Abstract
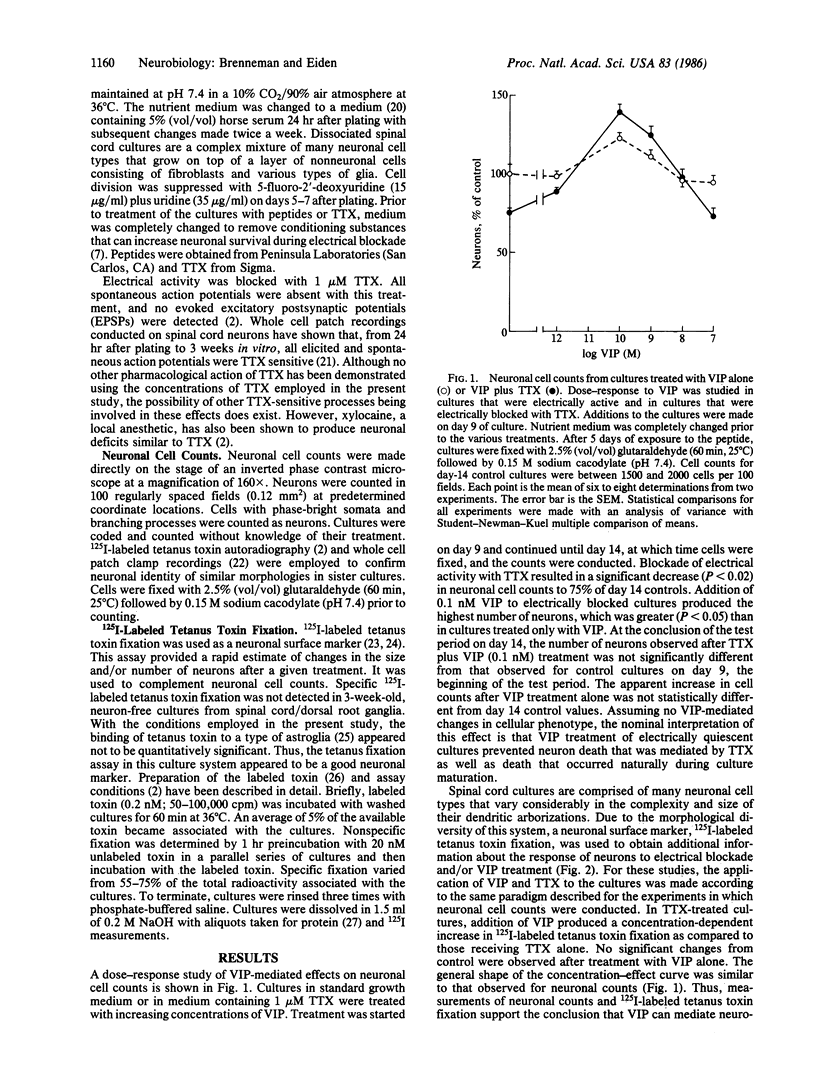
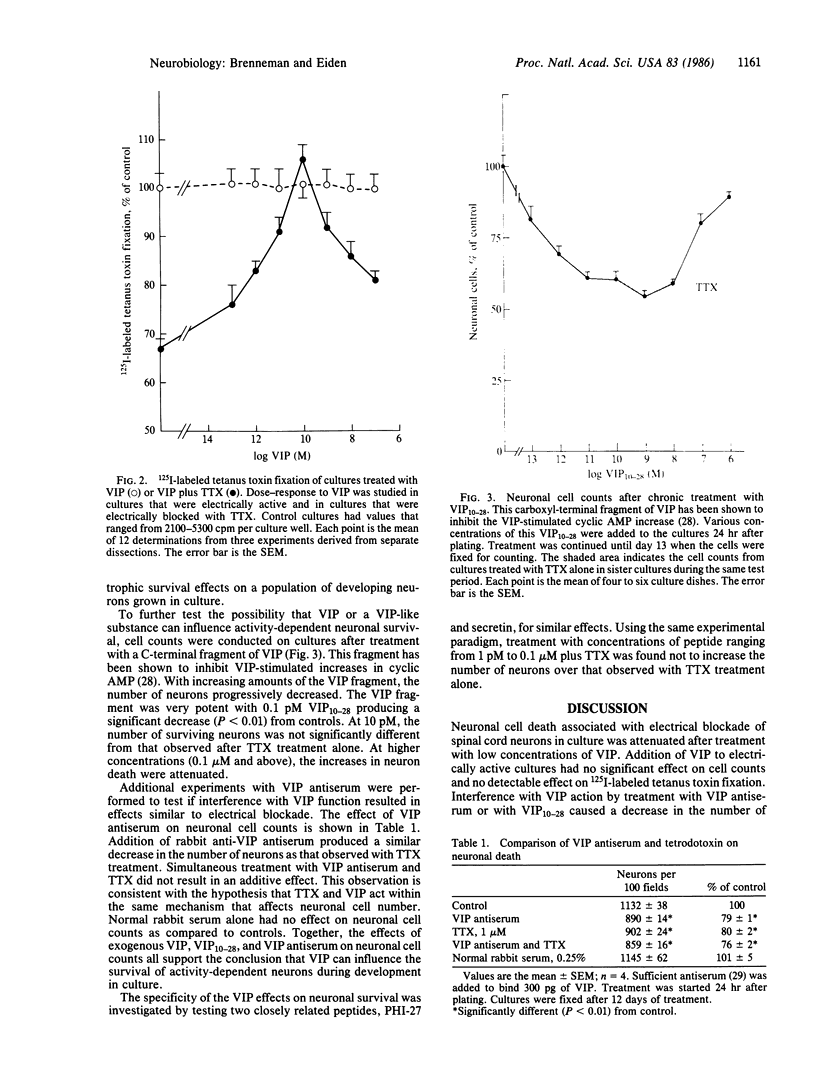
Blockade of electrical activity in dissociated spinal cord cultures results in a significant loss of neurons during a critical period in development. Decreases in neuronal cell numbers and 125I-labeled tetanus toxin fixation produced by electrical blockade with tetrodotoxin (TTX) were prevented by addition of vasoactive intestinal peptide (VIP) to the nutrient medium. The most effective concentration of VIP was 0.1 nM. At higher concentrations, the survival-enhancing effect of VIP on TTX-treated cultures was attenuated. Addition of the peptide alone had no significant effect on neuronal cell counts or tetanus toxin fixation. With the same experimental conditions, two closely related peptides, PHI-27 (peptide, histidylisoleucine amide) and secretin, were found not to increase the number of neurons in TTX-treated cultures. Interference with VIP action by VIP antiserum resulted in neuronal losses that were not significantly different from those observed after TTX treatment. VIP10-28, a fragment that inhibits VIP stimulation of adenylate cyclase, also produced a dose-dependent decrease in neuronal cell counts similar to that seen with TTX treatment. These data indicate that under conditions of electrical blockade a neurotrophic action of VIP on neuronal survival can be demonstrated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissonnette B. M., Collen M. J., Adachi H., Jensen R. T., Gardner J. D. Receptors for vasoactive intestinal peptide and secretin on rat pancreatic acini. Am J Physiol. 1984 Jun;246(6 Pt 1):G710–G717. doi: 10.1152/ajpgi.1984.246.6.G710. [DOI] [PubMed] [Google Scholar]
- Brenneman D. E., Eiden L. E., Siegel R. E. Neurotrophic action of VIP on spinal cord cultures. Peptides. 1985;6 (Suppl 2):35–39. doi: 10.1016/0196-9781(85)90132-9. [DOI] [PubMed] [Google Scholar]
- Brenneman D. E., Fitzgerald S., Litzinger M. J. Neuronal survival during electrical blockade is increased by 8-bromo cyclic adenosine 3',5' monophosphate. J Pharmacol Exp Ther. 1985 May;233(2):402–408. [PubMed] [Google Scholar]
- Brenneman D. E., Fitzgerald S., Nelson P. G. Interaction between trophic action and electrical activity in spinal cord cultures. Brain Res. 1984 Aug;317(2):211–217. doi: 10.1016/0165-3806(84)90098-1. [DOI] [PubMed] [Google Scholar]
- Brenneman D. E., Neale E. A., Habig W. H., Bowers L. M., Nelson P. G. Developmental and neurochemical specificity of neuronal deficits produced by electrical impulse blockade in dissociated spinal cord cultures. Brain Res. 1983 Jul;285(1):13–27. doi: 10.1016/0165-3806(83)90104-9. [DOI] [PubMed] [Google Scholar]
- Creazzo T. L., Sohal G. S. Effects of chronic injections of alpha-bungarotoxin on embryonic cell death. Exp Neurol. 1979 Oct;66(1):135–145. doi: 10.1016/0014-4886(79)90069-4. [DOI] [PubMed] [Google Scholar]
- Dimpfel W., Neale J. H., Habermann E. 125I-Labelled tetanus toxin as a neuronal marker in tissue cultures derived from embryonic CNS. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(2-3):329–333. doi: 10.1007/BF00510562. [DOI] [PubMed] [Google Scholar]
- Eagleson K. L., Raju T. R., Bennett M. R. Motoneurone survival is induced by immature astrocytes from developing avian spinal cord. Brain Res. 1985 Jan;349(1-2):95–104. doi: 10.1016/0165-3806(85)90135-x. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Nilaver G., Palkovits M. Distribution of vasoactive intestinal polypeptide (VIP) in the rat brain stem nuclei. Brain Res. 1982 Jan 14;231(2):472–477. doi: 10.1016/0006-8993(82)90386-9. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Brunso-Bechtold J. K., Yip J. W. Neuronal death in the spinal ganglia of the chick embryo and its reduction by nerve growth factor. J Neurosci. 1981 Jan;1(1):60–71. doi: 10.1523/JNEUROSCI.01-01-00060.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kato Y., Iwasaki Y., Iwasaki J., Abe H., Yanaihara N., Imura H. Prolactin release by vasoactive intestinal polypeptide in rats. Endocrinology. 1978 Aug;103(2):554–558. doi: 10.1210/endo-103-2-554. [DOI] [PubMed] [Google Scholar]
- Koh S. W., Kyritsis A., Chader G. J. Interaction of neuropeptides and cultured glial (Müller) cells of the chick retina: elevation of intracellular cyclic AMP by vasoactive intestinal peptide and glucagon. J Neurochem. 1984 Jul;43(1):199–203. doi: 10.1111/j.1471-4159.1984.tb06697.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ledley F. D., Lee G., Kohn L. D., Habig W. H., Hardegree M. C. Tetanus toxin interactions with thyroid plasma membranes. Implications for structure and function of tetanus toxin receptors and potential pathophysiological significance. J Biol Chem. 1977 Jun 25;252(12):4049–4055. [PubMed] [Google Scholar]
- Lorén I., Emson P. C., Fahrenkrug J., Björklund A., Alumets J., Håkanson R., Sundler F. Distribution of vasoactive intestinal polypeptide in the rat and mouse brain. Neuroscience. 1979;4(12):1953–1976. doi: 10.1016/0306-4522(79)90068-x. [DOI] [PubMed] [Google Scholar]
- Magistretti P. J., Manthorpe M., Bloom F. E., Varon S. Functional receptors for vasoactive intestinal polypeptide in cultured astroglia from neonatal rat brain. Regul Pept. 1983 Apr;6(1):71–80. doi: 10.1016/0167-0115(83)90136-2. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Wendon L. M., Black P., Stolkin C., Bray D. Tetanus toxin: a cell surface marker for neurones in culture. Brain Res. 1978 Jun 9;148(1):251–259. doi: 10.1016/0006-8993(78)90399-2. [DOI] [PubMed] [Google Scholar]
- Nornes H. O., Carry M. Neurogenesis in spinal cord of mouse: an autoradiographic analysis. Brain Res. 1978 Dec 22;159(1):1–6. doi: 10.1016/0006-8993(78)90105-1. [DOI] [PubMed] [Google Scholar]
- Nurcombe V., Bennett M. R. Embryonic chick retinal ganglion cells identified "in vitro". Their survival is dependent on a factor from the optic tectum. Exp Brain Res. 1981;44(3):249–258. doi: 10.1007/BF00236562. [DOI] [PubMed] [Google Scholar]
- Pittman R. H., Oppenheim R. W. Neuromuscular blockade increases motoneurone survival during normal cell death in the chick embryo. Nature. 1978 Jan 26;271(5643):364–366. doi: 10.1038/271364a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Neale E., Henkart M., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977 Sep;40(5):1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- Rougon G., Noble M., Mudge A. W. Neuropeptides modulate the beta-adrenergic response of purified astrocytes in vitro. Nature. 1983 Oct 20;305(5936):715–717. doi: 10.1038/305715a0. [DOI] [PubMed] [Google Scholar]
- Ruberg M., Rotsztejn W. H., Arancibia S., Besson J., Enjalbert A. Stimulation of prolactin release by vasoactive intestinal peptide (VIP). Eur J Pharmacol. 1978 Oct 1;51(3):319–320. doi: 10.1016/0014-2999(78)90421-1. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L., Arancibia S., Bluet-Pajot M. T., Enjalbert A., Epelbaum J., Priam M., Kordon C. Effect of vasoactive intestinal peptide (VIP) on somatostatin inhibition of pituitary growth hormone secretion in vitro. Eur J Pharmacol. 1980 May 2;63(2-3):235–236. doi: 10.1016/0014-2999(80)90453-7. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Mutt V. Isolation and characterization of the intestinal peptide porcine PHI (PHI-27), a new member of the glucagon--secretin family. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6603–6607. doi: 10.1073/pnas.78.11.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon S., Skaper S. D., Manthorpe M. Trophic activities for dorsal root and sympathetic ganglionic neurons in media conditioned by Schwann and other peripheral cells. Brain Res. 1981 Jan;227(1):73–87. doi: 10.1016/0165-3806(81)90095-x. [DOI] [PubMed] [Google Scholar]