Abstract
Posttraumatic stress disorder (PTSD) is typically accompanied by both acute and chronic alterations in the stress response. These alterations have mostly been described in individuals under baseline conditions, but studies have also used a challenge model to assess the role of the hypothalamic-pituitary-adrenal (HPA) axis in the stress response. The purpose of this article was to assess the effect of long-term treatment with the selective reuptake inhibitor (SSRI), paroxetine, on stress reactivity in patients with PTSD. We assessed diurnal salivary cortisol and urinary cortisol as well as cortisol, heart rate, and behavioral responses to a standardized cognitive stress challenge, in 13 female patients with chronic PTSD before and after 12 months of paroxetine treatment. Treatment resulted in a significant decrease in PTSD symptoms. Twenty-four-hour urinary cortisol was lower compared to base line after successful treatment. Treatment resulted in a decrease of salivary cortisol levels on all time points on a diurnal curve. Despite similar stress perception, cortisol response to the cognitive stress challenge resulted in a 26.5% relative decrease in stress-induced salivary cortisol with treatment. These results suggest that successful treatment with SSRI in chronic PTSD is associated with a trend for a decrease in baseline diurnal cortisol and with reduced cortisol reactivity to stress.
Keywords: cortisol, PTSD, stress, paroxetine, SSRI, challenge, HPA axis
INTRODUCTION
The last decade has seen a rapid expansion of studies on hypothalamic-pituitary-adrenal (HPA) axis reactivity in posttraumatic stress disorder (PTSD), These studies have shown an array of alterations related to HPA axis function, with varying specificity for the disorder. While, baseline studies of the HPA axis have yielded mostly mixed results, findings of increased corticotrophic-releasing hormone (CRH) in cerebrospinal fluid (CSF)1,2 and decreased plasma cortisol over a 24-h period3,4 have been more consistent. Mixed results have been reported in 24-h urine excretion5–7 and single time point plasma cortisol studies have been reported. For example, low cortisol in urine and plasma samples has been reported in some studies of adults with PTSD8–10 but not in others.11–19 In a recent study, cortisol was assessed in CSF showing increased levels in PTSD patients.20 Additionally, some studies demonstrated flattened free cortisol response after awakening.8–10
Several pharmacological challenge paradigms have been used to further assess HPA axis function.21 The outcome of CRH challenges,22–25 adrenocorticotrophic hormone (ACTH) challenges,22,24 and metyrapone challenges26–28 are so far inconclusive. In contrast, despite methodological differences, the low-dose (0.5 mg) dexamethasone suppression test in plasma has consistently found enhanced suppression of cortisol in subjects with PTSD,29–34 as well as enhanced suppression of ACTH.34,35 Only one study that measured salivary cortisol showed no group difference in cortisol suppression.36
Among the factors that contribute to inconsistencies in outcome in base line and challenge designs are differences in study populations, such as early childhood abuse versus (adult) combat trauma as well as differential contributions of early-life trauma on later-life psychopathology in general.
In PTSD, the response of the HPA axis to nonpharmacological challenges has been less extensively studied. To date, in PTSD patients, two studies assessing HPA axis reactivity to a cognitive stress challenge have been performed. Bremner et al., using a series of arithmetic tasks under time pressure showed significant differences between PTSD patients and healthy controls in cortisol levels during the anticipation phase as well as during and after challenge.22 Elzinga et al., assessed cortisol responsivity to traumatic reminders using a personalized traumatic script in abused women with and without current PTSD,37 and also showed increased cortisol levels in patients with PTSD during the challenge paradigm.
It has been hypothesized that the inability to dampen responses to cues that do not represent true threat, is related to stress-sensitized dysfunctional neural circuitry. These nonpharmacological challenge studies, when performed before and after a specific treatment intervention, can provide important information about the effects of that treatment on biological alterations and reactivity.
The purpose of the current study was to assess the salivary cortisol and autonomic response to a cognitive stress challenge paradigm following chronic paroxetine treatment. We hypothesized that PTSD patients who responded to treatment with paroxetine would show decreased salivary cortisol response after treatment.
While the efficacy of the selective reuptake inhibitor (SSRI), paroxetine, has been reported for clinical measures of PTSD,38,39 no studies have yet looked at the effects of SSRIs on HPA axis functioning in PTSD. An important question is whether the alterations found on HPA axis functioning in PTSD patients are reversible. Tucker et al. demonstrated that 10 weeks of open label administration of fluvoxamine dampened autonomic reactivity to trauma scripts in PTSD compared to controls, the reported efficacy of fluvoxamine.40 Nikisch et al. looked at long-term (16 weeks) citalopram administration in patients with major depression and found reduced responsiveness of HPA axis.41 Their results showed a time-dependent reduction of ACTH and cortisol response during the combined dexamethasone/CRH test both in treatment responders and nonresponders. Rinne et al. found that 12 weeks of fluvoxamine treatment42 in women with Borderline Personality Disorder (BPD) resulted in a reduction of the hyperresponsiveness of the HPA axis on the dexamethasone/CRH test.
SUBJECTS AND METHODS
Subjects
Patients in this study were part of a larger open label study on the effects of paroxetine on memory and hippocampal volume. Participating patients in this study were male and female outpatients diagnosed with PTSD. They were recruited through advertisements in the local newspapers. Diagnosis was on the basis of the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria for PTSD, as determined with the Structured Clinical Interview for DSM-IV (SCID43), consensus diagnosis by three research psychiatrists (EV, MV, and JDB), and a score of greater than 50 on the Clinician Administered PTSD Scale44 (CAPS, current symptoms version). In case of comorbid disorders, the PTSD symptom onset had to predate the onset of comorbid psychopathology. History of childhood trauma was assessed with the early trauma inventory45 (ETI).
Some patients (n = 6) in this study were part of a national double-blind trial of paroxetine versus placebo in PTSD. Following 12 weeks of participation in a double-blind study, subjects were offered participation in the current study of 9 months open trial treatment of 10–30 mg paroxetine per day. Assessments of these patients were performed before entering the double-blind phase; in all other patients this was performed before open label treatment. Patients with a serious medical or neurological illness were excluded, on the basis of history and physical examination, lab testing, and electrocardiogram. Patients with a history of psychotherapeutic treatment within 3 months of the study start, and a positive toxicology screening were also excluded. Female subjects were required to have a negative pregnancy test before the study. Patients with a history of alcohol or drug abuse 6 months prior to the study, as well as current alcohol abuse or drug use were excluded from participation. All subjects were medication free for at least 4 weeks before participation in the study. During the study no concomitant antidepressant or neuroleptic medication was allowed. After complete explanation of the study procedures and before entering the study, written informed consent was obtained. This study was approved by the Investigational Review Board (IRB) of Yale University and was prepared in accordance with the ethical standards laid down in the Declaration of Helsinki.
A total of 28 patients passed the inclusion criteria and started their participation in the open label study. Eleven patients (5 female, 6 male) of this group participated in the double-blind trial and received placebo or 20–50 mg of paroxetine for 12 weeks prior to open label treatment. Twenty-three completed the complete open label study protocol (9 male and 14 female). We were able to collect posttreatment data for this study in 5 male and 14 female patients. Two of the males, and one of the females had to be left out of the total subject sample, because either cortisol could not be assessed on account of too little saliva throughout the cognitive challenge, or noncompliance with the challenge protocol. Because including only three males would skew our sex distribution, we decided to leave the males out of further analysis. For the analysis we used data of 13 females, with an average age of 42.9 years (SD = 10.7). Four of the females were premenopausal, and nine were postmenopausal. Of these patients 12 were white, and 1 was Hispanic. The average score on the CAPS was 87.8 (SD = 21.7). Nine patients had a lifetime diagnosis of major depressive disorder (MDD), and six patients had a current depressive disorder. Three patients were also diagnosed with comorbid generalized anxiety (GAD) without agoraphobia, and two met criteria for social phobia. In four patients PTSD was the sole diagnosis. Onset of PTSD was typically in childhood, and in all cases preceded the other comorbid disorders, including affective disorders, by at least several years. In nine patients trauma occurred before the age of 18 years, in four patients primary trauma happened later in life. In all cases of early childhood trauma this was reported as sexual abuse. In later life traumas the witnessing of murder, assault, torture, and a motor vehicle accident were reported as the primary trauma. Mean score on the ETI-self report scale in the early childhood trauma group was 80 (SD = 37.8). For the other patients this was 42.5 (SD = 43).
Diurnal Cortisol and 24-h Urine Collection
All participating patients collected saliva over a 24-h time period using the same salivettes as in the cognitive challenge procedure. This involved collection of saliva at five time points (morning awaking, 8:00 am, 12:00 am, 4:00 pm, and 8:00 pm) before and after treatment. They were also instructed to collect urine over a 24-h time period and repeated this after treatment. Collection of saliva and urine and the cognitive challenge were performed on separate days.
Challenge Procedure
The cognitive challenge tests were on the basis of a protocol previously used in studies of aging46,47 and included arithmetic (multiplication, division, addition, and subtraction), cognitive tasks (Stroop task, e.g., looking at the word red spelled in color green and naming the color green), problem solving, matching figures to numbers and memory for figure–number pairing, and unscrambling words.22 All subjects were studied in the time interval from 1:00 pm to 3:30 pm. Subjects were placed in a hospital bed with application of a Dynamap cuff for measurement of heart rate and blood pressure, and baseline ratings of distress and salivary cortisol. Distress was assessed on a 10-point scale, ranging from 0 to 10. Subjects rested in a hospital bed for 60 min while listening to a tape with relaxing music and sounds (with headphone). Measurement of heart rate, blood pressure, and salivary cortisol was performed at 60 min, 10 min, and 5 min before the initiation of the cognitive challenge. At the start of the challenge a physician wearing a white laboratory coat entered the room, took place behind a desk, and initiated a series of computational tasks under time pressure, for each test in similar order. This lasted for 20 min. Each individual task was scored by the rater and performed under time pressure. Negative feedback regarding the score and the time spent in the task, was consistently given, and the level of difficulty was increased until subjects were unable to successfully complete the tasks. At the end of the cognitive challenge the physician left the room. The patient was debriefed by the research assistant who explained the procedure in terms of an “unfriendly physician,” not in terms of the setup. Heart rate and blood pressure were measured at 2, 4, 6, 7, 10, 12, 14, 15, 16, 18, 20, 30, 35, 45, 60, 75, and 90 min after the initiation of the challenge. Salivary cortisol was measured at 0, 10, 15, 20, 30, 35, 45, 60, 75, and 90 min after the initiation of the challenge. Saliva samples were collected using salivette collection devices, salivette tubes were centrifuged in 0 to 4°C to prepare the saliva and stored at −70°C. The test scores on the cognitive tasks were not included in the analysis. The physicians assessing the arithmetics in the pretreatment situation were different from those who assessed the arithmetics in the posttreatment situation.
Treatment Phase
In patients that participated in the double-blind clinical trial, paroxetine was started after a wash out of 2 weeks. In all other patients, paroxetine was prescribed in the first visit after the pretreatment assessments. All patients started open label with a dose of 10 mg daily and were titrated up to 20 mg in 4 days. If patients reported side effects or were concerned about any symptoms during treatment they could call a 24-h answering service that was linked to an on-call physician. If patients did not respond to 20 mg by week 8, the paroxetine dosage was increased up to a maximum of 30 mg/day. Follow-up visits were scheduled every other week from weeks 1 to 12 and every 4 weeks from weeks 12 to 36. No systematic psychotherapeutic procedure was administered in the study phase. Medication was dispensed every visit to enhance compliance and reduce chances of misuse. After successful treatment all assessments were performed again.
Data Analysis and Statistical Analysis
The saliva was analyzed for cortisol using an 125I immunoradiometric assay kit available from Diagnostic Products Corporation (Los Angeles, CA). Samples and standards (200 uL) were determined in duplicate; standard concentration ranged from 67 pg/mL to 3.0 ng/mL. Day-to-day coefficients of variation for low (398 pg/mL) and high (4.12 ng/mL) concentration quality assessment (QC) were made. After assessment of urinary volume, cortisol was assessed using the same assay kit. In subjects with a single inadequate or missing sample, data were interpolated where appropriate. Subjects with three or more adjacent samples missing were excluded from the analysis. The bulk of the samples was analyzed in one single run, some samples were run in a second analysis, and some samples were rerun in case of errors.
Clinical effect was analyzed using a paired samples t-test. Two-tailed tests of significance were used to assess group differences at discrete time points. In addition, a repeated measures analysis of variance (ANOVA) with time as repeated measure was used to compare cortisol, heart rate, and blood pressure response to the cognitive challenge, and in relation with diurnal cortisol and CAPS score, before and after treatment with paroxetine. The level of significance for all analyses was set at 0.05. We calculated the area under the curve (AUC) after normalizing both cortisol curves at the beginning of the mental arithmetic (T0), and compared the calculated AUC for the first 60 min after the start of the challenge (T0–T60).
RESULTS
Clinical Effect
Paroxetine treatment resulted in a mean 56% reduction (87.8 [SD = 21.7] to 38.8 [SD = 36.7]) in PTSD symptoms as measured with changes from base line on the CAPS total score (t = 4,77; df = 24; P < 0.001) among study completers. Improvement was significant for all symptom cluster scores (reexperiencing: 25.6 [SD = 8.3] to 9.0 [SD = 10.1] avoidance/numbing: 33.7 [SD = 10.0] to 13.5 [SD = 12.0], hyperarousal: 27.0 [SD = 6.4] to 10.8 [SD = 7.5].
Baseline Effects: 24-h Urinary and Diurnal Salivary Cortisol Effects
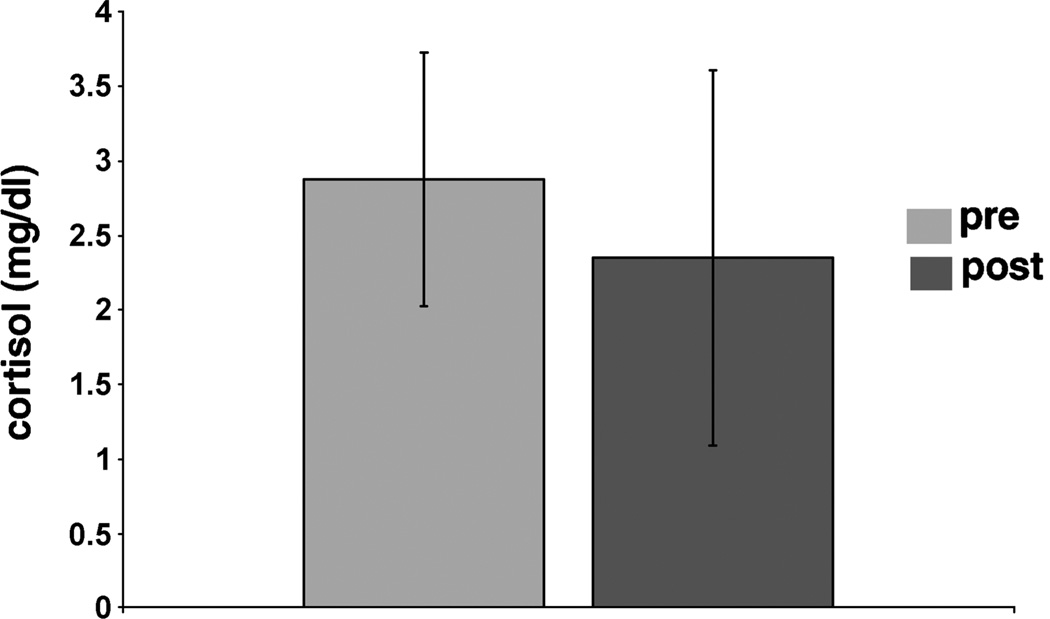
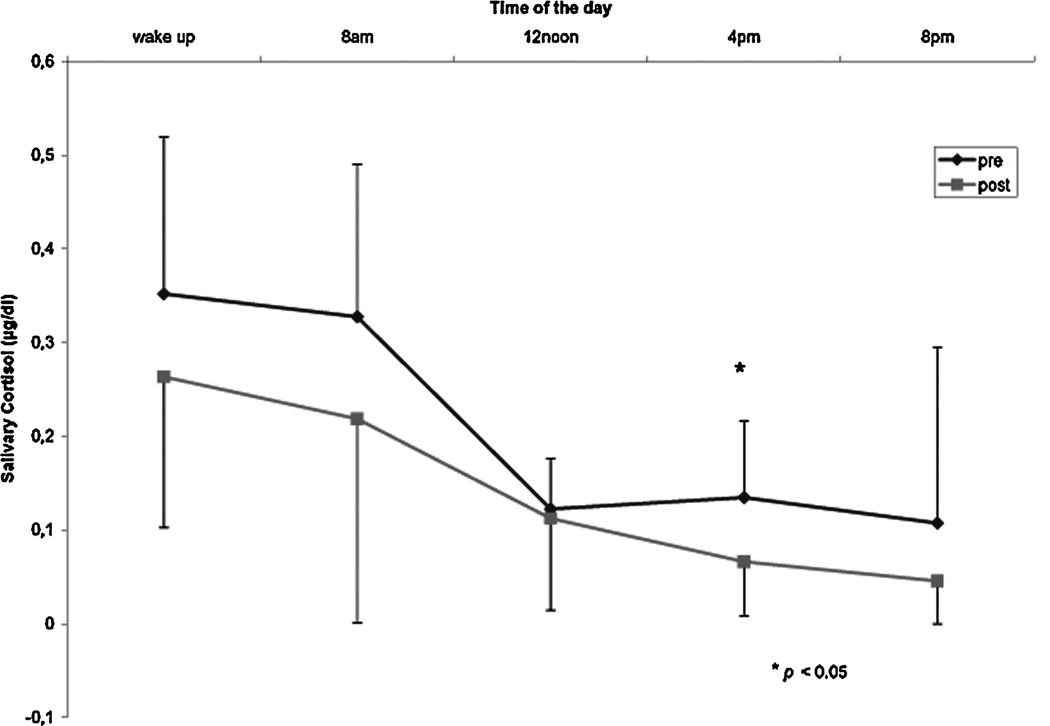
After treatment the mean baseline cortisol concentration in 24-h urine was reduced by 11%, the cortisol concentration changed from 2.88 (SD = 0.85) to 2.35 (SD = 1.26) µg/dL (NS) (Fig. 1). This reduction correlated significantly with the reduction in CAPS score (P < 0.01). Treatment also resulted in a decrease of salivary cortisol levels on all time points on the diurnal curve. Mean decrease on the five time points after treatment was 38%. Repeated measurements analysis showed a significant effect for treatment (F = 8,6; df = 9, P = 0.009). Regression analysis showed that the 4 pm time point best predicted the cortisol reduction in diurnal curve (t = −2.6; P = 0.02) (Table 1, Fig. 2).
FIGURE 1.
Twenty-four-hour cortisol in urine before and after treatment.
TABLE 1.
Twenty-four hour urinary cortisol and diurnal cortisol before and after long-treatment with paroxetine in PTSD
| Pretreatment | Posttreatment | ||||
|---|---|---|---|---|---|
| avg (µg/dl) | stdev | avg (µg/dl) | stdev | p | |
| 24 h urinary cortisol | 2.880 | 0.850 | 2.350 | 1.260 | ns |
| Diurnal cortisol | |||||
| wake up | 0.388 | 0.139 | 0.259 | 0.172 | ns |
| 8 am | 0.357 | 0.147 | 0.205 | 0.228 | ns |
| 12 pm | 0.124 | 0.057 | 0.109 | 0.103 | ns |
| 4 pm | 0.150 | 0.088 | 0.053 | 0.024 | 0.007 |
| 8 pm | 0.108 | 0.198 | 0.042 | 0.042 | ns |
FIGURE 2.
Diurnal curve for salivary cortisol before and after treatment.
Stressful Challenge Effects: Distress, Heart Rate, Blood Pressure, and Cortisol
Both cognitive challenges (pre- and posttreatment) were experienced as stressful. This was assessed by subjective units of distress (SUDS), ranging from 0 (not at all) to 100 (maximally stressed). In the pretreatment phase, the baseline SUDS increased from a mean of 21.5 (SD = 20.2) at −60 min and 13.5 (SD=16.1) at −5 min to 32.8 (SD=27.9) at 20 min after start of the challenge. Posttreatment the SUDS increased from 16.8 (SD = 15.9) at −60 min and 13.0 (SD = 16.1) at −5 min to 30.1 (SD = 28.8) at 20 min after start of the challenge. The individual difference between −60 and −5, and between −5 and 20 was significant in the pretreatment challenge (P < 0.05). In the posttreatment challenge only the −5- and 20-min comparison was significant (P < 0.05) (Fig. 3).
FIGURE 3.
Subjective distress before and after cognitive stress challenge, before and after treatment.
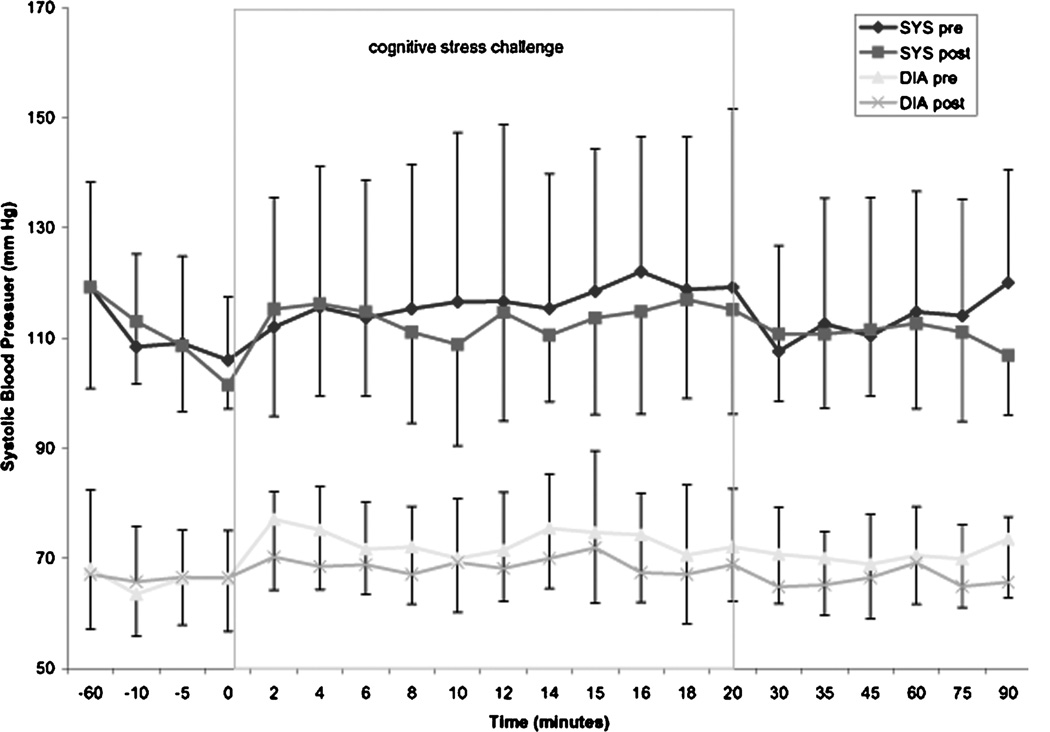
The pretreatment cognitive challenge resulted in a significant increase in heart rate when min “0” was compared with min 2, 6, 8, 10, 12, 14, 15, 16,18, and 20 (P < 0.05, Fig. 4). This was not observed in the posttreatment challenge. There was no significant effect of treatment on heart rate (F =0.85; P < 0.36). There was also no significant time effect on systolic blood pressure for either pre- or posttreatment challenge (Fig. 5).
FIGURE 4.
Heart rate during cognitive stress challenge before and after treatment.
FIGURE 5.
Blood pressure during cognitive stress challenge before and after treatment.
For diastolic blood pressure there was a positive time effect (F = 3.191; df = 20; P < 0.001). Time by treatment phase was not significant for either systolic or diastolic blood pressure, and there was no between-group effect for both measurements. There was no significant increase in systolic blood pressure after the start of the challenge in both treatment phases. Pretreatment cognitive challenge resulted in a significant increase in diastolic blood pressure when min “0” min was compared with 2, 4, 6, 10, and 14 min (P < 0.05) and 2, 4, 6, 8, 14, and 16 min for posttreatment diastolic blood pressure (Fig. 5).
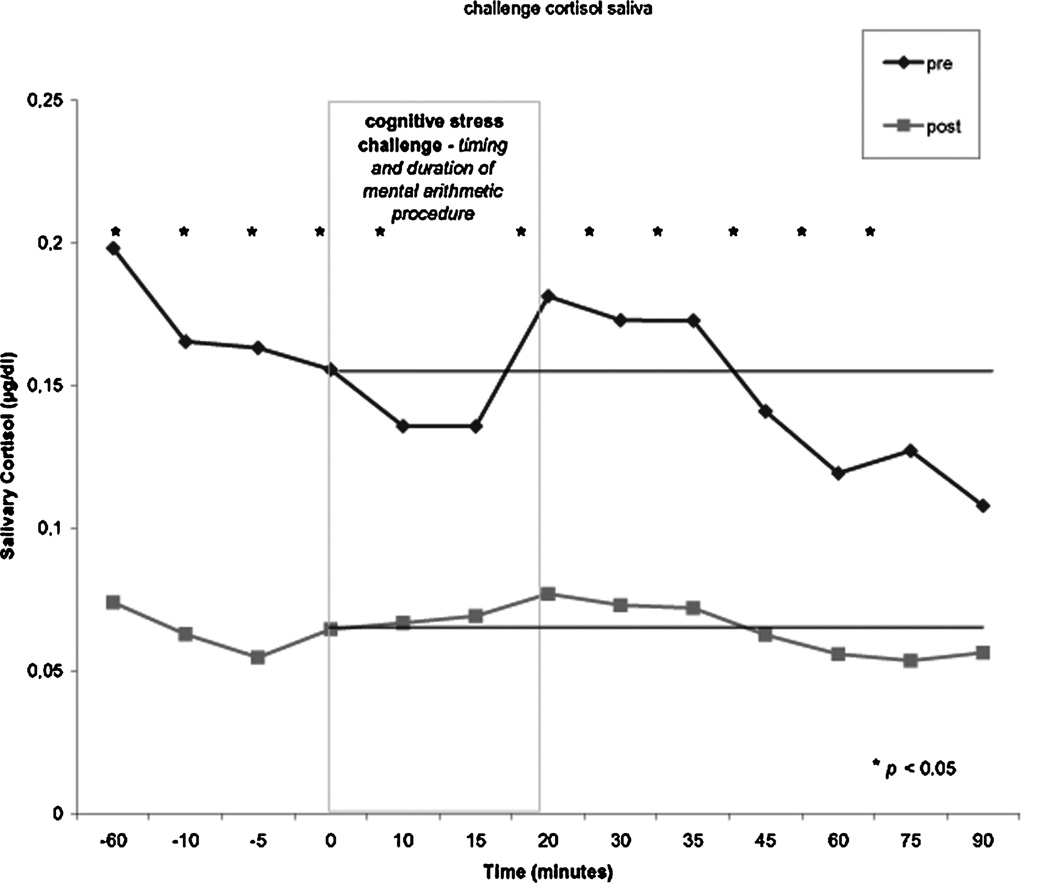
The pretreatment phase showed an increase of cortisol just after the start of the procedure. When the stress of mental arithmetic started, cortisol typically increased after 15 min, and peaked from 20 to 35 min after start of the challenge. Cortisol remained higher than base line after completion of the mental arithmetic. In the posttreatment phase, the cortisol elevation was almost completely absent. The peak concentration of cortisol occurred at the same time points as pretreatment, but at a significantly lower concentration. Repeated measures analysis showed a significant effect for treatment phase (F = 52.7; P = 0.007). Mean cortisol reduction across the challenge was 60%. At all time points except 15 and 90 min (respectively, P = 0.6 and P = 0.7) the difference between the pre- and posttreatment cortisol levels was significant (see Fig. 6). Cortisol dropped significantly from −60 to 15 min in the pretreatment (t = 2.45; df = 10; P = 0.034). When mean cortisol value at three time points T0, T10, and T15 was compared with mean of three subsequent time points T20, T30, and T35, there was a 5.4% differential increase when these time points were compared with the same ones in the posttreatment phase. There was no statistically significant difference between these time points in both the pre- and the posttreatment phase. When both salivary cortisol curves were normalized at the 0-min value, the calculated AUC for the first 60 min after the start of the challenge (0–60 min) showed a 26.5% relative decrease in stress-induced cortisol response between the pre- and posttreatment assessment (Fig. 6).
FIGURE 6.
Salivary cortisol during cognitive stress challenge before and after treatment.
DISCUSSION
In this study, successful clinical treatment with paroxetine in individual patients with PTSD led to a reduction (trend) in 24-h urinary and diurnal salivary cortisol, and a significantly lower cortisol response to cognitive stress challenge. The neuroendocrine changes were positively correlated with improvements in PTSD symptoms as measured with the CAPS. Diurnal salivary and 24-h urinary cortisol results in this study are especially interesting since several other studies have reported decreased baseline cortisol in PTSD when compared with control groups without PTSD. 4,48 As reported in the introduction, two studies have recently reported increased cortisol response to cognitive stress challenge22 and to traumatic reminders in abuse-related PTSD.37 These studies demonstrate intact stress-induced cortisol reactivity in the presence of lower baseline cortisol among individuals with PTSD. Our data show that in the presence of a subjectively similar stressful situation, successfully treated PTSD patients demonstrate a significant reduction of cortisol as expressed by the AUC. We also looked at the relative increase from T20, typically the moment cortisol starts to increase after prolonged stress, we found a 5.4% larger relative increase in cortisol posttreatment compared to the pretreatment. This not only suggests an intact HPA axis, but also a system in which the adrenals are producing a lower absolute amount of cortisol in response to the stressful mental arithmetic, after treatment with paxil compared to before treatment (i.e., a 26% decrease in the AUC from T0 to T60 after treatment). After treatment we also found lower total cortisol concentration in 24-h urine and diurnal cortisol, however, these findings were not significant. This may be partly on account of small sample size, since effect size analyses for these differences revealed that values (24-h cortisol: d = 0.66) might be significant with greater sample size.
Several interrelated mechanisms may have contributed to these findings. The role of serotonin in the regulation of the HPA axis has long been recognized.49,50 PTSD has been associated with alterations in platelet serotonin receptor binding.51–62 Serotonin has been shown to enhance glucocorticoid signaling,63 and increase the number and functional capacity of corticoid receptors in the hippocampus,64,65 which may influence glucocorticoid responsiveness.66 Recently, increased glucocorticoid sensitivity was found to be associated with hypocortisolism, reflected by the absence of cortisol response after awakening and lower daytime cortisol levels in Bosnian war refugees with PTSD.9 Low levels of cortisol in PTSD can be considered to disinhibit mediators that normally are downregulated by glucocorticoids. This is consistent with higher number of glucocorticoid receptor (GR) on peripheral lymphocytes in PTSD.48,67 Different study designs assessed lymphocyte GR function and number as a model for central GR functioning. In vitro measurements of lymphocyte sensitivity to dexamethasone showed enhanced GR sensitivity.9,67
In an earlier study, Rinne et al. reported a reduction in the hyperresponsiveness of the HPA axis after treatment with fluvoxamine in women with BPD, with the greatest changes seen in women with BPD and early childhood abuse.42 In another study using a metachlorophenylpiperazine (mCPP) challenge to probe serotonergic function in this research, Rinne and colleagues also found the greatest behavioral responses in women with BPD and early abuse.68 These studies suggest that it is not the neuropsychiatric disorder per se, but rather a history of traumatic childhood in combination with psychopathology that is an important determinant of this biological response pattern.69 One explanation may be that early-life events compromise glucocorticoid signaling, and contribute to elevated sympathetic tone, CRH hypersecretion, and changes in immune system function.66 These notions are consistent with the hypothesis that trauma spectrum disorders, including PTSD, depression, and BPD, all share a common biological basis that is linked to traumatic stress exposure.
The present study is the first to assess s long-term treatment effects on stress reactivity in PTSD. An earlier paper described positive effects of long-term paroxetine treatment on memory and hippocampal volume in patients with PTSD.70 The long-term treatment design used in this study provided an opportunity to measure effects of the medication on neurobiology that could be missed in studies of a shorter duration.
There are several limitations to the current study that warrant cautious interpretation. First, all patients were on paroxetine during the time of second cognitive challenge. The alterations in stress responsivity could therefore be a direct effect of the medication. A better design would involve tapering and stopping of medication before the second stressful challenge. Second, we do not know whether baseline cortisol was decreased or increased compared to that in a healthy control group, which makes it more difficult to interpret the cortisol change. Further, we did not include a placebo control group in this study for three reasons. Firstly, it is not known if relatively short-term treatment (e.g., 12 weeks) would result in alterations in stress-regulating systems; and second, it was felt that it would be unethical to provide long-term (12 months) placebo treatment to subjects with PTSD. Of note, after having experienced the first challenge, the second challenge could have been anticipated in a different way. Including a healthy control group in this study design, where subjects were challenged at the same time points as the PTSD patients, could have provided more information on the learning effect. It is possible that the lower cortisol effect would be explained by prior experience of the procedure rather than an effect of paroxetine. Our data on the SUDS, however, suggest that after treatment, the subjects perceived the procedure as equally distressing compared to the pretreatment challenge. It can be argued that subjects who had experienced the first challenge as unpleasant may have anticipated that the second challenge would be even more unpleasant. A recent study looking at habituation of cortisol responses to repeated psychosocial stress demonstrated that the general decline in cortisol response was only modest.71 In addition, to reduce habituation, and stimulate novelty, we chose to introduce another physician who performed the second challenge (posttreatment). A comparison of percentage increase in T0–T15 with T20–T35 showed an even larger cortisol increase in the posttreatment phase, however, this did not show in the AUC values that represent the effect of the total challenge procedure. As a final limitation cortisol was the only biological parameter assessed in this study. To study HPA axis regulation in more detail, the separate components of the stress axis should be measured, including corticotrophin-releasing factor (CRF), vasopressin, and ACTH. Moreover, the HPA axis and the locus coeruleus/noradrenergic system have mutual interconnections. PTSD may also be associated with dysfunctional connectivity between these systems. It would have been informative to assess these different stress systems within one study. Additionally, we did not collect a second control diurnal profile, to rule out day-to-day intraindividual variations.72 Finally, the sample size is small and therefore this study should be replicated with a larger number of patients. The age of onset of the traumatic experience in our sample varied, as did the trauma type; in nine patients trauma occurred before 18 years of age, in four the onset was in adult life. Our sample size was too small to calculate the magnitude of the effect on stress challenge in these two subgroups. It should also be mentioned that this was a completer analysis and all the males were left out of the analysis. Even though most women were postmenopausal, the data warrant caution in the absence of measurement of the phase of the menstrual cycle in the participating premenopausal women in the pre- and posttreatment time period. Gonadal steroids are known to exert influence on the reactivity of the HPA axis in humans.73,74
Finally, we would like to stress that the design of this study has important strengths that should be further explored in future studies. Future studies identifying biological alterations in relation to stress reactivity and HPA axis function should help to develop a better understanding of the direction and magnitude of neurohormonal changes and their relation to the pharmacological treatment of PTSD.
ACKNOWLEDGMENTS
We thank Sajid Siddiq, MD, Jahangir Adil, MD, Sarfraz Kahn, MD, Heather Douglas Palumberi, MA, Tammy Rowe-Padhy, BA, and Jacque Piscatelli, RN for help in data collection, and Kathy West for help in saliva processing. This study was supported by NIMH 1R01MH56120-01A1, a grant from Glaxo Smith Kline, and a Veterans Administration Career Development Award to Dr. Bremner.
REFERENCES
- 1.Bremner JD, Licinio J, Darnell A, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am. J. Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker DG, West SA, Nicholson WE, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am. J. Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 3.Yehuda R, Teicher MH, Trestman RL, et al. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol. Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- 4.Bremner JD, Vythilingam M, Anderson G, et al. Assessment of the hypothalamic-pituitary-adrenal axis over a 24-hour diurnal period and in response to neuroendocrine challenges in women with and without childhood sexual abuse and posttraumatic stress disorder. Biol. Psychiatry. 2003;54:710–718. doi: 10.1016/s0006-3223(02)01912-1. [DOI] [PubMed] [Google Scholar]
- 5.Mason JW, Giller EL, Kosten TR, et al. Urinary free-cortisol levels in posttraumatic stress disorder patients. J. Nerv. Ment. Dis. 1986;174:145–149. doi: 10.1097/00005053-198603000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Yehuda R, Southwick SM, Nussbaum G, et al. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J. Nerv. Ment. Dis. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Yehuda R, Kahana B, Binder-Brynes K, et al. Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. Am. J. Psychiatry. 1995;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- 8.Neylan TC, Schuff N, Lenoci M, et al. Cortisol levels are positively correlated with hippocampal N-acetylaspartate. Biol. Psychiatry. 2003;54:1118–1121. doi: 10.1016/S0006-3223(03)01974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol. Psychiatry. 2004;55:745–751. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:209–215. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Mason JW, Giller EL, Kosten TR, Harkness L. Elevation of urinary norepinephrine/cortisol ratio in posttraumatic stress disorder. J. Nerv. Ment. Dis. 1988;176:498–502. doi: 10.1097/00005053-198808000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Pitman RK, Orr SP. Twenty-four hour urinary cortisol and catecholamine excretion in combat-related posttraumatic stress disorder. Biol. Psychiatry. 1990;27:245–247. doi: 10.1016/0006-3223(90)90654-k. [DOI] [PubMed] [Google Scholar]
- 13.Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: evidence for chronic neuroendocrine activation in women. Psychosom. Med. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Maes M, Lin A, Bonaccorso S, et al. Increased 24-hour urinary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta Psychiatr. Scand. 1998;98:328–335. doi: 10.1111/j.1600-0447.1998.tb10092.x. [DOI] [PubMed] [Google Scholar]
- 15.Thaller V, Vrkljan M, Hotujac L, Thakore J. The potential role of hypocortisolism in the pathophysiology of PTSD and psoriasis. Coll. Antropol. 1999;23:611–619. [PubMed] [Google Scholar]
- 16.Young EA, Breslau N. Cortisol and catecholamines in posttraumatic stress disorder: an epidemiologic community study. Arch. Gen. Psychiatry. 2004;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- 17.Lundin T. Naval rescue workers and post-traumatic stress syndrome. Relieving conversation made coping easier. Lakartidningen. 1997;94:1204–1206. [PubMed] [Google Scholar]
- 18.Siegel AJ, Chu JA. Factitious anemia due to self-administered blood-letting in psychiatric patients. J. Nerv. Ment. Dis. 2002;190:788–789. doi: 10.1097/00005053-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Mayou RA, Ehlers A, Hobbs M. Psychological debriefing for road traffic accident victims. Three-year follow-up of a randomised controlled trial. Br. J. Psychiatry. 2000;176:589–593. doi: 10.1192/bjp.176.6.589. [DOI] [PubMed] [Google Scholar]
- 20.Baker DG, Ekhator NN, Kasckow JW, et al. Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. Am. J. Psychiatry. 2005;162:992–994. doi: 10.1176/appi.ajp.162.5.992. [DOI] [PubMed] [Google Scholar]
- 21.De Kloet CS, Vermetten E, Geuze E, et al. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J. Psychiatr. Res. 2005 Oct 6; doi: 10.1016/j.jpsychires.2005.08.002. 2005 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Bremner JD, Vythilingam M, Vermetten E, et al. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28:733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- 23.Smith MA, Davidson J, Ritchie JC, et al. The corticotropin-releasing hormone test in patients with posttraumatic stress disorder. Biol. Psychiatry. 1989;26:349–355. doi: 10.1016/0006-3223(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 24.Rasmusson AM, Lipschitz DS, Wang S, et al. Increased pituitary and adrenal reactivity in premenopausal women with posttraumatic stress disorder. Biol. Psychiatry. 2001;50:965–977. doi: 10.1016/s0006-3223(01)01264-1. [DOI] [PubMed] [Google Scholar]
- 25.Kellner M, Yassouridis A, Hubner R, et al. Endocrine and cardiovascular responses to corticotropin-releasing hormone in patients with posttraumatic stress disorder: a role for atrial natriuretic peptide? Neuropsychobiology. 2003;47:102–108. doi: 10.1159/000070018. [DOI] [PubMed] [Google Scholar]
- 26.Kanter ED, Wilkinson CW, Radant AD, et al. Glucocorticoid feedback sensitivity and adrenocortical responsiveness in posttraumatic stress disorder. Biol. Psychiatry. 2001;50:238–245. doi: 10.1016/s0006-3223(01)01158-1. [DOI] [PubMed] [Google Scholar]
- 27.Yehuda R, Levengood RA, Schmeidler J, et al. Increased pituitary activation following metyrapone administration in post-traumatic stress disorder. Psychoneuroendocrinology. 1996;21:1–16. doi: 10.1016/0306-4530(95)00055-0. [DOI] [PubMed] [Google Scholar]
- 28.Kellner M, Otte C, Yassouridis A, et al. Overnight metyrapone and combined dexamethasone/metyrapone tests in post-traumatic stress disorder: preliminary findings. Eur. Neuropsychopharmacol. 2004;14:337–339. doi: 10.1016/j.euroneuro.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Yehuda R, Southwick SM, Krystal JH, et al. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am. J. Psychiatry. 1993;150:83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- 30.Yehuda R, Boisoneau D, Lowy MT, Giller EL., Jr Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch. Gen. Psychiatry. 1995;52:583–593. doi: 10.1001/archpsyc.1995.03950190065010. [DOI] [PubMed] [Google Scholar]
- 31.Stein MB, Yehuda R, Koverola C, Hanna C. Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol. Psychiatry. 1997;42:680–686. doi: 10.1016/s0006-3223(96)00489-1. [DOI] [PubMed] [Google Scholar]
- 32.Yehuda R, Halligan SL, Grossman R, et al. The cortisol and gluco-corticoid receptor response to low dose dexamethasone administration in aging combat veterans and holocaust survivors with and without posttraumatic stress disorder. Biol. Psychiatry. 2002;52:393–403. doi: 10.1016/s0006-3223(02)01357-4. [DOI] [PubMed] [Google Scholar]
- 33.Yehuda R, Halligan SL, Golier JA, et al. Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology. 2004;29:389–404. doi: 10.1016/s0306-4530(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 34.Newport DJ, Heim C, Bonsall R, et al. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biol. Psychiatry. 2004;55:10–20. doi: 10.1016/s0006-3223(03)00692-9. [DOI] [PubMed] [Google Scholar]
- 35.Yehuda R, Golier JA, Halligan SL, et al. The ACTH response to dexamethasone in PTSD. Am. J. Psychiatry. 2004;161:1397–1403. doi: 10.1176/appi.ajp.161.8.1397. [DOI] [PubMed] [Google Scholar]
- 36.Lindley SE, Carlson EB, Benoit M. Basal and dexamethasone suppressed salivary cortisol concentrations in a community sample of patients with posttraumatic stress disorder. Biol. Psychiatry. 2004;55:940–945. doi: 10.1016/j.biopsych.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Elzinga BM, Schmahl CG, Vermetten E, et al. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- 38.Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am. J. Psychiatry. 2001;158:1982–1988. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- 39.Tucker P, Zaninelli R, Yehuda R, et al. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J. Clin. Psychiatry. 2001;62:860–868. doi: 10.4088/jcp.v62n1105. [DOI] [PubMed] [Google Scholar]
- 40.Tucker P, Smith KL, Marx B, et al. Fluvoxamine reduces physiologic reactivity to trauma scripts in posttraumatic stress disorder. J. Clin. Psychopharmacol. 2000;20:367–372. doi: 10.1097/00004714-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Nikisch G, Mathe AA, Czernik A, et al. Long-term citalopram administration reduces responsiveness of HPA axis in patients with major depression: relationship with s-citalopram concentrations in plasma and cerebrospinal fluid (CSF) and clinical response. Psychopharmacology (Berl.) 2005;181:751–760. doi: 10.1007/s00213-005-0034-3. [DOI] [PubMed] [Google Scholar]
- 42.Rinne T, De Kloet ER, Wouters L, et al. Fluvoxamine reduces responsiveness of HPA axis in adult female BPD patients with a history of sustained childhood abuse. Neuropsychopharmacology. 2003;28:126–132. doi: 10.1038/sj.npp.1300003. [DOI] [PubMed] [Google Scholar]
- 43.Spitzer R. Structural Clinical Interview for DSMIV. New York: New York State Psychiatric Institute; 1994. [Google Scholar]
- 44.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J. Trauma. Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 45.Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress. Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 46.Seeman TE, Singer B, Charpentier P. Gender differences in patterns of HPA axis response to challenge: Macarthur studies of successful aging. Psychoneuroendocrinology. 1995;20:711–725. doi: 10.1016/0306-4530(95)00024-0. [DOI] [PubMed] [Google Scholar]
- 47.Seeman TE, Berkman LF, Charpentier PA, et al. Behavioral and psychosocial predictors of physical performance: Macarthur studies of successful aging. J. Gerontol. A Biol. Sci. Med. Sci. 1995;50:M177–M183. doi: 10.1093/gerona/50a.4.m177. [DOI] [PubMed] [Google Scholar]
- 48.Yehuda R, Lowy MT, Southwick SM, et al. Lymphocyte glucocorticoid receptor number in posttraumatic stress disorder. Am. J. Psychiatry. 1991;148:499–504. doi: 10.1176/ajp.148.4.499. [DOI] [PubMed] [Google Scholar]
- 49.Lopez JF, Vazquez DM, Chalmers DT, Watson SJ. Regulation of 5-HT receptors and the hypothalamic-pituitary-adrenal axis. Implications for the neurobiology of suicide. Ann. N. Y. Acad. Sci. 1997;836:106–134. doi: 10.1111/j.1749-6632.1997.tb52357.x. [DOI] [PubMed] [Google Scholar]
- 50.Veenema AH, Koolhaas JM, De Kloet ER. Basal and stress-induced differences in HPA axis, 5-HT responsiveness, and hippocampal cell proliferation in two mouse lines. Ann. N. Y. Acad. Sci. 2004;1018:255–265. doi: 10.1196/annals.1296.030. [DOI] [PubMed] [Google Scholar]
- 51.Troiani TA, Boland RT. Critical incident stress debriefing: keeping your flight crew healthy. J. Air Med.. Transp. 1992;11:21–24. doi: 10.1016/s1046-9095(05)80202-8. [DOI] [PubMed] [Google Scholar]
- 52.Arora RC, Fichtner CG, O’connor F, Crayton JW. Paroxetine binding in the blood platelets of post-traumatic stress disorder patients. Life Sci. 1993;53:919–928. doi: 10.1016/0024-3205(93)90444-8. [DOI] [PubMed] [Google Scholar]
- 53.Fichtner CG, Arora RC, O’connor FL, Crayton JW. Platelet paroxetine binding and fluoxetine pharmacotherapy in posttraumatic stress disorder: preliminary observations on a possible predictor of clinical treatment response. Life Sci. 1994;54:PL39–PL44. doi: 10.1016/0024-3205(94)00592-3. [DOI] [PubMed] [Google Scholar]
- 54.Weizman R, Laor N, Schujovitsky A, et al. Platelet imipramine binding in patients with posttraumatic stress disorder before and after phenelzine treatment. Psychiatry Res. 1996;63:143–150. doi: 10.1016/0165-1781(96)02760-6. [DOI] [PubMed] [Google Scholar]
- 55.Maguire K, Norman T, Burrows G, et al. Platelet paroxetine binding in post-traumatic stress disorder. Psychiatry Res. 1998;77:1–7. doi: 10.1016/s0165-1781(97)00133-9. [DOI] [PubMed] [Google Scholar]
- 56.Spivak B, Vered Y, Graff E, et al. Low platelet-poor plasma concentrations of serotonin in patients with combat-related posttraumatic stress disorder. Biol. Psychiatry. 1999;45:840–845. doi: 10.1016/s0006-3223(98)00231-5. [DOI] [PubMed] [Google Scholar]
- 57.Cicin-Sain L, Mimica N, Hranilovic D, et al. Posttraumatic stress disorder and platelet serotonin measures. J. Psychiatr. Res. 2000;34:155–161. doi: 10.1016/s0022-3956(99)00049-7. [DOI] [PubMed] [Google Scholar]
- 58.Hondius AJ, Van Willigen LH, Kleijn WC, Van Der Ploeg HM. Health problems among Latin-American and middle-eastern refugees in the Netherlands: relations with violence exposure and ongoing sociopsychological strain. J. Trauma. Stress. 2000;13:619–634. doi: 10.1023/A:1007858116390. [DOI] [PubMed] [Google Scholar]
- 59.Holbrook TL, Hoyt DB, Stein MB, Sieber WJ. Perceived threat to life predicts posttraumatic stress disorder after major trauma: risk factors and functional outcome. J. Trauma. 2001;51:287–292. doi: 10.1097/00005373-200108000-00010. Discussion 292–293. [DOI] [PubMed] [Google Scholar]
- 60.Pivac N, Muck-Seler D, Sagud M, Jakovljevic M. Platelet serotonergic markers in posttraumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:1193–1198. doi: 10.1016/s0278-5846(02)00261-0. [DOI] [PubMed] [Google Scholar]
- 61.Cohen E, Dekel R, Solomon Z, Lavie T. Posttraumatic stress symptoms and fear of intimacy among treated and non-treated survivors who were children during the Holocaust Soc. Psychiatry Psychiatr. Epidemiol. 2003;38:611–617. doi: 10.1007/s00127-003-0681-9. [DOI] [PubMed] [Google Scholar]
- 62.Roy-Byrne P, Berliner L, Russo J, et al. Treatment preferences and determinants in victims of sexual and physical assault. J. Nerv. Ment. Dis. 2003;191:161–165. doi: 10.1097/01.NMD.0000055343.62310.73. [DOI] [PubMed] [Google Scholar]
- 63.Herr AS, Tsolakidou AF, Yassouridis A, et al. Antidepressants differentially influence the transcriptional activity of the glucocorticoid receptor in vitro. Neuroendocrinology. 2003;78:12–22. doi: 10.1159/000071701. [DOI] [PubMed] [Google Scholar]
- 64.Vedder H, Weiss I, Holsboer F, Reul JM. Glucocorticoid and mineralocorticoid receptors in rat neocortical and hippocampal brain cells in culture: characterization and regulatory studies. Brain Res. 1993;605:18–24. doi: 10.1016/0006-8993(93)91351-r. [DOI] [PubMed] [Google Scholar]
- 65.Przegalinski E, Budziszewska B. The effect of long-term treatment with antidepressant drugs on the hippocampal mineralocorticoid and glucocorticoid receptors in rats. Neurosci. Lett. 1993;161:215–218. doi: 10.1016/0304-3940(93)90297-x. [DOI] [PubMed] [Google Scholar]
- 66.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am. J. Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 67.Yehuda R, Golier JA, Yang RK, Tischler L. Enhanced sensitivity to glucocorticoids in peripheral mononuclear leukocytes in posttraumatic stress disorder. Biol. Psychiatry. 2004;55:1110–1116. doi: 10.1016/j.biopsych.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 68.Rinne T, Westenberg HG, Den Boer JA, Van Den Brink W. Serotonergic blunting to meta-chlorophenylpiperazine (m-CPP) highly correlates with sustained childhood abuse in impulsive and autoaggressive female borderline patients. Biol. Psychiatry. 2000;47:548–555. doi: 10.1016/s0006-3223(99)00181-x. [DOI] [PubMed] [Google Scholar]
- 69.Heim C, Newport DJ, Wagner D, et al. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress. Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- 70.Vermetten E, Vythilingam M, Southwick SM, et al. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wust S, Federenko IS, Van Rossum EF, et al. Habituation of cortisol responses to repeated psychosocial stress-further characterization and impact of genetic factors. Psychoneuroendocrinology. 2005;30:199–211. doi: 10.1016/j.psyneuen.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 72.Kirschbaum C, Hellhammer D. Response variability of salivary cortisol under psychological stimulation. J. Clin. Chem. Clin. Biochem. 1989;27:237. [PubMed] [Google Scholar]
- 73.Kirschbaum C, Kudielka BM, Gaab J, et al. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 74.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]