Bernier M, Bron D. Adhesion to bone marrow stroma inhibits apoptosis of chronic lymphocytic leukemia cells. Leukemia & lymphoma. 1999;35:445–453. doi: 10.1080/10428199909169609. [DOI] [PubMed] [Google Scholar]
- 20.Eistere W, Hilbe W, Stauder R, Bechter O, Fend F, Thaler J. An aggressive subtype of B-CLL is characterized by strong CD44 expression and lack of CD11c. British journal of haematology. 1996;93:661–669. doi: 10.1046/j.1365-2141.1996.d01-1704.x. [DOI] [PubMed] [Google Scholar]
- 21.Damiano JS, Dalton WS. Integrin-mediated drug resistance in multiple myeloma. Leukemia & lymphoma. 2000;38:71–81. doi: 10.3109/10428190009060320. [DOI] [PubMed] [Google Scholar]
- 22.Hazlehurst LA, Damiano JS, Buyuksal I, Pledger WJ, Dalton WS. Adhesion to fibronectin via beta1 integrins regulates p27kip1 levels and contributes to cell adhesion mediated drug resistance (CAM-DR) Oncogene. 2000;19:4319–4327. doi: 10.1038/sj.onc.1203782. [DOI] [PubMed] [Google Scholar]
- 23.Hazlehurst LA, Enkemann SA, Beam CA, Argilagos RF, Painter J, Shain KH, et al. Genotypic and phenotypic comparisons of de novo and acquired melphalan resistance in an isogenic multiple myeloma cell line model. Cancer research. 2003;63:7900–7906. [PubMed] [Google Scholar]
- 24.Landowski TH, Olashaw NE, Agrawal D, Dalton WS. Cell adhesion-mediated drug resistance (CAM-DR) is associated with activation of NFkappaB (RelB/p50) in myeloma cells. Oncogene. 2003;22:2417–2421. doi: 10.1038/sj.onc.1206315. [DOI] [PubMed] [Google Scholar]
- 25.Manteca C, Daube G, Jauniaux T, Linden A, Pirson V, Detilleux J, et al. A role for the Clostridium perfringens beta2 toxin in bovine enterotoxaemia? Vet Microbiol. 2002;86:191–202. doi: 10.1016/S0378-1135(02)00008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruber G, Hess J, Stiefel C, Aebersold DM, Zimmer Y, Greiner RH, et al. Correlation between the tumoral expression of beta3-integrin and outcome in cervical cancer patients who had undergone radiotherapy. Br J Cancer. 2005;92:41–46. doi: 10.1038/sj.bjc.6602278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart DA, Cooper CR, Sikes RA. Changes in extra-cellular matrix (ECM) and ECM-associated proteins in the metastatic progression of prostate cancer. Reprod Biol Endocrinol. 2004;2:2. doi: 10.1186/1477-7827-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyt DG, Rusnak JM, Mannix RJ, Modzelewski RA, Johnson CS, Lazo JS. Integrin activation suppresses etoposide-induced DNA strand breakage in cultured murine tumor-derived endothelial cells. Cancer Res. 1996;56:4146–4149. [PubMed] [Google Scholar]
- 29.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nature reviews. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 30.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nature reviews. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 31.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell'Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–2663. [PubMed] [Google Scholar]
- 32.Nishio M, Endo T, Tsukada N, Ohata J, Kitada S, Reed JC, et al. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood. 2005;106:1012–1020. doi: 10.1182/blood-2004-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Satyamoorthy K, Meier F, Berking C, Bogenrieder T, Herlyn M. Function and regulation of melanomastromal fibroblast interactions: when seeds meet soil. Oncogene. 2003;22:3162–3171. doi: 10.1038/sj.onc.1206455. [DOI] [PubMed] [Google Scholar]
- 35.Satyamoorthy K, Li G, Vaidya B, Patel D, Herlyn M. Insulin-like growth factor-1 induces survival and growth of biologically early melanoma cells through both the mitogen-activated protein kinase and betacatenin pathways. Cancer Res. 2001;61:7318–7324. [PubMed] [Google Scholar]
- 36.Zhong L, Roybal J, Chaerkady R, Zhang W, Choi K, Alvarez CA, et al. Identification of secreted proteins that mediate cell-cell interactions in an in vitro model of the lung cancer microenvironment. Cancer Res. 2008;68:7237–7245. doi: 10.1158/0008-5472.CAN-08-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satyamoorthy K, Li G, Vaidya B, Kalabis J, Herlyn M. Insulin-like growth factor-I-induced migration of melanoma cells is mediated by interleukin-8 induction. Cell Growth Differ. 2002;13:87–93. [PubMed] [Google Scholar]
- 38.Podar K, Tai YT, Davies FE, Lentzsch S, Sattler M, Hideshima T, et al. Vascular endothelial growth factor triggers signaling cascades mediating multiple myeloma cell growth and migration. Blood. 2001;98:428–435. doi: 10.1182/blood.v98.2.428. [DOI] [PubMed] [Google Scholar]
- 39.Ogata A, Chauhan D, Teoh G, Treon SP, Urashima M, Schlossman RL, et al. IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J Immunol. 1997;159:2212–2221. [PubMed] [Google Scholar]
- 40.Podar K, Tai YT, Lin BK, Narsimhan RP, Sattler M, Kijima T, et al. Vascular endothelial growth factor-induced migration of multiple myeloma cells is associated with beta1 integrin- and phosphatidylinositol3-kinase-dependent PKCalpha activation. J Biol Chem. 2002;277:7875–7881. doi: 10.1074/jbc.M109068200. [DOI] [PubMed] [Google Scholar]
- 41.Hideshima T, Chauhan D, Schlossman R, Richardson P, Anderson KC. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene. 2001;20:4519–4527. doi: 10.1038/sj.onc.1204623. [DOI] [PubMed] [Google Scholar]
- 42.Ge NL, Rudikoff S. Insulin-like growth factor I is a dual effector of multiple myeloma cell growth. Blood. 2000;96:2856–2861. [PubMed] [Google Scholar]
- 43.Qiang YW, Kopantzev E, Rudikoff S. Insulinlike growth factor-I signaling in multiple myeloma: downstream elements, functional correlates and pathway cross-talk. Blood. 2002;99:4138–4146. doi: 10.1182/blood.v99.11.4138. [DOI] [PubMed] [Google Scholar]
- 44.Dancescu M, Rubio-Trujillo M, Biron G, Bron D, Delespesse G, Sarfati M. Interleukin 4 protects chronic lymphocytic leukemic B cells from death by apoptosis and upregulates Bcl-2 expression. The Journal of experimental medicine. 1992;176:1319–1326. doi: 10.1084/jem.176.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jewell AP, Worman CP, Lydyard PM, Yong KL, Giles FJ, Goldstone AH. Interferon-alpha upregulates bcl-2 expression and protects B-CLL cells from apoptosis in vitro and in vivo. British journal of haematology. 1994;88:268–274. doi: 10.1111/j.1365-2141.1994.tb05017.x. [DOI] [PubMed] [Google Scholar]
- 46.Buschle M, Campana D, Carding SR, Richard C, Hoffbrand AV, Brenner MK. Interferon gamma inhibits apoptotic cell death in B cell chronic lymphocytic leukemia. The Journal of experimental medicine. 1993;177:213–218. doi: 10.1084/jem.177.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konig A, Menzel T, Lynen S, Wrazel L, Rosen A, Al-Katib A, et al. Basic fibroblast growth factor (bFGF) upregulates the expression of bcl-2 in B cell chronic lymphocytic leukemia cell lines resulting in delaying apoptosis. Leukemia. 1997;11:258–265. doi: 10.1038/sj.leu.2400556. [DOI] [PubMed] [Google Scholar]
- 48.Panayiotidis P, Ganeshaguru K, Jabbar SA, Hoffbrand AV. Interleukin-4 inhibits apoptotic cell death and loss of the bcl-2 protein in B-chronic lymphocytic leukaemia cells in vitro. Br J Haematol. 1993;85:439–445. doi: 10.1111/j.1365-2141.1993.tb03330.x. [DOI] [PubMed] [Google Scholar]
- 49.Mainou-Fowler T, Craig VA, Copplestone AJ, Hamon MD, Prentice AG. Effect of anti-APO1 on spontaneous apoptosis of B cells in chronic lymphocytic leukaemia: the role of bcl-2 and interleukin 4. Leukemia & lymphoma. 1995;19:301–308. doi: 10.3109/10428199509107902. [DOI] [PubMed] [Google Scholar]
- 50.Levesque MC, Misukonis MA, O'Loughlin CW, Chen Y, Beasley BE, Wilson DL, et al. IL-4 and interferon gamma regulate expression of inducible nitric oxide synthase in chronic lymphocytic leukemia cells. Leukemia. 2003;17:442–450. doi: 10.1038/sj.leu.2402783. [DOI] [PubMed] [Google Scholar]
- 51.Kolb JP, Roman V, Mentz F, Zhao H, Rouillard D, Dugas N, et al. Contribution of nitric oxide to the apoptotic process in human B cell chronic lymphocytic leukaemia. Leukemia & lymphoma. 2001;40:243–257. doi: 10.3109/10428190109057923. [DOI] [PubMed] [Google Scholar]
- 52.Lee YK, Shanafelt TD, Bone ND, Strege AK, Jelinek DF, Kay NE. VEGF receptors on chronic lymphocytic leukemia (CLL) B cells interact with STAT 1 and 3: implication for apoptosis resistance. Leukemia. 2005;19:513–523. doi: 10.1038/sj.leu.2403667. [DOI] [PubMed] [Google Scholar]
- 53.Lindemann RK. Stroma-initiated hedgehog signaling takes center stage in B-cell lymphoma. Cancer Res. 2008;68:961–964. doi: 10.1158/0008-5472.CAN-07-5500. [DOI] [PubMed] [Google Scholar]
- 54.Dierks C, Grbic J, Zirlik K, Beigi R, Englund NP, Guo GR, et al. Essential role of stromally induced hedgehog signaling in B-cell malignancies. Nat Med. 2007;13:944–951. doi: 10.1038/nm1614. [DOI] [PubMed] [Google Scholar]
- 55.Hegde GV, Peterson KJ, Emanuel K, Mittal AK, Joshi AD, Dickinson JD, et al. Hedgehog-induced survival of B-cell chronic lymphocytic leukemia cells in a stromal cell microenvironment: a potential new therapeutic target. Mol Cancer Res. 2008;6:1928–1936. doi: 10.1158/1541-7786.MCR-08-0142. [DOI] [PubMed] [Google Scholar]
- 56.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 57.Kaelin WG, Jr, Thompson CB. Q&A: Cancer: clues from cell metabolism. Nature. 465:562–564. doi: 10.1038/465562a. [DOI] [PubMed] [Google Scholar]
- 58.Oettgen HF, Old LJ, Boyse EA, Campbell HA, Philips FS, Clarkson BD, et al. Inhibition of leukemias in man by L-asparaginase. Cancer Res. 1967;27:2619–2631. [PubMed] [Google Scholar]
- 59.Whitecar JP, Jr, Bodey GP, Harris JE, Freireich EJ. L-asparaginase. The New England journal of medicine. 1970;282:732–734. doi: 10.1056/NEJM197003262821307. [DOI] [PubMed] [Google Scholar]
- 60.Avramis VI, Tiwari PN. Asparaginase (native ASNase or pegylated ASNase) in the treatment of acute lymphoblastic leukemia. Int J Nanomedicine. 2006;1:241–254. [PMC free article] [PubMed] [Google Scholar]
- 61.Hutson RG, Kitoh T, Moraga Amador DA, Cosic S, Schuster SM, Kilberg MS. Amino acid control of asparagine synthetase: relation to asparaginase resistance in human leukemia cells. Am J Physiol. 1997;272:1691–1699. doi: 10.1152/ajpcell.1997.272.5.C1691. [DOI] [PubMed] [Google Scholar]
- 62.Aslanian AM, Fletcher BS, Kilberg MS. Asparagine synthetase expression alone is sufficient to induce l-asparaginase resistance in MOLT-4 human leukaemia cells. Biochem J. 2001;357:321–328. doi: 10.1042/0264-6021:3570321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Appel IM, den Boer ML, Meijerink JP, Veerman AJ, Reniers NC, Pieters R. Upregulation of asparagine synthetase expression is not linked to the clinical response L-asparaginase in pediatric acute lymphoblastic leukemia. Blood. 2006;107:4244–4249. doi: 10.1182/blood-2005-06-2597. [DOI] [PubMed] [Google Scholar]
- 64.Williams DA. A new mechanism of leukemia drug resistance? The New England journal of medicine. 2007;357:77–78. doi: 10.1056/NEJMcibr072412. [DOI] [PubMed] [Google Scholar]
- 65.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 66.Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 67.Pan S, Berk BC. Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circ Res. 2007;100:213–219. doi: 10.1161/01.RES.0000256089.30318.20. [DOI] [PubMed] [Google Scholar]
- 68.Trachootham D, Zhang H, Zhang W, Feng L, Du M, Zhou Y, et al. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood. 2008;112:1912–1922. doi: 10.1182/blood-2008-04-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franco R, DeHaven WI, Sifre MI, Bortner CD, Cidlowski JA. Glutathione depletion and disruption of intracellular ionic homeostasis regulate lymphoid cell apoptosis. J Biol Chem. 2008;283:36071–36087. doi: 10.1074/jbc.M807061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu SC. Regulation of glutathione synthesis. Curr Top Cell Regul. 2000;36:95–116. doi: 10.1016/s0070-2137(01)80004-2. [DOI] [PubMed] [Google Scholar]
- 71.Rosado JO, Salvador M, Bonatto D. Importance of the trans-sulfuration pathway in cancer prevention and promotion. Mol Cell Biochem. 2007;301:1–12. doi: 10.1007/s11010-006-9389-y. [DOI] [PubMed] [Google Scholar]
- 72.Hyde R, Taylor PM, Hundal HS. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem J. 2003;373:1–18. doi: 10.1042/bj20030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bannai S, Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. J Membr Biol. 1986;89:1–8. doi: 10.1007/BF01870891. [DOI] [PubMed] [Google Scholar]
- 74.Brodie AE, Potter J, Reed DJ. Unique characteristics of rat spleen lymphocyte, L1210 lymphoma and HeLa cells in glutathione biosynthesis from sulfur-containing amino acids. Eur J Biochem. 1982;123:159–164. doi: 10.1111/j.1432-1033.1982.tb06512.x. [DOI] [PubMed] [Google Scholar]
- 75.Edinger AL, Thompson CB. Antigen-presenting cells control T cell proliferation by regulating amino acid availability. Proc Natl Acad Sci USA. 2002;99:1107–1109. doi: 10.1073/pnas.042707999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gmunder H, Eck HP, Benninghoff B, Roth S, Droge W. Macrophages regulate intracellular glutathione levels of lymphocytes. Evidence for an immunoregulatory role of cysteine. Cell Immunol. 1990;129:32–46. doi: 10.1016/0008-8749(90)90184-s. [DOI] [PubMed] [Google Scholar]
- 77.Bannai S, Ishii T. Formation of sulfhydryl groups in the culture medium by human diploid fibroblasts. J Cell Physiol. 1980;104:215–223. doi: 10.1002/jcp.1041040211. [DOI] [PubMed] [Google Scholar]
- 78.MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM, et al. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–3289. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25:4675–4682. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- 80.Garber K. Oncometabolite? IDH1 discoveries raise possibility of new metabolism targets in brain cancers and leukemia. J Natl Cancer Inst. 102:926–928. doi: 10.1093/jnci/djq262. [DOI] [PubMed] [Google Scholar]
- 81.Paolillo M, Russo MA, Serra M, Colombo L, Schinelli S. Small molecule integrin antagonists in cancer therapy. Mini Rev Med Chem. 2009;9:1439–1446. doi: 10.2174/138955709789957404. [DOI] [PubMed] [Google Scholar]
- 82.Burger M, Hartmann T, Krome M, Rawluk J, Tamamura H, Fujii N, et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106:1824–1830. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 83.Lin B, Podar K, Gupta D, Tai YT, Li S, Weller E, et al. The vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584 inhibits growth and migration of multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2002;62:5019–5026. [PubMed] [Google Scholar]
- 84.Bisping G, Wenning D, Kropff M, Gustavus D, Muller-Tidow C, Stelljes M, et al. Bortezomib, dexamethasone and fibroblast growth factor receptor 3-specific tyrosine kinase inhibitor in t(4;14) myeloma. Clin Cancer Res. 2009;15:520–531. doi: 10.1158/1078-0432.CCR-08-1612. [DOI] [PubMed] [Google Scholar]
- 85.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]