In this study, multispectral imaging was used to show relationships between spectral absorption properties of the neuroretinal rim and visual field loss in glaucoma. Multispectral imaging may provide clinically useful information for assessment of glaucoma.
Abstract
Purpose.
To investigate the relationship between neuroretinal rim (NRR) differential light absorption (DLA, a measure of spectral absorption properties) and visual field (VF) sensitivity in primary open-angle glaucoma (POAG).
Methods.
Patients diagnosed with (n = 22) or suspected of having (n = 7) POAG were imaged with a multispectral system incorporating a modified digital fundus camera, 250-W tungsten-halogen lamp, and fast-tuneable liquid crystal filter. Five images were captured sequentially within 1.0 second at wavelengths selected according to absorption properties of hemoglobin (range, 570–610 nm), and a Beer-Lambert law model was used to produce DLA maps of residual NRR from the images. Patients also underwent VF testing. Differences in NRR DLA in vertically opposing 180° and 45° sectors either side of the horizontal midline were compared with corresponding differences in VF sensitivity on both decibel and linear scales by Spearman's rank correlation.
Results.
The decibel VF sensitivity scale showed significant relationships between superior–inferior NRR DLA difference and sensitivity differences between corresponding VF areas in 180° NRR sectors (Spearman ρ = 0.68; P < 0.0001), superior-/inferior-temporal 45° NRR sectors (ρ = 0.57; P < 0.002), and superior-/inferior-nasal 45° NRR sectors (ρ = 0.59; P < 0.001). Using the linear VF sensitivity scale significant relationships were found for 180° NRR sectors (ρ = 0.62; P < 0.0002) and superior–inferior–nasal 45° NRR sectors (ρ = 0.53; P < 0.002). No significant difference was found between correlations using the linear or decibel VF sensitivity scales.
Conclusions.
Residual NRR DLA is related to VF sensitivity in POAG. Multispectral imaging may provide clinically important information for the assessment and management of POAG.
Progressive structural changes in the optic disc and functional losses in the visual field (VF) caused by loss of retinal ganglion cells are hallmarks of primary open-angle glaucoma (POAG), one of the world's leading causes of blindness.1,2 There is no universally accurate test for diagnosis of POAG3,4 or detection of its progression,5 and available tests often produce noisy measurements with large between-subject variability. Clinicians therefore commonly rely on building complementary information from a battery of objective and subjective structural and functional tests for both diagnosis and monitoring.
Objective imaging tools established for use in glaucoma, such as optical coherence tomography, confocal scanning laser ophthalmoscopy, and scanning laser polarimetry, measure optic disc topography and/or retinal nerve fiber layer thickness in varying resolution. These measurements correlate with VF loss to various degrees,6 but considerable scatter is common to all relationships.7 Supplementary objective imaging information on optic disc health would therefore be desirable for assessment of glaucomatous and suspect eyes. One potential source of additional information comes from the spectral absorption characteristics of the neuroretinal rim (NRR) which are not objectively quantified by current imaging tools. Multispectral imaging (also called hyperspectral imaging) can provide detailed, objective information on spectral absorption properties of imaged tissue.
Several groups have previously used multispectral imaging techniques to estimate oxygen saturation in retinal blood vessels,8,9 peripapillary retina,10 and monkey optic disc tissue.11,12 Retinal vessel oxygen saturation changes in response to treatment of human glaucoma have also been investigated with multispectral imaging.13–15 There is, however, a gap in current knowledge as to how spectral information from multispectral imaging of the NRR itself might relate to VF losses in POAG and whether multispectral imaging of the NRR might therefore provide useful information for assessing patients who have or are at risk of having glaucoma.
In the present study, we use multispectral imaging to explore the relationship between local wavelength-specific differences in light absorption by NRR tissue and VF sensitivity in patients diagnosed with or suspected of having POAG. The multispectral imaging technique described may provide useful information for assessment of those with or suspected of having POAG, and we hope to stimulate further study in this promising area.
Methods
The study adhered to the tenets of the Declaration of Helsinki and was approved by the NHS Central Manchester Research Ethics Committee. All patients provided written informed consent to participate after explanation of the nature and possible consequences of the study.
Patients
Patients were recruited from the glaucoma clinics of the Manchester Royal Eye Hospital (Manchester, UK). Recruited patients were either diagnosed with or suspected of having POAG. General inclusion criteria for all patients were older than 40 years, no other ocular disease except mild cataract, clear ocular media; refractive error within ±5.00 DS equivalent and/or 1.50 DC, visual acuity better than 6/12, and experience of at least two prior VF tests.
Additional inclusion criteria for those diagnosed with POAG were clinical diagnosis of POAG confirmed by an experienced glaucoma subspecialist ophthalmologist (CHF) and receiving bilateral treatment for the disease; worse eye with (1) VF mean deviation (MD) worse than (<) −2.00 dB, (2) pattern standard deviation (PSD) worse than (>) 2.00 dB, and (3) an abnormal glaucoma hemifield test16; and at least one of the three criteria in the fellow eye.
Additional inclusion criteria for those suspected of POAG were suspected of having or deemed at high risk of having POAG by an experienced glaucoma subspecialist ophthalmologist (CHF) based on both eyes having (1) an optic disc and/or retinal nerve fiber layer appearance suggestive of glaucomatous damage, (2) VF damage suggestive of early POAG, (3) intraocular pressure consistently >22 mm Hg, or a combination of 3 with 1 or 2; receiving bilateral prophylactic medication.
All VFs were subjectively examined to exclude any clearly nonglaucomatous losses such as hemianopia. Note that to recruit eyes with a range of disease stages, where both eyes of a patient diagnosed with POAG met the general inclusion criteria, both were enrolled in the study. For all suspects, both eyes were enrolled. Fellow eyes were separated for data analysis (see later section on Statistical Analysis).
Patients underwent confocal scanning laser tomography (HRT3; Heidelberg Retina Tomograph, Heidelberg Engineering, GmbH, Heidelberg, Germany) and multispectral imaging of the optic discs of both eyes in the same visit. VF data (SITA Standard 24-2 program of the Humphrey Field Analyzer; Carl Zeiss Meditec, Dublin, CA) was collected retrospectively from the clinic database (most recent VF test). Patients with no reliable VF test results obtained within 6 weeks before imaging were excluded. Reliability was ascertained on the basis of fixation losses (<30%), false-positive errors (<20%), and false-negative errors (<30%), as well as the technician's written notes.
Multispectral Imaging
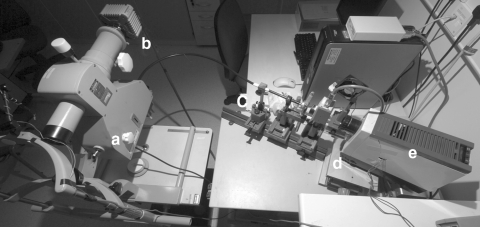
Multispectral images were obtained with a custom-modified digital fundus camera system (Fig. 1) which has been described in detail elsewhere.17 Briefly, the system incorporates a 250-W tungsten halogen lamp filtered by a fast tuneable liquid crystal filter (Varispec VIS 07-20 STD; Cambridge Research and Instrumentation, Cambridge, UK). Images were captured with a low-noise, Peltier-cooled CCD array (Orca C4742-80-12AG; Hamamatsu Photonics, Hamamatsu, Japan) with a spatial resolution of 336 × 256 pixels (after 4 × 4 binning).
Figure 1.
The multispectral imaging system. The system incorporates (a) a modified fundus camera with (b) a Peltier-cooled CCD array; (c) a fluid optic; (d) a fast-tuneable liquid crystal filter; and (e) a 250-W tungsten halogen lamp.
After pupillary dilation, narrow-band (7-nm half width) images were taken sequentially at five different central wavelengths selected according to the absorption properties of hemoglobin in water18 (range, 570–610 nm; total acquisition time, <1.0 second). Specifically, we captured images at central wavelengths of 570 nm where absorption is equal between oxygenated and deoxygenated hemoglobin in water, and at wavelengths where the ratio between absorption by oxygenated and deoxygenated hemoglobin in water is large (575, 580, 586, and 610 nm). The wavelengths chosen are similar to those in studies that used multispectral imaging for oximetry in the eye8–12,14 and brain.19–21 All images were subjectively reviewed for eye movement artifacts as they were captured, and any poor image sets were recaptured.
Calculation of Differential Light Absorption
Captured images were corrected for systematic variations in the spectral sensitivity of the system with a correction factor derived from images of a white surface with a flat reflection spectrum. The corrected monochromatic images were then aligned assuming that degradation due to eye movement can be approximated by a combination of translation, rotation, and scaling.22 Alignment was subjectively reviewed and repeated if movement artifacts remained.
Using a Beer-Lambert law model19–21 (equation 1) we then calculated our metric differential light absorption (DLA) on a pixel-by-pixel basis from the aligned images. DLA is a measure of the relative wavelength-specific, light-absorption properties of the imaged tissue, which is dependent on the concentration of wavelength-specific absorbers in the tissue. Specifically, we find the least-squares solution to equation 1 in which the constants a and b are the molar extinction coefficients of oxygenated and deoxygenated hemoglobin in water,18 covariate ω is a second absorber inversely related to DLA. Iλ and I570nm represent the pixel intensities of the images obtained at each wavelength and at 570 nm, respectively. By calculating DLA on a pixel-by-pixel basis we can produce spatial maps of DLA by the imaged tissue.
Similar approaches have been used to estimate oxygen saturation in animal brain tissue19–21 and human retinal vessels8–10,13–15 using instruments calibrated manometrically or by oxygen-breathing experiments. Because of the similarities between our methods and those of previous studies, we expect that our DLA metric is related to oxygen saturation. However, variations in the reflective characteristics of individual eyes make accurate in vivo calibration to oxygen saturation extremely difficult. Since in the present study we are solely concerned with determining the presence or absence of correlations with VF sensitivity, we conservatively report DLA in arbitrary relative units.
Data Analysis
Differential Light Absorption Maps.
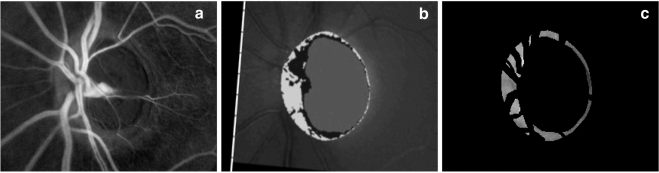
Image processing was performed in commercial software (R2008a; MatLab; The Mathworks, Inc., Natick, MA). The optic disc topography images were resampled and aligned with the optic disc DLA maps using the same method as described above. Masks were then created based on the NRR area defined in the aligned retinal topography (the area within the optic disc whose surface height is above a reference plane located parallel to the peripapillary retinal surface and 50 μm below the retinal surface at the point where the papillomacular bundle and optic disc margin coincide). These masks were then applied to the DLA maps to isolate only the NRR tissue and overlying blood vessels (i.e., peripapillary retina and optic cups were removed). Additional masks were then applied to remove visible blood vessels from the DLA maps, leaving only NRR tissue (Fig. 2). Masking was performed manually with reference to the original monochromatic images and relevant retinal topographies. Mean DLA was then calculated in the eight 45° sectors shown in Figure 3 from the masked maps. These measurements have been found to be repeatable in healthy subjects, with coefficient of variation <5% for all sectors repeated both on the same day and on different days and no significant difference between measurements taken on the same day and different days.23
Figure 2.
Example of an optic disc DLA map (a), resampled and aligned optic disc topography (b), and a masked DLA map, leaving only neuroretinal rim tissue (c). In the DLA maps brighter areas represent areas of greater DLA. In the topographic image, the neuroretinal rim is represented by the light gray and black areas combined, and it was on this area that the mask used to create (c) was based.
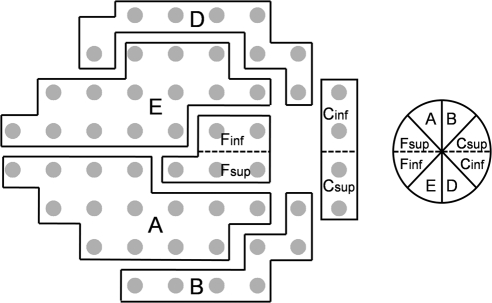
Figure 3.
Diagram of optic disc sectors (right) and corresponding visual field locations (left) for a right eye. Adapted from Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2213–2220. © ARVO. (See Ref. 24.) Dashed lines: the location of the split between the original sectors described by Garway-Heath et al.24 at the horizontal midline. The 45° optic disc sectors and corresponding visual field locations are labeled as they are referred to in the text: (A) superior-temporal, (B) superior-nasal, (Csup and Cinf) nasal, (D) inferior-nasal, and (E) inferior-temporal (Fsup and Finf), temporal.
Comparison with VF Sensitivity.
VF sensitivities were grouped according to a slight modification (addition of a horizontal center line) of a published map24 which describes the anatomic relationship between optic disc sectors and 24-2 test locations (Fig. 3). The SITA algorithm reports sensitivities on a decibel scale in which the decibel is 10log(1/Lambert). For this study, measured sensitivities at points within each group were averaged on both the decibel and linear (1/Lambert) scales for comparison to DLA of corresponding optic disc sectors, similar to the approach of Garway-Heath et al.24
Altitudinal differences in VF sensitivity were compared with differences in NRR DLA in opposing sectors above and below the horizontal midline of the optic disc. This approach of comparing altitudinal differences in VF sensitivity with differences in NRR DLA and area above and below the horizontal midline is based on the common finding of vertical within-eye asymmetry of both structural and functional damage in glaucoma.16,25–28 It holds the advantage of using each eye as its own reference, thus avoiding the problem of wide between-subject variation in the measure.
Statistical Analysis
All left eyes were converted to right eye format, and statistical analysis was performed in the open-source environment R (ver. 2.12.1).29 We regarded all eyes as part of a disease continuum and therefore did not separate the POAG-diagnosed and -suspect eyes in our analysis. Rather than simply selecting one eye from each patient to include in the analysis we used a bootstrapping approach to more fully explore the available data while keeping fellow eyes separate. One thousand samples of one eye per patient (patients with only one enrolled eye were included in every sample) were taken and the correlation measured in each sample. The mean correlation and central 95% range from the 1000 samples are reported for each relationship, and P values for mean correlations were calculated from the t-distribution with appropriate degrees of freedom for a single sample. This approach therefore reports the correlation that can be expected on average, and the range in which correlation will fall 95% of the time when one eye per patient is selected.
To limit the effect of outliers on the correlations found we used Spearman's rank correlation coefficient which computes correlation between the ranked order of the variables, rather than their raw values and, unlike Pearson correlation, is unaffected by the nonlinearity of monotonic relationships between variables.
Specifically, the following correlations were assessed on both the decibel and linear VF sensitivity scales: superior–inferior difference in NRR DLA and inferior–superior difference in VF sensitivity; sectors A/E differences in NRR DLA and VF sensitivity; sectors B/D differences in NRR DLA and VF sensitivity; sectors Csup–Cinf differences in NRR DLA and VF sensitivity; and sectors Fsup–Finf differences in NRR DLA and VF sensitivity. A Wilcoxon signed rank test was used to test whether one VF sensitivity scale yielded stronger correlations than the other. Statistical significance was assumed at P < 0.0045 after Bonferroni correction for multiple (n = 11) comparisons. However, since the relationships are not independent, this is a rather conservative cutoff and so we also report exact P values for all relationships to two significant figures.
Results
Study Population
Seven patients were excluded from the study after initial enrollment because of poor pupillary dilation preventing good-quality imaging (n = 3), no reliable VF test results available within 6 weeks before imaging (n = 3), and recruitment in error (ocular media not clear, n = 1). Twenty-nine patients comprised our final dataset, of which four POAG patients had only one enrolled eye. All the remaining patients (18 POAG, 7 suspect) had both eyes enrolled. Hence, our final dataset included 54 eyes of 29 patients comprising 14 eyes of 7 suspects and 40 eyes of 22 POAG patients. VF tests were performed within 10 days before imaging for 21 patients and within 6 weeks for all. Since eyes from both groups were analyzed together as part of a disease continuum, demographics of all eyes are shown in Table 1.
Table 1.
Demographic Information for All Eyes
| Number of eyes (patients) | 54 (29) |
| Median age (range) | 69 years (45 to 84 years) |
| Median (range) VF MD | −2.20 dB (−17.18 to 1.93) |
| Median (range) VF PSD | 3.34 dB (1.21 to 14.15) |
| Median (range) total neuroretinal rim area | 1.13 mm2 (0.31 to 2.63) |
| Median (range) mean neuroretinal rim DLA | 30.69 units (13.94 to 42.77) |
| Median (range) elapsed time between VF test and imaging | 4 days (0 to 42) |
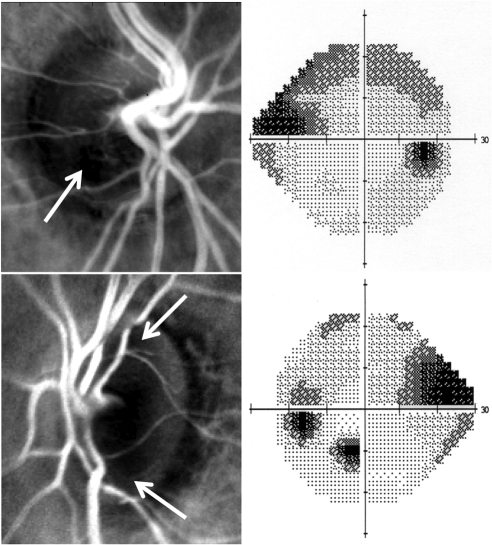
Figure 4 shows spatial DLA maps from two patients, with their corresponding VFs. In the DLA maps, the brighter areas represent areas with greater DLA. Anecdotally, patients were asked whether they found the multispectral imaging comfortable and all responded positively.
Figure 4.
Spatial DLA maps of two patient's optic discs (left) with corresponding visual field grayscale plots (right). In the DLA maps, brighter areas represent areas with greater DLA. Arrows: darker areas (areas of lower DLA) that subjectively correspond to the VF defects shown. The areas of very low DLA adjacent to the optic discs correspond to areas of peripapillary atrophy.
Altitudinal Relationships
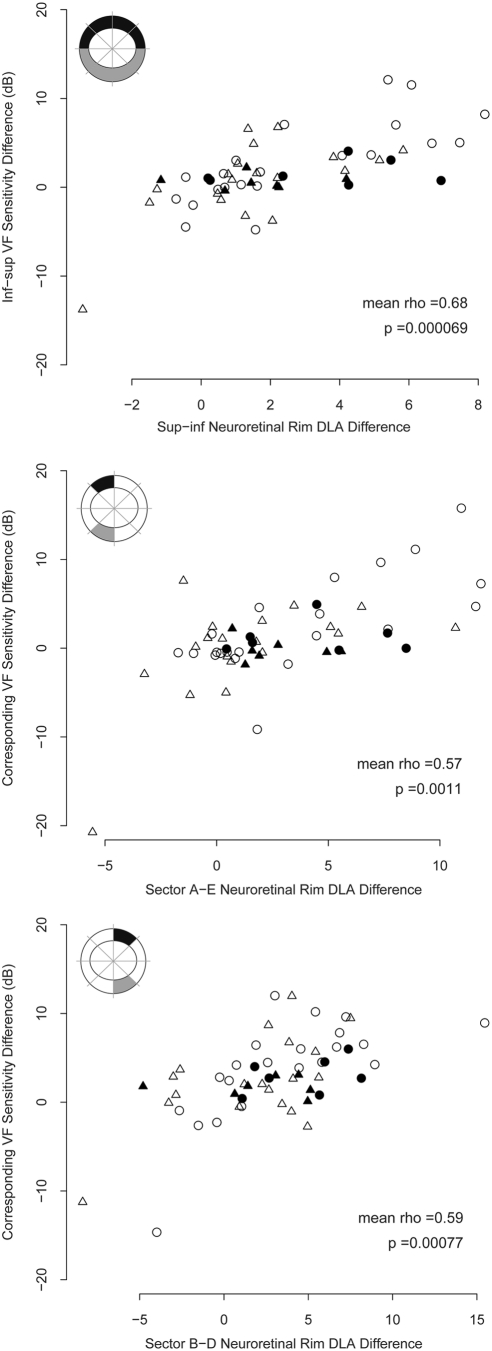
Table 2 summarizes the correlations found between altitudinal differences in VF sensitivity on both the decibel and linear (1/Lambert) scales and superior–inferior differences in NRR mean DLA for all corresponding vertically opposing sectors and grouped sectors, as defined in Figure 3.
Table 2.
Mean Spearman's Rho (Central 95% Range; n = 1000 bootstrap samples) and P Values
| Decibel Scale | Linear Scale | |
|---|---|---|
| Superior-Inferior | 0.68 (0.58 to 0.77), P = 0.000069* | 0.62 (0.50 to 0.73), P = 0.00015* |
| A–E | 0.57 (0.39 to 0.76), P = 0.0011* | 0.22 (−0.02 to 0.44), P = 0.125 |
| B–D | 0.59 (0.44 to 0.74), P = 0.00077* | 0.53 (0.36 to 0.69), P = 0.0016* |
| Csup-Cinf | 0.37 (0.15 to 0.60), P = 0.050 | 0.25 (0.01 to 0.48), P = 0.096 |
| Fsup-Finf | 0.06 (−0.15 to 0.28), P = 0.38 | 0.06 (−0.19 to 0.32), P = 0.38 |
n = 29. Data are for correlations between altitudinal differences in VF sensitivity in decibels and linear (1/Lambert) scales and superior-inferior differences in mean neuroretinal rim DLA of corresponding optic disc sectors (labeled as in Fig. 3).
Statistically significant correlations (P < 0.0045).
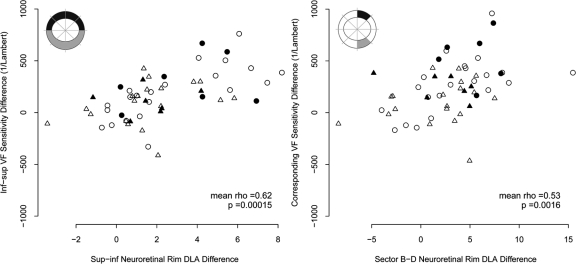
On average when one eye per patient was sampled, statistically significant correlations were found between altitudinal differences in decibel VF sensitivity and superior–inferior differences in mean NRR DLA for the entire superior and inferior hemifields (excluding blind-spot points), sectors A/E and sectors B/D. For correlations between altitudinal differences in linear (1/Lambert) VF sensitivity and superior–inferior differences in mean NRR DLA statistical significance was reached for the entire superior hemifields (excluding blind spot points) and sectors B/D. No statistically significant difference was found between the correlations using the decibel and linear scales (Wilcoxon signed-rank test, P = 0.10).
All significantly correlated relationships between altitudinal differences in decibel VF sensitivity and superior–inferior differences in mean NRR DLA are shown in Figure 5, and the same for linear VF sensitivity is shown in Figure 6. Patients diagnosed with or suspected of having POAG are represented by different plotting symbols in Figures 5 and 6 for clarity, although POAG-diagnosed/suspect eyes were treated as belonging to a disease continuum rather than separated in analysis of all correlations. All eyes are represented in Figures 5 and 6, but right and left eyes are represented by different plotting symbols, as fellow eyes were separated for analysis of all correlations.
Figure 5.
Superior–inferior differences in neuroretinal rim differential light absorption (DLA, arbitrary units) versus differences in corresponding visual field sensitivity (decibel scale) for all sectors where significant relationships were found. The plots include a diagrammatic representation of the neuroretinal rim sectors considered (shaded) for a right eye; the sectors are labeled on the axes as in Figure 3. The mean Spearman's rank correlation is shown (n = 1000 bootstrap samples of one eye per patient) and P values (n = 29). For clarity, the symbols of the study population are as follows: Open symbols, POAG-diagnosed patients; solid symbols POAG-suspect patients; circles, right eyes; triangles, left eyes (separated for all data analyses).
Figure 6.
Superior–inferior differences in neuroretinal rim differential light absorption (DLA, arbitrary units) versus the differences in corresponding visual field sensitivity (linear scale) for all sectors where significant relationships were found. See Figure 5 for description of the symbols.
Discussion
The objective of this study was to investigate whether spectral absorption properties of the residual NRR (DLA) are related to functional VF measures in eyes with established or suspected POAG. Multispectral imaging of the NRR was performed successfully on a group of patients with suspected or diagnosed POAG and a range of VF loss. The technique used was noninvasive, comfortable for the patient, and easily performed. It produced good-quality spatial DLA maps of the residual NRR, which allowed discrimination of local differences in DLA.
A comparison of within-eye altitudinal differences in decibel VF sensitivity to superior–inferior differences in NRR DLA yielded correlations that were statistically significant for 180° and 45° sectors of the superior–inferior portions of the NRR. When a linear scale was used for VF sensitivity, the same correlations reached statistical significance for 180° sectors and the superior-/inferior-nasal 45° sectors of the optic disc. NRR DLA was compared with VF sensitivity using both decibel and linear scales, as there is no a priori framework for how the two measures should theoretically relate. This is in contrast to structural tests, which are often considered as surrogate measures of the number of remaining retinal ganglion cells, and therefore it is sensible to expect that these should be linearly related to VF sensitivity. The results of this study show that NRR DLA is related to VF sensitivity measured in either decibel or linear units and has not provided strong evidence of either scale being more strongly related. Another difference between DLA and structural measures is that DLA may reasonably be expected to increase or decrease over time, or in response to changes in treatment or local ocular–systemic stress, whereas structural measures generally do not increase once retinal ganglion cells are lost. This is one reason for why we currently do not attempt to fit models to the relationships found in this moderately sized study population, as model coefficients are likely be highly dependent on the particular data used, including the time point at which the data were collected relative to the patient's treatment and local ocular–systemic stresses. Despite this inherent source of noise in these relationships, our results show that, on average, NRR DLA is related to VF sensitivity, and further study into these factors may allow a generalizable model to be fitted similar to that used by Hood et al.7 Similarly, further study may also investigate whether spectral information from multispectral imaging can help to explain discordance in structural and functional measures seen in some patients.
Significant correlations were not found between NRR DLA differences in the nasal and temporal optic disc sectors and sensitivity differences in their corresponding VF areas using either decibel or linear scales. These portions of the NRR are underrepresented in the 24-2 VF test pattern; stronger associations might be found if the VF were better sampled in these areas, such as with a 10-2 test pattern for the temporal sectors.
One source of variability in the multispectral imaging process, which limits the strength of the correlations found in this study, is that longer wavelength light penetrates deeper into tissues before it is back scattered.30,31 We make no correction for this at present with the narrow range of wavelengths used. The use of sequential image capture may also be seen as a limitation of our technique, since it necessitates alignment of the captured images. However, alignment was achieved satisfactorily using an established technique,22 and sequential capture holds the advantage of individual adjustment of exposure time for each wavelength.
Another notable source of variability limiting the correlations found is the response variability in VF tests which increases with decreasing sensitivity.32 We also make no correction for age to the VF sensitivity or NRR DLA estimates, however; since we look for within-eye differences in most measures, such age correction is not necessary.
The use of Spearman's rank correlation is important in limiting the effect of outliers on correlations found in this study. Consider, for example, the first plot in Figure 5, where an outlier appears toward the origin. The Pearson correlation coefficient may be artificially increased by this outlier, whereas Spearman's correlation gives it no more weight than if it lay on the edge of the rest of the data.
We suggest that the bootstrapping approach used in this study to test correlation in multiple samples of one eye per patient is a more satisfactory exploration of available data than the convention of simply selecting one eye per patient at random or based on criteria (such as worse eye) which may induce bias. The method is particularly advantageous in smaller studies as correlations found by selecting one eye per patient can vary significantly depending on which eyes are selected (note that there are >5.2 million possible selections of one eye from each patient in a study population of the present size). In these cases a single selection of one eye per patient may not be representative of the dataset as a whole.
Our measure DLA is based on methods used in other studies to measure oxygen saturation, and so we expect the two to be related. Experiments are currently being performed in our laboratory to investigate how DLA relates to blood perfusion and oxygen saturation and to calibrate our system to allow assignment of SI units to the measure. The Beer-Lambert law model used to calculate DLA presently includes constants based on spectral extinction coefficients of hemoglobin in water. Clearly, adjustment of these constants pertaining to the scattering and absorption properties of the eye may improve the model and more closely relate DLA to oxygen saturation and/or blood perfusion.
In conclusion, this is the first report known to us of correlations between spectral information from multispectral imaging of the NRR and VF sensitivity in patients diagnosed with or suspected of having POAG. The correlations found suggest that multispectral optic disc imaging may provide clinically useful information for assessment of those with or at risk of POAG. Further study is indicated to evaluate the mechanisms behind this relationship.
Acknowledgments
The authors thank both anonymous reviewers for constructive comments that helped improve the manuscript.
Footnotes
Supported by a College of Optometrists (UK) PhD Studentship (JD), Central Manchester NHS Foundation Trust Grant RO1180, the Manchester Academic Health Sciences Centre (MAHSC), and the NIHR Manchester Biomedical Research Centre.
Disclosure: J. Denniss, None; I. Schiessl, None; V. Nourrit, None; C.H. Fenerty, None; R. Gautam, None; D.B. Henson, None
References
- 1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851 [PMC free article] [PubMed] [Google Scholar]
- 3. Mowatt G, Burr JM, Cook JA, et al. Screening tests for detecting open-angle glaucoma: systematic review and meta-analysis. Invest Ophthalmol Vis Sci. 2008;49:5373–5385 [DOI] [PubMed] [Google Scholar]
- 4. DeLeón-Ortega JE, Arthur SN, McGwin G, Jr, Xie A, Monheit BE, Girkin CA. Discrimination between glaucomatous and nonglaucomatous eyes using quantitative imaging devices and subjective optic nerve head assessment. Invest Ophthalmol Vis Sci. 2006;47:3374–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Artes PH, Chauhan BC. Longitudinal changes in the visual field and optic disc in glaucoma. Prog Retin Eye Res. 2005;24:333–354 [DOI] [PubMed] [Google Scholar]
- 6. Bowd C, Zangwill LM, Madeiros FA, et al. Structure-function relationships using confocal scanning laser ophthalmoscopy, optical coherence tomography and scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2006;47:2889–2895 [DOI] [PubMed] [Google Scholar]
- 7. Hood DC, Anderson SC, Wall M, Raza AS, Kardon RH. A test of a linear model of glaucomatous structure–function loss reveals sources of variability in retinal nerve fiber and visual field measurements. Invest Ophthalmol Vis Sci. 2009;50:4254–4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beach JM, Schwenzer KJ, Srinivas S, Kim D, Tiedeman JS. Oximetry of retinal vessels by dual-wavelength imaging: calibration and influence of pigmentation. J Appl Physiol. 1999;86:748–758 [DOI] [PubMed] [Google Scholar]
- 9. Hardarson SH, Harris A, Karlsson RA, et al. Automatic retinal oximetry. Invest Ophthalmol Vis Sci. 2006;47:5011–5016 [DOI] [PubMed] [Google Scholar]
- 10. Ito M, Murayama K, Deguchi T, et al. Oxygen saturation levels in the juxta-papillary retina in eyes with glaucoma. Exp Eye Res. 2008;86:512–518 [DOI] [PubMed] [Google Scholar]
- 11. Khoobehi B, Beach JM, Kawano H. Hyperspectral imaging for measurement of oxygen saturation in the optic nerve head. Invest Ophthalmol Vis Sci. 2004;45:1464–1472 [DOI] [PubMed] [Google Scholar]
- 12. Beach J, Ning J, Khoobehi B. Oxygen saturation in optic nerve head structures by hyperspectral image analysis. Curr Eye Res. 2007;32:161–170 [DOI] [PubMed] [Google Scholar]
- 13. Siesky B, Harris A, Cantor LB, et al. A comparative study of the effects of brinzolamide and dorzolamide on retinal oxygen saturation and ocular microcirculation in patients with primary open-angle glaucoma. Br J Ophthalmol. 2008;92:500–504 [DOI] [PubMed] [Google Scholar]
- 14. Traustason S, Hardarson SH, Gottfredsdottir MS, et al. Dorzolamide-timolol combination and retinal vessel oxygen saturation in patients with glaucoma or ocular hypertension. Br J Ophthalmol. 2009;93:1064–1067 [DOI] [PubMed] [Google Scholar]
- 15. Hardarson SH, Gottfredsdottir MS, Halldorsson GH, et al. Glaucoma filtration surgery and retinal oxygen saturation. Invest Ophthalmol Vis Sci. 2009;50:5247–5250 [DOI] [PubMed] [Google Scholar]
- 16. Asman P, Heijl A. Glaucoma hemifield test: automated visual field evaluation. Arch Ophthalmol. 1992;110:812–819 [DOI] [PubMed] [Google Scholar]
- 17. Nourrit V, Denniss J, Muqit MMK, et al. High resolution hyperspectral imaging of the retina with a modified fundus camera. Fr J Ophtalmol. 2010;33:686–692 [DOI] [PubMed] [Google Scholar]
- 18. Oregon Medical Laser Center Tabulated molar extinction coefficient for hemoglobin in water. Portland, OR: Oregon Health and Science University; 1998. Available at: http://omlc.ogi.edu/spectra/hemoglobin/summary.html Accessed September 9, 2009 [Google Scholar]
- 19. Berwick J, Johnston D, Jones M, et al. Neurovascular coupling investigated with two-dimensional optical imaging spectroscopy in rat whisker barrel cortex. Eur J Neurosci. 2005;22:1655–1666 [DOI] [PubMed] [Google Scholar]
- 20. Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996;272:551–554 [DOI] [PubMed] [Google Scholar]
- 21. Mayhew J, Zheng Y, Hou Y, et al. Spectroscopic analysis of changes in remitted illumination: the response to increased neural activity in brain. Neuroimage. 1999;10:304–326 [DOI] [PubMed] [Google Scholar]
- 22. Nourrit V, Bueno J, Vohnsen B, Artal P. Non linear registration for scanned retinal images: application to ocular polarimetry. Appl Opt. 2008;47:5341–5347 [DOI] [PubMed] [Google Scholar]
- 23. Denniss J. Diagnostic imaging and the structure-function relationship in glaucoma (thesis). Manchester, UK: University of Manchester; 2010 [Google Scholar]
- 24. Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2213–2220 [PubMed] [Google Scholar]
- 25. Jonas JB, Budde WM, Panda-Jonas S. Ophthalmoscopic evaluation of the optic nerve head. Surv Ophthalmol. 1999;43:293–320 [DOI] [PubMed] [Google Scholar]
- 26. Jonas JB, Budde WM. Diagnosis and pathogenesis of glaucomatous optic neuropathy: morphological aspects1. Prog Retin Eye Res. 2000;19:1–40 [DOI] [PubMed] [Google Scholar]
- 27. Mikelberg FS, Drance SM. The mode of progression of visual field defects in glaucoma. Am J Ophthalmol. 1984;98:443. [DOI] [PubMed] [Google Scholar]
- 28. Hart WM, Becker B. The onset and evolution of glaucomatous visual field defects. Ophthalmology. 1982;89:268–279 [DOI] [PubMed] [Google Scholar]
- 29. R Development Core Team R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org/ Accessed June 15, 2011 [Google Scholar]
- 30. Delori FC, Gragoudas ES, Francisco R, Pruett RC. Monochromatic ophthalmoscopy and fundus photography. Arch Ophthalmol. 1977;95:861–868 [DOI] [PubMed] [Google Scholar]
- 31. Berendschot TTJM, DeLint PJ, van Norren D. Fundus reflectance: historical and present ideas. Prog Retin Eye Res. 2003;22:171–200 [DOI] [PubMed] [Google Scholar]
- 32. Henson DB, Chaudry S, Artes PH, Faragher EB, Ansons A. Response variability in the visual field: comparison of optic neuritis, glaucoma, ocular hypertension, and normal eyes. Invest Ophthalmol Vis Sci. 2000;41:417–421 [PubMed] [Google Scholar]