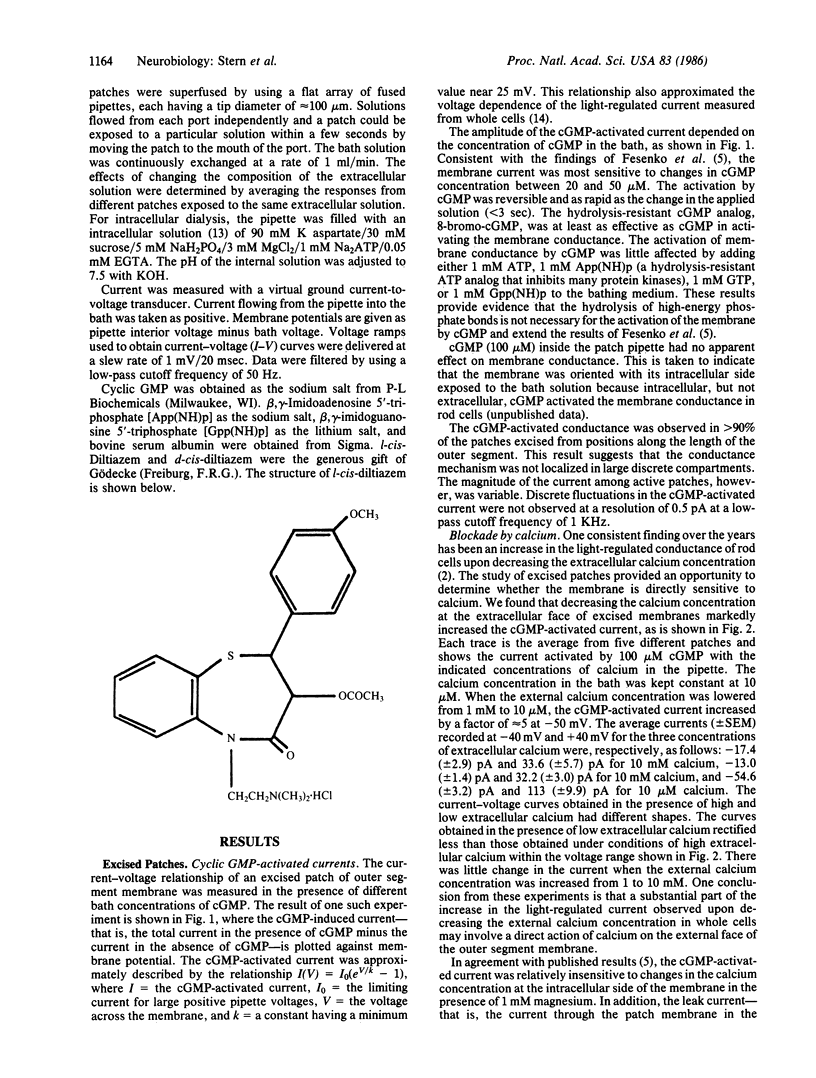
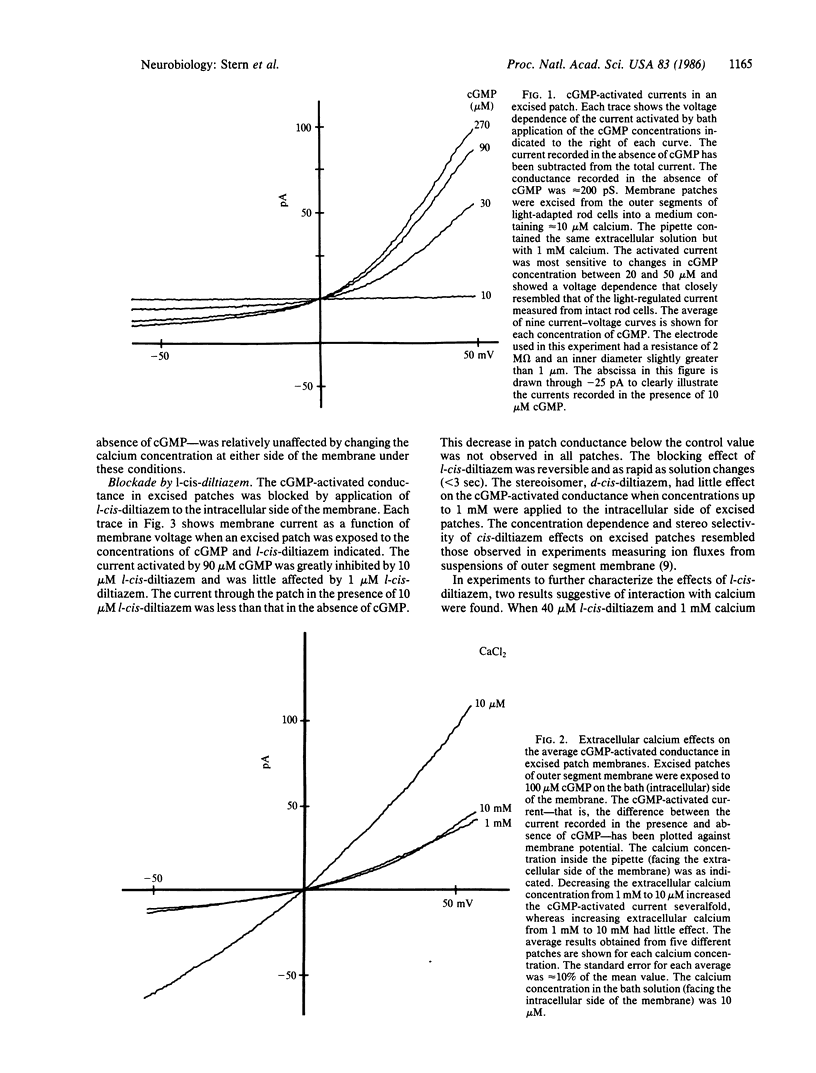
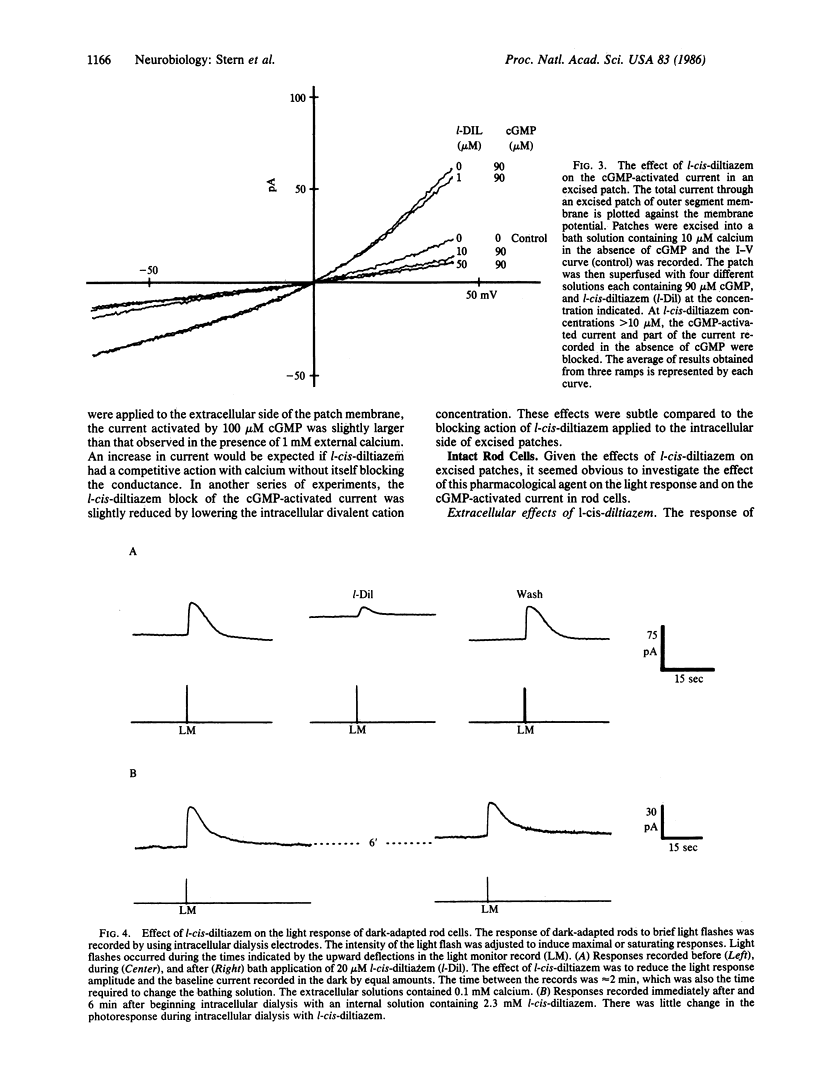
Abstract
The effect of calcium ions on the cGMP-activated current of outer segment membrane was examined by the excised-patch technique. Changes in the extracellular calcium concentration had marked effects on the cGMP-activated current, while changes in intracellular calcium concentration were ineffective. Changes in calcium concentration in the absence of cGMP had little, if any, effect on membrane conductance. These results suggest that both intracellular cGMP and extracellular calcium can directly affect the conductance underlying the light response in rod cells. The pharmacological agent l-cis-diltiazem reversibly inhibited the cGMP-activated current when applied to the intracellular side of an excised patch. When superfused over intact rod cells, l-cis-diltiazem reversibly blocked much of the normal light response. The isomer, d-cis-diltiazem, did not significantly affect either patches or intact rod cells. Thus, the light-regulated conductance has binding sites for both calcium and cGMP that may interact during the normal light response in rod cells and a site specific for l-cis-diltiazem that can be used to identify and further study the conductance mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta A., Cavaggioni A., Sorbi R. T. Cyclic GMP and the permeability of the disks of the frog photoreceptors. J Physiol. 1979 Oct;295:171–178. doi: 10.1113/jphysiol.1979.sp012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. I., Hall I. A., Ferrendelli J. A. Calcium and cyclic nucleotide regulation in incubated mouse retinas. J Gen Physiol. 1978 May;71(5):595–612. doi: 10.1085/jgp.71.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., Bownds M. D. Visual transduction in vertebrate photoreceptors. Annu Rev Neurosci. 1979;2:17–34. doi: 10.1146/annurev.ne.02.030179.000313. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Kaupp U. B. Cyclic GMP directly regulates a cation conductance in membranes of bovine rods by a cooperative mechanism. J Biol Chem. 1985 Jun 10;260(11):6788–6800. [PubMed] [Google Scholar]
- Lam D. M. Biosynthesis of acetylcholine in turtle photoreceptors. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1987–1991. doi: 10.1073/pnas.69.7.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeish P. R., Schwartz E. A., Tachibana M. Control of the generator current in solitary rods of the Ambystoma tigrinum retina. J Physiol. 1984 Mar;348:645–664. doi: 10.1113/jphysiol.1984.sp015131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J. H., Lisman J. E. Internal dialysis of Limulus ventral photoreceptors. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7580–7584. doi: 10.1073/pnas.79.23.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]



