Abstract
A radiolabeling method for bioconjugation based on the Diels-Alder reaction between 3,6-diaryl-s-tetrazines and an 18F-labeled trans-cyclooctene is described. The reaction proceeds with exceptionally fast rates, making it an effective conjugation method within seconds at low micromolar concentrations.
Positron emission tomography (PET) is non-invasive imaging modality that utilizes positron-emitting radionuclides (C-11, N-13, O-15 and F-18).1 F-18 PET has a number of attributes that make it clinically attractive, including 100% positron efficiency, a very high specific radioactivity, and a short half-life of ~110 min.2 However, the short half-life of F-18 and the poor nucleophilicity of fluoride render it difficult to incorporate F-18 in complex molecules. Currently, radiochemistry is a major limiting factor for the field of PET.3 Despite recent advances,4,5 challenges exist for improving F-18 incorporation with respect to reaction rates, efficiency, and selectivity.
Recently, we introduced the tetrazine-trans-cyclooctene ligation (‘TTCO ligation’, Fig. 1) as a method of bioconjugation that proceeds with fast reaction rates without need for catalysis.6,7 trans-Cyclooctene derivatives are readily prepared from cis-cyclooctenes using a photochemical flow-reaction that we developed.8 While a variety of s-tetrazine derivatives were known react with strained alkenes,9 we have found that 3,6-diaryl-s-tetrazines offer an excellent combination of fast reactivity and stability for both the conjugate and starting material.6 In particular, 3,6-di(2-pyridyl)-s-tetrazines were shown to display excellent characteristics.6,10 Thus, the reaction between trans-cyclooctene and 1a proceeds with a rapid rate (k2 ~ 2000 M-1 s-1 in 9:1 MeOH:water), and is successful in cell media and cell lysate.6 3,6-Di(2-pyridyl)-s-tetrazines can easily be functionalized as their amido derivatives (1b),6 which display excellent stability toward water and biological nucleophiles.10
Fig. 1.

Tetrazine-trans-cyclooctene ligation (‘TTCO-ligation’)
After we described the TTCO-ligation, the groups of Hilderbrand[11a] and Pipkorn and Braun[11b] described ligations between tetrazines and less reactive dienophiles. However, the use of trans-cyclooctene is the key to fast rates of reactivity, and other groups have subsequently developed applications for the TTCO-ligation.11c-d,12
Because of the fast rate of reactivity, the TTCO-ligation offers opportunities for the rapid conjugation of radionuclides to biomolecules, both of which are often available only at low concentration. Very recently, Robillard and coworkers elegantly demonstrated that our system for TTCO-ligation could be utilized in In111–imaging of tumors in live mice using a DOTA-derivative of 1b.12 Herein, we report the development of an extremely fast and reactive method for generating 18F labeled probes based on the TTCO-ligation.
While a number of indirect methods for 18F-incorporation into tetrazines or trans-cyclooctene derivatives could be envisioned (e.g. derivatization by N-succinimidyl-4-18F-fluorobenzoate13), such approaches are complicated by the need to carry 18F through added manipulations. Thus, we focused on the development of direct methods for 18F-incorporation via reactions with fluoride ion, with an initial focus on the synthesis of 18F-labeled tetrazines (Scheme 1). Our attempts to convert nitrotetrazine derivatives 2a and 2b into 18F-labeled substitution products 3 using 18F-fluoride/kryptofix or 18F-TBAF gave decomposition products and only traces of radiolabeled products. We also combined mesylate 4 with fluoride: in the most successful experiment (18F-TBAF at 85 °C for 15 min), 18F-labeled product 5 was obtained in ~1% labeling yield.
Scheme 1.

Synthesis of 18F-labeled tetrazine. Attempts to prepare 3 by nucleophilic substitution were low yielding. Fluorination of 4 (18F-fluoride, MeCN, 85 °C, 15 min) gives 5 in only 1% radiochemical yield.
The instability of tetrazine precursors to F-labeling conditions prompted us to consider methods for preparing 18F-labeled trans-cyclooctenes. To this end, we synthesized the nosylate 8 as shown in Scheme 2. The key step in the synthesis was photoisomerization of 6 to 7 using a flow reactor that continuously removes the trans-isomers through selective metal complexation.7 The major diastereomer of 7 was carried forward in the synthesis of 8.
Scheme 2.
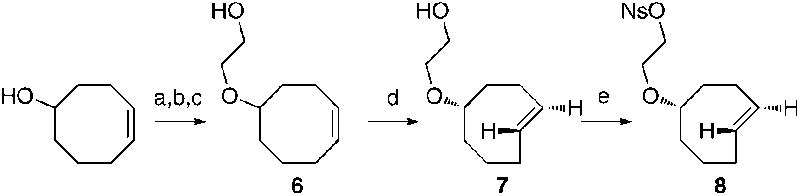
Synthesis of trans-cyclooctene nosylate 8. Reagents and conditions: (a) NaH, α-bromoacetic acid. (b) CH2N2, 69%, 2 steps (c) DIBAL, 78% (d) Methyl benzoate-sensitized photoisomerization with active removal of trans-isomers, AgNO3, 55%. (e) NsCl, Et3N, 87%.
Nosylate 8 reacted efficiently with TBAF to provide 19F-9 in high yield. With this standard in hand, conditions for the preparation of 18F-9 were optimized (Table 1). It was found that 18F-9 could be obtained in good yield by adding nosylate 8 to a mixture of [18F]-fluoride (100 mCi)/ tetrabutylammonium bicarbonate (TBAB) in acetonitrile (0.8 mL). We determined the effect of varying the concentration of 8 in reactions conducted for 15 min at 75 °C (Table 1, entries 1–4). The highest labeling yield (71%) was achieved with 7.0 mM 8, but a useful radiochemical yield (18%) was still obtained with 0.35 mM 8. Different reaction temperatures were studied, and 75 °C was found to be optimal (Table 1, entries 3, 5–7). Finally, we investigated the efficiency of the 18F labeling as a function of time using 2 mg of 8 at 75 °C (Table 1, entries 8–10). While the labeling yield was optimal (71%) in an experiment conducted for 15 min, useful labeling yields were also obtained after 3 min (43%) and 7 min (68%). The conclusion of the experiments summarized in Table 1 is that the conditions of entry 3 (7.0 mM 8, 75 °C, 15 min) are optimal for 18F labeling.
Table 1.
Optimization of synthesis of 18F-labeled trans-cyclooctene
| Entry | Amount of 8 | Temp | Reaction Time | Radiochemical Yield |
|---|---|---|---|---|
| 1 | 100 μg (0.35 mM) | 75 °C | 15 min | 18% |
| 2 | 500 μg (1.8 mM) | 75 °C | 15 min | 25% |
|
| ||||
| 3 | 2.0 mg (7.0 mM) | 75 °C | 15 min | 71% |
|
| ||||
| 4 | 3.0 mg (11 mM) | 75 °C | 15 min | 71% |
|
| ||||
| 5 | 2.0 mg (7.0 mM) | 40 °C | 15 min | 24% |
|
| ||||
| 6 | 2.0 mg (7.0 mM) | 55 °C | 15 min | 34% |
|
| ||||
| 7a | 2.0 mg (7.0 mM) | 90 °C | 15 min | 71% |
|
| ||||
| 8 | 2.0 mg (7.0 mM) | 75 °C | 3 min | 43% |
|
| ||||
| 9 | 2.0 mg (7.0 mM) | 75 °C | 7 min | 68% |
|
| ||||
| 10 | 2.0 mg (7.0 mM) | 75 °C | 30 min | 71% |
Comparable results (70% RCY) were obtained under similar conditions using K2CO3/K222 instead of TBAB.
As derivatives of 3,6-di(2-pyridyl)-s-tetrazine (e.g. 1b, Figure 1) are readily accessible.6,10,12 3,6-di(2-pyridyl)-s-tetrazine (1a) was used to test the efficiency of the 18F-labeled trans-cyclooctene 9 in the TTCO-ligation. A ‘cold’ study with 19F-9 was initially conducted. As expected, the conjugate 19F-10 formed immediately, and then slowly isomerized to 1,4-dihydropyrazine 19F-11 as a mixture of isomers6 (Scheme 3a). These isotopically stable conjugates served as coinjection standards for analysis of reactions with 18F-9.
Scheme 3.
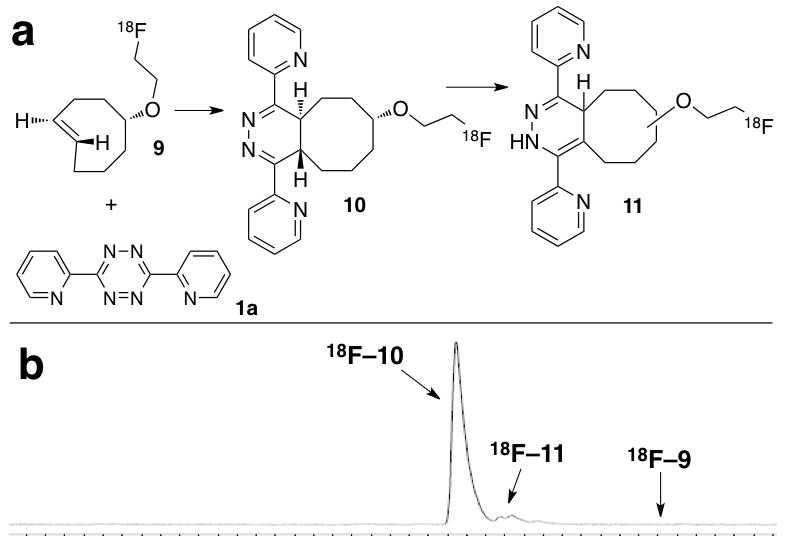
(a) Conjugation between 9 and 1a occurs rapidly to form conjugate 10, which slowly rearranges to 11 as a mixture of regio- and stereoisomers. (b) Radio-HPLC analysis of the crude product from Table 2 Entry 3
The conjugations between 1a and 18F-9 were carried out in 1:1 acetonitrile/water, and were analyzed within 10 seconds of mixing. Prior to the conjugation, 18F-9 was purified by HPLC and easily separated from unreacted precursor 8. When 18F-9 (1 mCi, 2 μM) was combined with 1a (concentrations of ≥21 μM), 18F-9 was completely consumed with 10 s, and 18F-10 had formed in 98% radiochemical yield, accompanied by 18F-11 (1%) (Table 2, entries 1-2). When the concentration of 18F-9 was decreased to 0.1 mCi (0.2 μM), the conjugate 18F-10/11 was still formed in excellent radiochemical yield (98%, entry 3). Useful radiochemical yields could also be obtained with even lower concentrations of 1a (entries 4-5). We also investigated the efficiency of the conjugation between 1a (21 μM) and 18F-9 (1 mCi, 2 μM) in PBS buffer and serum media: 18 F-10 is formed in quantitative yield within 10 seconds (entries 6-7).
Table 2.
The effect of concentration on the formation of 18F-10 through the conjugation between 18F-9 and 3,6-di(2-pyridyl)-s-tetrazine. All reactions were performed at room temperature.
| Entry | 18F-9a | 1a | Solvent | Reaction time | Radiochemical Yield (18F-10 + 18F-11) |
|---|---|---|---|---|---|
| 1 | 1 mCi (2 μM) | 210 μM | MeCN/H2O | <10 s | >98 % |
| 2 | 1 mCi (2 μM) | 21 μM | MeCN/H2O | <10 s | >98 % |
| 3 | 0.1 mCi (0.2 μM) | 21 μM | MeCN/H2O | <10 s | 98 % |
| 4 | 0.1 mCi (0.2 μM) | 2.1 μM | MeCN/H2O | <10 s | 56 % |
| 5 | 0.1 mCi (0.2 μM) | 0.21 μM | MeCN/H2O | <10 s | 15 % |
| 6 | 1 mCi (2 μM) | 21 μM | PBS buffer | <10 s | >98 % |
| 7 | 1 mCi (2 μM) | 21 μM | Serum | <10 s | >98 % |
The concentration of 18F-9 was estimated based on the specific activity of fluoride after bombardment (~4 Ci/μmol), taking into account a correction for the rate of radioactive decay.
The Diels-Alder conjugate 10 was found to be stable in water, and the benign isomerization to 11 was the only side reaction. Thus, 19F-10 was the only product detected by 1H NMR analysis immediately after the conjugation. In CD3CN/H2O, the rearrangement of 19F-10 to 19F-11 proceeded to 11% conversion after 4 hours, and >95% conversion after 48 hours. The stability of the radiolabeled conjugation product was monitored in PBS buffer and serum media for 4 hours, and no degradation products of 18F-10 were observed.
In conclusion, a new radiolabeling method for bioconjugation based on the TTCO-ligation has been described. The method utilizes a novel F-18 labeled trans-cyclooctene probe, and the efficiency of the reaction has been demonstrated with 3,6-di(2-pyridyl)-s-tetrazine, which can be readily functionalized via amido derivatives. The reaction proceeds with exceptionally fast rates, making it an effective conjugation method at low concentration. We anticipate that the TTCO-ligation will provide the foundation for a new class of reliable methods for 18F-labeling of biomolecules in PET applications.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant Number P20 RR017716 from the COBRE Program of the NCRR, and by the USC Department of Radiology. Spectra were obtained with instrumentation supported by NSF CRIF:MU grants: CHE 0840401 and CHE-0541775.
Contributor Information
Zibo Li, Email: ziboli@usc.edu.
Joseph M. Fox, Email: jmfox@udel.edu.
Notes and references
- 1.Schlyer DJ. Ann Acad Med Singapore. 2004;33:146–154. [PubMed] [Google Scholar]
- 2.Schirrmacher R, Wängler C, Schirrmacher E. Mini-Reviews in Organic Chemistry. 2007;4:317–329. [Google Scholar]
- 3.Elsinga PH. Methods. 2002;27:208–217. doi: 10.1016/s1046-2023(02)00076-2. [DOI] [PubMed] [Google Scholar]; Schirrmacher R, Wängler C, Schirrmacher E. Eur J Org Chem. 2008:2853–2873. [Google Scholar]
- 4.Marik J, Sutcliffe JL. Tetrahedron Lett. 2006;47:6681–6684. [Google Scholar]
- 5.Li ZB, Wu Z, Chen K, Chin FT, Chen X. Bioconjug Chem. 2007;18:1987–1994. doi: 10.1021/bc700226v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackman ML, Royzen M, Fox JM. J Am Chem Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For reviews of Cu-free bioconjugation chemistry: Jewett JC, Bertozzi CR. Chem Soc Rev. 2010;39:1272–1279. doi: 10.1039/b901970g.; Becer CR, Hoogenboom R, Schubert US. Angew Chem Int Ed. 2009;48:4900–4908. doi: 10.1002/anie.200900755.; Reviews of bioorthogonal chemistry: Lim RKV, Lin Q. Chem Commun. 2010;46:1589. doi: 10.1039/b925931g.; Kalia J, Raines RT. Curr Org Chem. 2010;14:138–147. doi: 10.2174/138527210790069839.. Sletten EM, Bertozzi CR. Angew Chem Int Ed. 2009;48:6974. doi: 10.1002/anie.200900942.
- 8.Royzen M, Yap GPA, Fox JM. J Am Chem Soc. 2008;130:3760–3761. doi: 10.1021/ja8001919. [DOI] [PubMed] [Google Scholar]
- 9.Thalhammer F, Wallfahrer U, Sauer J. Tetrahedron Lett. 1990;31:6851. [Google Scholar]; b) Boger DL. Chem Rev. 1986;86:781. [Google Scholar]
- 10.Acetamido-substituted dipyridyltetrazines (1b) display fast kinetics12, and are stable for prolonged periods (>24 h) in water, and toward amines and thiols in MeOH: Selvaraj R, Blackman ML, Fox JM , unpublished results.
- 11.a) Devaraj NK, Weissleder R, Hilderbrand SA. Bioconj Chem. 2008;19:2297–2299. doi: 10.1021/bc8004446. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Pipkorn R, Waldeck W, Didinger B, Koch M, Mueller G, Wiessler M, Braun K. J Peptide Sci. 2010;15:235–241. doi: 10.1002/psc.1108. [DOI] [PubMed] [Google Scholar]; c) Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Angew Chem Int Ed. 2009;48:7013. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Devaraj NK, Hilderbrand S, Upadhyay R, Mazitschek R, Weissleder R. Angew Chem Int Ed. 2010;19:2869–2872. doi: 10.1002/anie.200906120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossin R, Verkerk PR, Bosch SMvd, Vulders RCM, Verel I, Lub J, Robillard MS. Angew Chem Int Ed. 2010;49:3375–3378. doi: 10.1002/anie.200906294. [DOI] [PubMed] [Google Scholar]
- 13.Vaidyanathan G, Zalutsky MR. Nature Protocols. 2006;1:1655–1661. doi: 10.1038/nprot.2006.264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


