Abstract
Acquiring resistance against transforming growth factor β (TGFβ)-induced growth inhibition at early stages of carcinogenesis and shifting to TGFβ's tumour-promoting functions at later stages is a pre-requisite for malignant tumour progression and metastasis. We have identified the transcription factor distal-less homeobox 2 (Dlx2) to exert critical functions during this switch. Dlx2 counteracts TGFβ-induced cell-cycle arrest and apoptosis in mammary epithelial cells by at least two molecular mechanisms: Dlx2 acts as a direct transcriptional repressor of TGFβ receptor II (TGFβRII) gene expression and reduces canonical, Smad-dependent TGFβ signalling and expression of the cell-cycle inhibitor p21CIP1 and increases expression of the mitogenic transcription factor c-Myc. On the other hand, Dlx2 directly induces the expression of the epidermal growth factor (EGF) family member betacellulin, which promotes cell survival by stimulating EGF receptor signalling. Finally, Dlx2 expression supports experimental tumour growth and metastasis of B16 melanoma cells and correlates with tumour malignancy in a variety of human cancer types. These results establish Dlx2 as one critical player in shifting TGFβ from its tumour suppressive to its tumour-promoting functions.
Keywords: betacellulin, Dlx2, EGFR, metastasis, TGFβ
Introduction
Transforming growth factor β (TGFβ) plays a central role in various biological processes such as development, tissue homeostasis, fibrosis, and cancer. During gastrulation and neural crest cell migration, TGFβ induces cell motility and invasiveness, thus enabling cells to migrate to distant sites within the developing embryo. In contrast, in differentiated epithelial tissue, TGFβ primarily maintains tissue homeostasis by promoting growth arrest and apoptosis, thus exerting tumour suppressor function (Massague, 2008).
This ambivalent nature of TGFβ signalling also plays a critical role in cancer initiation and progression. At early stages of tumourigenesis, TGFβ functions as a tumour suppressor by promoting cell-cycle arrest and apoptosis. In contrast, during late stage tumourigenesis, TGFβ exerts malignant activities, such as inducing an epithelial–mesenchymal transition (EMT), supporting tumour angiogenesis, and suppressing anti-tumuoral immune responses (Wakefield and Roberts, 2002; Siegel and Massagué, 2003; Pardali and Moustakas, 2007; Massague, 2008). The switch of TGFβ signalling from its tumour suppressor activity to a tumour-promoting factor is achieved by at least two major modifications: the attenuation of pro-apoptotic TGFβ signalling and the activation of phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) signalling pathways (Huber et al, 2005). Tumour-suppressive TGFβ signalling is mediated by canonical, Smad-dependent TGFβ signalling. Upon ligand binding to TGFβ receptors I and II, the receptor-associated Smad proteins (Smad2/3) are phosphorylated, dissociate from the receptor complex, and translocate to the nucleus in association with Smad4, where they modulate the expression of specific target genes. Expression of genes encoding anti-proliferative and pro-apoptotic factors is induced, such as the cell-cycle inhibitors p15INK4B and p21CIP1, while the expression of mitogenic factors like c-Myc is repressed (Massague, 2004). In tumours, canonical TGFβ signalling is often suppressed, and cell-cycle arrest and apoptosis are bypassed by reduced TGFβ receptor II (TGFβRII) expression or by mutational inactivation of Smad proteins (Massague, 2008). Yet, cancer cells utilize TGFβ to promote tumour progression and survival by non-canonical TGFβ signalling, which mainly results in the activation of the MAPK and the PI3K pathways (Gotzmann et al, 2002; Lee et al, 2007b). A total loss of TGFβ signalling impairs late stage tumour progression and metastasis formation, demonstrating a critical role of TGFβ signalling for cancer malignancy (Cui et al, 1996; Oft et al, 1998; Moustakas and Heldin, 2005). However, the molecular mechanisms underlying the switch from TGFβ's growth inhibitory functions to its tumour-suppressive activities are only poorly understood.
Here, we report that the transcription factor distal-less homeobox 2 (Dlx2) is upregulated upon TGFβ treatment and attenuates growth-suppressive TGFβ signalling in a negative feedback loop. Moreover, Dlx2 induces mitogenic epidermal growth factor receptor (EGFR) signalling by directly inducing the expression of the EGFR-ligand betacellulin. Together, these Dlx2 functions protect cells from TGFβ-induced cell-cycle arrest and apoptosis and supports primary tumour growth and metastasis of B16 melanoma cells. Finally, the clinical relevance of Dlx2 is underscored by the observation that its expression correlates with the malignant progression of various human cancer types.
Results
Dlx2 expression is induced by canonical TGFβ signalling
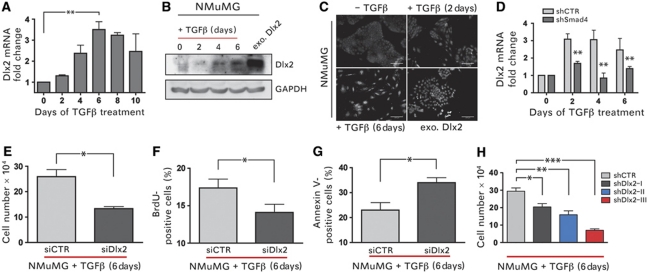
We have employed the normal murine mammary gland (NMuMG) cells as an experimental system to dissect the molecular mechanisms that enable epithelial cells to switch TGFβ signalling from proliferation suppressive to pro-survival functions. These non-transformed, epithelial cells respond with cell-cycle arrest and, partially, apoptosis during the early phases of TGFβ treatment. However, with ongoing TGFβ treatment, NMuMG cells overcome growth-suppressive TGFβ signalling and undergo EMT (Gal et al, 2008). To identify genes critically required to overcome TGFβ-mediated growth suppression, NMuMG cells were treated with TGFβ, and changes in gene expression during TGFβ treatment were determined. Among a large number of genes changing in their expression during TGFβ treatment, Dlx2 mRNA was found increasingly expressed after 2 days, with highest levels after 6 days (Figure 1A), a time period during which TGFβ-induced cell-cycle arrest and apoptosis was most prominent. Amplified Dlx2 mRNA levels were accompanied by increased levels of Dlx2 protein as determined by immunoblotting analysis (Figure 1B). Notably, increased levels of Dlx2 were predominantly localized to the nucleus in TGFβ-treated cells (Figure 1C). That Dlx2 is a general target of TGFβ signalling was further confirmed in a murine breast cancer cell line established from a tumour of a MMTV-Polyoma Middle T (MMTV-PyMT) transgenic mouse and in B16 melanoma cells (Supplementary Figure S1A). Dlx2 mRNA expression was not significantly induced upon TGFβ treatment in NMuMG cells harbouring a stable knockdown of Smad4 (shSmad4-NMuMG) (Figure 1D; Deckers et al, 2006), indicating that Dlx2 gene expression depended on canonical TGFβ signalling.
Figure 1.
Dlx2 is a target of canonical TGFβ signalling and is critical for survival during TGFβ treatment of NMuMG cells. (A) Dlx2 mRNA levels were determined by quantitative RT–PCR in NMuMG cells treated with TGFβ for the days indicated. (B) Immunoblotting analysis of Dlx2 protein levels in NMuMG cells treated with TGFβ for the days indicated and of cells stably expressing Dlx2 is shown. GAPDH was used as loading control. (C) Subcellular localization of Dlx2 in NMuMG cells treated with TGFβ or stably expressing Dlx2 (exo. Dlx2) was determined by fluorescence microscopy. Scale bar=100 μm. (D) Dlx2 mRNA levels were determined by quantitative RT–PCR in stable Smad4 knockdown (shSmad4) and control (shCTR) NMuMG cells treated with TGFβ for the days indicated. (E–G) Dlx2-depleted (siDlx2) and control (siCTR) NMuMG cells were treated with TGFβ (2 ng/ml) for 6 days. Viable cells were counted by trypan blue exclusion using a Neubauer cell counting chamber (E). Proliferation rates were determined by BrdU incorporation and flow cytometry (F). The rates of apoptosis were measured by Annexin V staining and flow cytometry (G). (H) NMuMG cells stably expressing three independent shRNAs against Dlx2 (shDlx2 I–III) or control shRNA (shCTR) were treated with TGFβ (1 ng/ml), and cell numbers were determined using a Neubauer counting chamber at day 6 of TGFβ treatment. Data are shown as mean±s.d. and are representative of three independent experiments. Statistical values are calculated by using an unpaired, two-tailed t-test. *P⩽0.05; **P⩽0.01; ***P⩽0.005.
Dlx2 promotes cell survival and proliferation during TGFβ treatment
We next investigated whether Dlx2 function was required for cell survival during TGFβ treatment. NMuMG cells were transfected with siRNA against Dlx2 (siDlx2) or with control siRNA (siCTR) (Supplementary Figure S1B), and changes in proliferation and apoptosis were determined. The potential protective function of Dlx2 was analysed at 6 days of TGFβ treatment, when cell death and detachment of cells from the cell culture plate were most prominent. Notably, siDlx2-NMuMG cells exhibited reduced cell numbers as compared with control siRNA-transfected cells (Figure 1E).
To assess whether loss of Dlx2 function affected proliferation and/or apoptosis during TGFβ treatment, we compared the levels of proliferation (BrdU incorporation) and of apoptosis (Annexin V staining) between siCTR- and siDlx2-treated NMuMG cells upon TGFβ treatment. Proliferation was significantly reduced and apoptosis was significantly increased in the absence of Dlx2 upon TGFβ treatment (Figure 1F and G), explaining the reduced cell number in TGFβ-treated siDlx2-NMuMG cells. These results were confirmed by the diminished growth rate of TGFβ-treated NMuMG cells in which Dlx2 expression was ablated by stable expression of shRNAs against Dlx2 (shDlx2-NMuMG) as compared with control shRNA-transfected cells (shCTR-NMuMG) (Figure 1H; Supplementary Figure S1C).
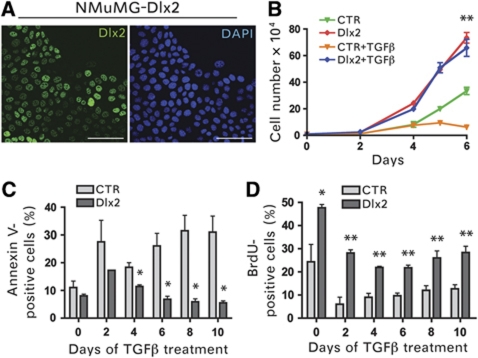
Next, we analysed whether the forced expression of Dlx2 affected proliferation and/or apoptosis of NMuMG cells in the presence or absence of TGFβ. We stably infected NMuMG cells with lentiviral vectors encoding HA-tagged, murine Dlx2 or firefly luciferase as control and used infected cell pools for further analysis. Dlx2 was exclusively expressed in the nucleus of stably infected NMuMG cells (Figure 2A). Dlx2-expressing NMuMG cells exhibited a significantly increased cell proliferation rate, as compared with control cells, and showed no sensitivity towards TGFβ-mediated growth inhibition (Figure 2B). Indeed, while control NMuMG cells ceased growing in the presence of TGFβ, Dlx2-expressing cells increased in numbers in the absence as well as in the presence of TGFβ. Annexin V staining and BrdU incorporation analysis revealed that, in comparison to control-transfected cells, Dlx2-expressing NMuMG cells exhibited decreased levels of apoptosis and proliferated at higher rates, respectively (Figure 2C and D).
Figure 2.
Dlx2 protects from TGFβ-induced cell-cycle arrest and apoptosis. (A) Confocal laser scanning microscopy of NMuMG cells stably expressing N-terminal HA-tagged Dlx2. Dlx2 was detected by anti-HA immunofluorescence staining (green). Blue DAPI staining visualizes nuclei. Scale bar=100 μm. (B) Dlx2-expressing (Dlx2) and control (CTR) NMuMG cells were treated with or without TGFβ for the days indicated and counted by trypan blue exclusion using a Neubauer cell counting chamber. The time point day 6 was used to determine statistical significance between Dlx2-expressing and control cells. (C) Dlx2-expressing and control NMuMG cells were treated with TGFβ for the days indicated. Apoptosis was measured by Annexin V staining and flow cytometry. (D) Dlx2-expressing and control NMuMG cells were treated with TGFβ for the days indicated, and proliferation rates were determined by BrdU incorporation and flow cytometry. Data are shown as mean values±s.d. and are representative of three independent experiments. Statistical values are calculated by using an unpaired, two-tailed t-test. *P⩽0.05; **P⩽0.01.
Together, these gain and loss-of-function experiments demonstrate that Dlx2 is critical for cell survival and proliferation during the growth-suppressive phase of TGFβ treatment.
Dlx2 inhibits canonical TGFβ signalling
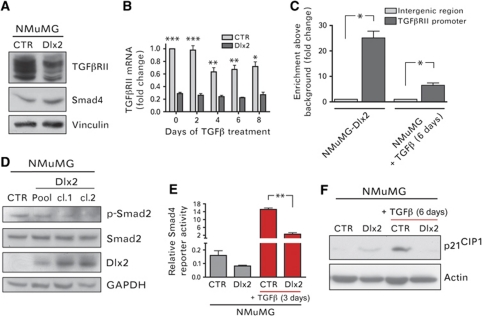
An attenuation of the canonical pro-apoptotic TGFβ signalling pathway is frequently found responsible for TGFβ-resistant growth (Massague, 2008). Hence, we determined the expression levels and activities of different molecules known to play critical roles in canonical TGFβ signalling. Notably, the protein levels of TGFβRII were found decreased in Dlx2-expressing NMuMG cells, whereas Smad4 protein levels were unchanged (Figure 3A). Reduced TGFβRII mRNA levels pointed to a Dlx2-mediated transcriptional repression of TGFβRII expression in NMuMG cells (Figure 3B). That Dlx2 is indeed a transcriptional repressor of the TGFβRII gene was further underlined by the observation that ablation of Dlx2 function counteracted the TGFβ-induced repression of the TGFβRII gene (Supplementary Figure S2A). Furthermore, forced expression of Dlx2 in HEK293 and NMuMG cells resulted in reduced TGFβRII promoter activity (Supplementary Figure S2B and C). Chromatin immunoprecipitation (ChIP) experiments with NMuMG cells either stably expressing HA-tagged Dlx2 or treated with TGFβ for 6 days demonstrated that Dlx2 directly bound to the TGFβRII gene promoter (Figure 3C). The specific binding of endogenous Dlx2 to the TGFβRII promoter was also observed in B16 melanoma cells (Supplementary Figure S2D) and in Py2T murine breast cancer cells (data not shown). Changes in the expression of inhibitory Smads, such as Smad7, or of the Smad-specific E3 ubiquitin protein ligase 1 (Smurf1) were not detected (data not shown), both of which have been shown to inhibit TGFβ receptor signalling (Di Guglielmo et al, 2003; Zhang et al, 2007).
Figure 3.
Dlx2 expression attenuates Smad-dependent, canonical TGFβ signalling. (A) Immunoblotting analysis of TGFβRII and Smad4 protein levels in Dlx2-expressing and control NMuMG cells is shown. Immunoblotting against vinculin was used as loading control. (B) TGFβRII mRNA levels in Dlx2-expressing and control NMuMG cells were determined by quantitative RT–PCR at different days of TGFβ treatment as indicated. Values were normalized to endogenous RPL19 levels. (C) Dlx2 binds directly the TGFβRII promoter. ChIP of Dlx2 was performed either on Dlx2-expressing NMuMG cells or on NMuMG cells treated for 6 days with TGFβ. Immunoprecipitated DNA fragments were quantified by quantitative PCR using primers covering basepairs −386 to −204 of the TGFβRII promoter region and primers covering an intergenic region as negative control. (D) Lysates of Dlx2-expressing cell pools or cell clones and control (CTR) NMuMG cells were analysed by immunoblotting analysis with antibodies against p-Smad2, total Smad2, HA to determine Dlx2 expression, and GAPDH as a loading control. (E) Dlx2-expressing and control NMuMG cells were transfected with a reporter plasmid where repetitive Smad4-binding motifs control the expression of firefly luciferase and then treated with or without TGFβ for 3 days. Luciferase activity values were normalized to co-transfected Renilla luciferase activities. (F) Immunoblotting analysis of Dlx2-expressing and control NMuMG cells in the absence or presence of TGFβ for 6 days with antibodies against p21CIP1 and against actin as loading control. Data are shown as mean±s.d. and are representative of three independent experiments. Statistical values are calculated by using an unpaired, two-tailed t-test. *P⩽0.05; **P⩽0.01; ***P⩽0.001.
As a consequence of the Dlx2-mediated decrease in TGFβRII protein levels, canonical TGFβ signal transduction was found attenuated. The levels of phosphorylated Smad2 were reduced in clones and pools of Dlx2-expressing NMuMG cells (Figure 3D). Concomitantly, the transcriptional activity of the common mediator Smad4 was diminished in Dlx2-expressing cells, as revealed by Smad4-specific luciferase-reporter (CAGA box reporter) analysis (Dennler et al, 1998; Figure 3E). This decrease in TGFβ signalling lead to changes in the expression of bona fide TGFβ target genes, exemplified by the reduced expression of p21CIP1 and the increased expression of c-Myc (Figures 3F and 4A).
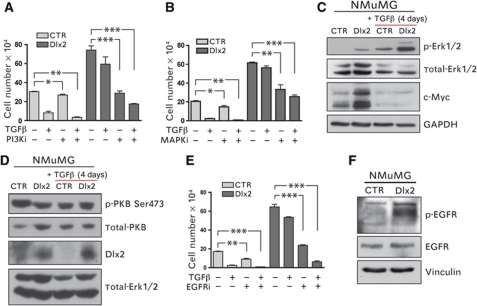
Figure 4.
Dlx2 promotes resistance against TGFβ-mediated growth inhibition via activation of EGFR signalling. (A) Dlx2-expressing and control NMuMG cells were treated with TGFβ for 4 days in combination with the PI3K inhibitor ZSTK474 (0.239 μM) or DMSO (solvent control) and counted using a Neubauer chamber. (B) Dlx2-expressing and control NMuMG cells were treated with TGFβ for 4 days in combination with the MEK1/2 inhibitor PD98059 (9.35 μM) or DMSO (solvent control), and cell numbers were determined using a Neubauer cell counting chamber. (C) Dlx2 expression increases phosphorylation of the MAPK Erk1/2 as well as c-Myc total protein levels. Immunoblotting analysis of cell lysates from Dlx2-expressing and control NMuMG cells treated with or without TGFβ for 4 days. Immunoblotting against total Erk1/2 and GAPDH was used as loading control. (D) Dlx2 expression has no effect on the phosphorylation of PKB at Ser473, as determined by immunoblotting with an antibody specific for PKB phosphorylated at serine 473. Immunoblotting against total PKB and total-Erk1/2 was used as loading control. (E) Dlx2-expressing and control NMuMG cells were treated with TGFβ for 4 days in combination with the EGFR inhibitor AG1478 (3 μM) or DMSO (solvent control) and cell numbers were determined using a Neubauer chamber. (F) Dlx2 expression leads to increased phosphorylation of the EGFR at its activating tyrosine 1173. Immunoblotting analysis of cell lysates from Dlx2-expressing and control NMuMG cells with antibodies against pY1173-EGFR and total EGFR. Immunoblotting against vinculin was used as a loading control. Data are shown as mean±s.d. and are representative of at least three independent experiments. Statistical values are calculated by using an unpaired, two-tailed t-test **P⩽0.01; ***P⩽0.001.
In summary, expression of Dlx2 attenuates apoptotic TGFβ signalling via direct transcriptional repression of the TGFβRII gene, resulting in reduced TGFβ signalling and Smad4 transcriptional activity and, thus, diminished expression of the cell-cycle inhibitor p21CIP1 and increased expression of mitogenic c-Myc.
Dlx2 engages EGFR to promote cell survival and proliferation
Inhibition of apoptotic, canonical TGFβ signalling explains why Dlx2 confers resistance towards TGFβ-mediated growth inhibition. However, it does not explain why Dlx2 expression increases cell proliferation and survival in the presence and in the absence of TGFβ. Recently, several reports have demonstrated that the MAPK and PI3K pathways are interactively engaged to ensure cell survival and proliferation in the presence of tumour-suppressive TGFβ (Janda et al, 2002; Lee et al, 2007b). Hence, we investigated whether these pathways were activated in TGFβ-resistant Dlx2-expressing NMuMG cells.
To investigate whether the MAPK and PI3K pathways were involved in TGFβ-resistant growth, we treated control and Dlx2-expressing NMuMG cells with chemical inhibitors for the MAPK kinase MEK1/2 (PD98059) or for PI3K (ZSTK474). Treatment with either inhibitor significantly reduced cell growth of Dlx2-expressing NMuMG cells as compared with control cells, and these effects were markedly increased upon combined treatment with TGFβ (Figure 4A and B). Thus, Dlx2-mediated proliferation as well as TGFβ-resistant growth substantially relies on the activity of the MAPK and PI3K signalling pathways. Immunoblotting analysis revealed that the levels of the activated (phosphorylated) forms of the MAPK Erk1/2 but not of the PI3K effector protein kinase B (PKB) were higher in Dlx2-expressing NMuMG cells as compared with control cells, in the absence as well as in the presence of TGFβ (Figure 4C and D). Yet, depletion of Dlx2 in NMuMG cells did not affect the overall activation of PKB or Erk1/2 (Supplementary Figure S3).
Various growth factor receptors are known to induce MAPK and PI3K activities upon TGFβ treatment to promote survival and proliferation, including platelet-derived growth factor receptor (PDGFR), vascular endothelial growth factor receptor (VEGFR), and EGFR/ErbB family members (Fabregat et al, 1996, 2000; Murillo et al, 2005; Del Castillo et al, 2006). Hence, we utilized chemical inhibitors against these growth factor receptors to identify a potential upstream activator of MAPK and PI3K signalling. Among the inhibitors tested (VEGFR, PDGFR, IGFR, EGFR), exclusively inhibition of EGFR by the chemical inhibitor Tyrphostin AG1478 significantly repressed Dlx2-mediated, TGFβ-resistant proliferation of NMuMG cells (Figure 4E, data not shown). Dlx2-dependent, elevated activity of EGFR was confirmed by immunoblotting analysis using an antibody against phosphorylated EGFR (tyrosine 1173; Figure 4F).
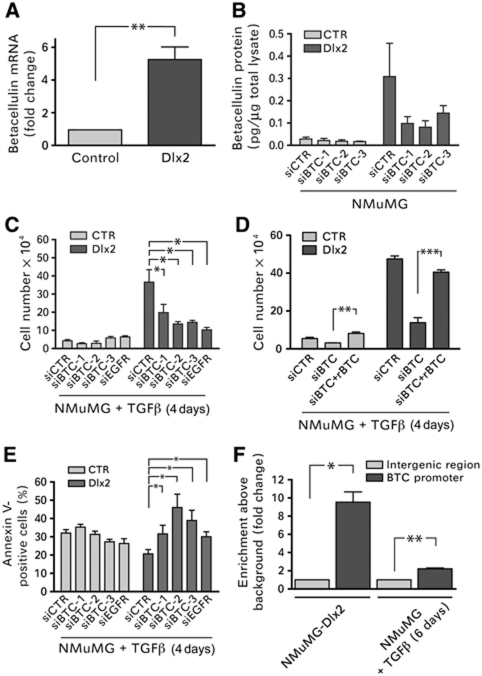
Since total EGFR protein levels were not changed in Dlx2-expressing NMuMG cells (Figure 4E), we assessed whether the expression of members of the EGF family was upregulated by the expression of Dlx2. Gene expression analysis revealed that the EGFR-ligand betacellulin was significantly upregulated in Dlx2-expressing NMuMG cells as compared with control cells. Quantitative RT–PCR and ELISA analysis confirmed the Dlx2-dependent increased expression of betacellulin at the mRNA and protein levels, respectively (Figure 5A and B). To assess whether betacellulin was responsible for the stimulation of EGFR and increased cell survival and proliferation, control and Dlx2-expressing NMuMG cells were transfected with siRNAs against betacellulin and concomitantly treated with TGFβ. The extent of the ablation of betacellulin expression was determined by ELISA and by quantitative RT–PCR (Figure 5B; Supplementary Figure S4A). Reduced betacellulin levels significantly reduced cell numbers of Dlx2-expressing NMuMG cells but not in control cells and thus abrogated a major part of Dlx2-mediated cell proliferation during TGFβ treatment (Figure 5C). Notably, siRNA-mediated ablation of EGFR expression comparably repressed Dlx2-mediated cell proliferation, suggesting that betacellulin was the only inducer of EGFR in Dlx2-expressing cells (Figure 5C; Supplementary Figure S4B). Indeed, addition of recombinant betacellulin to Dlx2-expressing NMuMG cells that had been ablated for betacellulin expression by siRNA transfection restored cell proliferation in the presence of TGFβ (Figure 5D). As expected by their expression of EGFR (Figure 4F), recombinant betacellulin also exerted a proliferative effect on control cells, yet failed to promote cell proliferation to numbers comparable to Dlx2-expressing cells (Figure 5D), indicating that betacellulin by itself was not sufficient to overcome TGFβ-induced growth arrest and apoptosis. Conversely, siRNA-mediated ablation of betacellulin expression in Dlx2-expressing NMuMG cells significantly increased apoptosis upon TGFβ treatment, underscoring its importance for Dlx2-mediated TGFβ resistance (Figure 5E). Finally, ChIP experiments revealed that Dlx2 directly bound to the betacellulin gene promoter in NMuMG cells (Figure 5F), in B16 melanoma cells (Supplementary Figure S4C) and Py2T murine breast cancer cells (not shown), suggesting that it directly induced its expression.
Figure 5.
Betacellulin expression is induced by Dlx2 and provides cell survival and proliferation by stimulating EGFR. (A) Betacellulin mRNA levels were determined by quantitative RT–PCR in NMuMG cells stably expressing either GFP (Control) or Dlx2. (B) The protein levels of betacellulin are increased in Dlx2-expressing NMuMG cells, as determined in cell lysates of GFP and Dlx2-expressing NMuMG cells by ELISA. The high levels of betacellulin induced by Dlx2 expression in NMuMG cells are efficiently reduced by siRNA-mediated knockdown of betacellulin expression (siBTC), as determined by ELISA. (C) Betacellulin (BTC) and its receptor EGFR are required for TGFβ-resistant growth of Dlx2-expressing NMuMG cells. siRNA-mediated ablation of either betacellulin or EGFR expression reduces TGFβ-resistant growth of Dlx2-expressing NMuMG cells with comparable efficacies. Viable cells were counted by trypan blue exclusion using a Neubauer cell counting chamber. (D) siRNA-mediated ablation of betacellulin expression (mixture of the three siRNAs used in (B, C)) in Dlx2-expressing cells results in TGFβ-mediated growth arrest and apoptosis, which can be rescued by addition of recombinant betacellulin (rBTC; 10 ng/ml). Viable cells were counted by trypan blue exclusion using a Neubauer cell counting chamber. (E) TGFβ-resistant growth of Dlx2-expressing NMuMG cells requires betacellulin. The rates of apoptosis in siCTR, siBTC, and siEGFR transfected control or Dlx2-expressing cells were measured by Annexin V staining and flow cytometry. (F) Betacellulin is a direct transcriptional target of Dlx2. ChIP of Dlx2 was performed on either Dlx2-expressing cells or NMuMG cells treated for 6 days with TGFβ. Immunoprecipitated DNA fragments were quantified by quantitative RT–PCR using primers amplifying the promoter region of the betacellulin gene and primers covering an intergenic region as negative control. Data are shown as mean±s.d. and are representative of at least three independent experiments. Statistical values are calculated by using an unpaired, two-tailed t-test. *P⩽0.05; **P⩽0.01; ***P⩽0.001.
In conclusion, Dlx2-mediated TGFβ resistance appears to rely on two mechanisms, the inhibition of apoptotic, canonical TGFβ signalling via direct transcriptional repression of the TGFβRII gene and the activation of mitogenic and pro-survival EGFR signalling via the direct transcriptional induction of betacellulin gene expression.
Dlx2 promotes tumour growth and metastasis
Next, we investigated whether increased expression of Dlx2 correlated with human cancer progression and metastasis by surveying gene expression profiles of human cancer biopsies for Dlx2 expression using the NextBio database (nextbio.com). Significant correlations of increased Dlx2 expression with the potential of melanoma and lung cancers to metastasize and with advanced tumour stages in prostate and lung cancers were detected (Table I). Moreover, treatment of human glioma cells with a specific inhibitor for TGFβRI has reduced Dlx2 expression, indicating that Dlx2 is also a target of TGFβ signalling in glioma cells (Table I). Finally, Dlx2 has been found highly expressed in human breast cancer and in breast cancer-initiating cells (Zhang et al, 2008; Rhodes et al, 2009). These results support the hypothesis that Dlx2 also plays a critical role for cell survival and proliferation during tumour progression and metastasis formation in patients.
Table 1. Dlx2 expression in human cancers.
| Cancer type | Bioset name | P-value | Fold upregulation | NCBI-GEO accession number |
|---|---|---|---|---|
| Melanoma tumours | Intermediate metastatic potential melanoma versus foreskin melanocyte normal | 0.0451 | 4.19 | GSE4845 |
| High metastatic potential melanoma versus foreskin melanocyte normal | 0.0361 | 4.93 | GSE4845 | |
| Intermediate metastatic potential melanoma versus low metastatic potential melanoma | 0.0196 | 2.52 | GSE4845 | |
| High metastatic potential melanoma versus low metastatic potential melanoma | 0.0028 | 3.03 | GSE4845 | |
| High metastatic potential melanoma versus low metastatic potential melanoma | 0.0067 | 2.96 | GSE4845 | |
| Metastatic melanoma versus normal melanocytes | 0.0021 | 18.8 | GSE4570 | |
| Lung cancer | Stage 4 versus stage 1 | 0.0123 | 3.847 | GSE2109 |
| Distant metastasis versus no metastasis | 0.0064 | 3.045 | GSE2109 | |
| Stage 4 versus stage 1 | 0.012 | 3.087 | GSE2109 | |
| Stage 2A versus stage 1b | 0.0351 | 3.29 | GSE3141 | |
| Breast cancer | T2 versus T1 | 0.0227 | 2.32 | GSE10810_19 |
| T2 versus normal tissue | 0.0269 | 2.6 | GSE10810_3 | |
| ER-positive versus normal | 0.0102 | 2.34 | GSE10810_15 | |
| Breast tumour versus normal | 0.007 | 1.91 | GSE10810_1 | |
| Lobular versus ductal | 0.021 | 3.39 | GSE5460_7 | |
| ER-3 versus ER-0 | 0.0001 | 2.8 | GSE3143_1 | |
| Mouse mammary tumour initiating cells | 0.0095 | 3.63 | GSE8863_11 | |
| Metastasis versus primary breast cancer | 0.0086 | 3.9 | GSE8863_7 | |
| 0.0242 | 1.62 | GSE3521_GPL885_7 | ||
| Lobular versus normal | 2.5E−5 | 1.73 | GSE3971_2 | |
| 2.5E−5 | 1.63 | GSE3971_1 | ||
| Prostate cancer | Stage 4 versus stage 2 | 0.0039 | 3.27 | GSE6919 |
| Glioma | TGFβ inhibition leads to Dlx2 downregulation | 0.0037 | −21.9 | Bruna et al (2007) |
| Expression of Dlx2 correlates significantly with advanced tumour progression and the metastatic potential of melanoma, glioma, lung, and prostate cancers. Microarray data are accessible at the NCBI Gene Expression Omnibus (GEO) database. | ||||
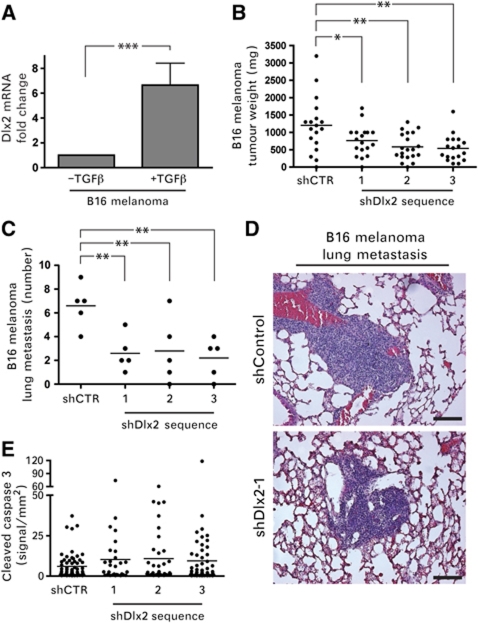
The findings that various cancer types, including melanoma, develop resistance against TGFβ-mediated growth inhibition during malignant progression (reviewed in Teicher, 2001) and that Dlx2 expression correlates with melanoma malignancy (Table I) motivated us to investigate whether Dlx2 expression played a significant role in melanoma growth and metastasis formation. Cultured B16 melanoma cells, when treated with TGFβ for 4 days, exhibited increased Dlx2 mRNA levels, revealing that Dlx2 expression is upregulated by TGFβ signalling also in these cells (Figure 6A). Next, we investigated whether ablation of Dlx2 expression impaired the ability of B16 melanoma cells to form tumours and to metastasize to the lungs upon subcutaneous implantation into syngeneic C57Bl/6 mice. Three cell pools of B16 melanoma cells stably transfected with independent shRNA constructs against Dlx2 (shDlx2-B16 I–III) and one control shRNA (shCTR) cell pool (Supplementary Figure S5A) were implanted into the flanks of mice (five mice per cell pool). Primary tumour volumes and the incidence of lung metastasis were quantified 2 weeks after implantation. shRNA-mediated knockdown of Dlx2 resulted in a significant reduction in primary tumour growth (Figure 6B) and in micrometastatic lesions in the lungs (Figure 6C and D) in comparison to shCTR cells. Dlx2 was found expressed at high levels in the nuclei of control B16 cells and at reduced levels in Dlx2-depleted cells (Supplementary Figure S5B). Moreover, the rate of tumour cell apoptosis, as determined by immunohistochemical staining for cleaved caspase 3, was markedly, yet not significantly increased in Dlx2-depleted B16 tumours as compared with control tumours (Figure 6E), while the proliferation rate, as determined by staining for Ki67, was unaffected (Supplementary Figure S5C). Consistent with our findings that Dlx2 promotes cell survival and proliferation, these results demonstrate that Dlx2 expression is also required for primary tumour growth and metastatic outgrowth of B16 melanoma cells.
Figure 6.
Dlx2 is required for B16 melanoma primary tumour growth and lung metastasis. (A) Dlx2 expression is induced by TGFβ in melanoma cells. B16 melanoma cells were treated with TGFβ for 6 days and Dlx2 mRNA levels were determined by quantitative RT–PCR. Values were normalized to endogenous RPL19 mRNA levels. (B) Reduced primary tumour growth in B16 melanoma cells transfected to stably express shRNA against Dlx2. Three independent Dlx2-specific shRNA sequences (shDlx2 1–3) and one control shRNA sequence (shCTR) were used to establish stable cell pools. Cells were injected into both flanks of 9–10 C57Bl/6 mice per cell pool and tumour weights were measured 2 weeks after implantation. (C) Reduced metastatic outgrowth of B16 melanoma cells depleted for Dlx2 expression. Micrometastatic lesions were counted on histological sections (shown in D) of the lungs of the mice described in (B) (five lungs per cell pool were analysed). (D) Serial histological sections of lungs from C57/Bl6 injected subcutaneously with shDlx2-1 and shCTR B16 melanomas cells were stained with haematoxylin/eosin. Scale bar=100 μm. (E) Tumour sections were stained against cleaved caspase 3 to quantify the rate of apoptosis. The moderate increase in apoptosis observed in Dlx2-depleted tumours was not statistically significant. Data are shown as mean±s.d. Statistical values are calculated by using an unpaired, two-tailed t-test. *P⩽0.05; **P⩽0.01; ***P⩽0.001.
Discussion
The TGFβ signalling pathway exerts a dual function during tumour development and progression. At early stages of tumourigenesis, TGFβ functions as a tumour suppressor by inducing cell-cycle arrest and apoptosis. During late stage tumourigenesis, TGFβ promotes tumour cell invasion and metastasis by inducing an EMT, immunosuppression, and angiogenesis (Thiery and Sleeman, 2006; Massague, 2008; Yang and Weinberg, 2008). Hence, the breakdown of TGFβ-mediated growth restrains plays an important role during tumour formation and progression. Cancer cells evade this TGFβ-mediated tumour-suppressive barrier via downregulation of the TGFβ receptors by a yet poorly understood mechanism (Kang et al, 1999; Kim et al, 2000; Lee et al, 2007a). Hence, the delineation of the molecular pathways enabling cancer cells to overcome TGFβ-mediated growth inhibition and to convert it into a tumour-promoting factor is a critical milestone for the design and development of adequate therapeutic interventions.
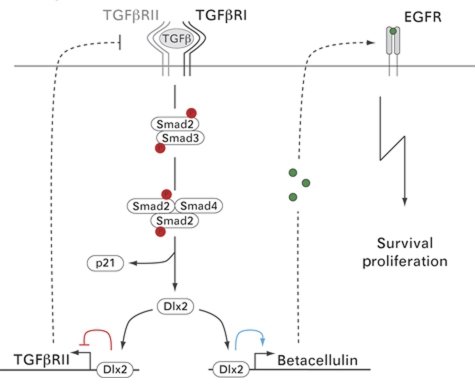
Here, we have identified the transcription factor Dlx2 to enable non-transformed, non-tumourigenic NMuMG cells and B16 melanoma cells to overcome TGFβ-mediated growth inhibition in vitro and in vivo, respectively. This Dlx2-mediated TGFβ-resistant growth is achieved by two major regulatory modifications (i) the inhibition of the pro-apoptotic TGFβ signalling pathway and (ii) the simultaneous activation of the pro-survival and mitogenic EGF receptor signalling pathway by the direct transcriptional induction of betacellulin expression (Figure 7).
Figure 7.
A working model of the molecular mechanisms underlying Dlx2-mediated resistance to TGFβ-induced cell-cycle arrest and apoptosis. Binding of TGFβ to TGFβ receptors induces phosphorylation and activation of the receptor-associated signal transducers Smad2/3. Activated Smad2/3 form a trimeric complex with Smad4 and enter the nucleus to induce expression of TGFβ target genes, such as Dlx2 and the cell-cycle inhibitor p21CIP1. Subsequently, Dlx2 directly binds and represses transcription of the TGFβRII gene (red line) and directly binds and activates expression of the gene for the EGFR-ligand betacellulin (blue line). Reduced TGFβRII expression results into diminished Smad2/3 activation, reduced Smad4 transcriptional activity and, finally, into an attenuation of cytostatic TGFβ signalling. On the other hand, increased expression of betacellulin leads to the activation of EGFR-mediated signal transduction and to cell proliferation and survival.
The molecular and cellular analysis presented in this study reveals that Dlx2-mediated attenuation of the canonical TGFβ signalling pathway is a consequence of a direct transcriptional repression of the TGFβRII gene and leads to changes in the expression of TGFβ target genes, such as decreased expression of the cell-cycle inhibitor p21CIP1 and increased expression of the mitogenic transcription factor c-Myc. Furthermore, we show that Dlx2 itself is a target of canonical TGFβ signalling and, thus, is exerting its function in a negative feedback loop. In summary, we identify Dlx2 as a novel TGFβ-inducible transcriptional repressor that attenuates Smad-dependent TGFβ signalling and thereby promotes cell-cycle arrest and apoptosis (Figure 7).
This mechanistic model of Dlx2-mediated TGFβRII gene repression is consistent with previous reports on autocrine negative feedback loops of TGFβ signalling downregulating TGFβRII expression (Gazit et al, 1993; Woodward et al, 1995; Nishikawa et al, 1998; Truty et al, 2009). The model also resembles the recently described function of KLF14 in human pancreatic epithelial cancer (PANC1) cells, in which KLF14 has been shown to be a TGFβ-inducible repressor of the TGFβRII gene (Truty et al, 2009). Notably, in a recent comprehensive genome analysis of cancer cell lines, the TGFβRII gene was found to be one of the prominent recessive cancer genes suffering from homozygous deletion during carcinogenesis (Bignell et al, 2010).
The capability of Dlx-family transcription factors to interfere with TGFβ signalling is further supported by observations showing that Dlx1 inhibits activin-mediated signalling by blocking Smad4 activity in haematopoietic cells (Chiba et al, 2003), and that Dlx2 expression correlates with decreased TGFβRI and Smad4 levels in a thoracic aortic aneurysm model (Jones et al, 2008). Interestingly, a recent report shows that Dlx4 blocks the growth-suppressive effects of TGFβ by binding to Smad4 and thus preventing canonical TGFβ signalling and the expression of the cell-cycle inhibitors p15INK4B and p21CIP1 (Trinh et al, 2011). Moreover, Dlx4 can activate the expression of c-Myc in a Smad-independent manner. Finally, Dlx2 has also been shown to interact with Smad proteins to control the expression of various target genes (Maira et al, 2010), indicating that Dlx-family members are not only an autonomous transcriptional regulators but also Smad interaction partners.
Besides directly repressing transcription of the TGFβRII gene and inhibiting the canonical TGFβ signalling pathway, we here demonstrate that Dlx2 binds the promoter region and induces transcription of the betacellulin gene, an EGF-family member and specific ligand of EGFR and ErbB4. Betacellulin is well known for its roles in cell differentiation and cancer (Shing et al, 1993; Dunbar and Goddard, 2000). Increased expression and synthesis of betacellulin leads to the stimulation of EGFR and to the activation of its effector signalling pathways, which are essential for Dlx2-mediated TGFβ-resistant growth, notably the MAPK and PI3K pathways. However, it should be noted that the pharmacological inhibition of the MAPK and the PI3K pathways not only represses Dlx2-mediated signalling but also signalling mediated by other inducers activated during TGFβ treatment of NMuMG cells and thus, their effects are much stronger than the ablation of Dlx2. In fact, depletion of Dlx2 does not have a major effect on the overall activation of PKB or Erk1/2 (Supplementary Figure S3). Other pathways may include FGF receptor signalling, and with it MAPK signalling, induced by neuronal cell adhesion molecule in TGFβ-treated NMuMG cells (and other cells undergoing EMT) (Lehembre et al, 2008). From these insights, we conclude that other pathways are also stimulating MAPK and PI3K signalling and that the loss of Dlx2 cannot replicate the complete repression of PI3K or MEK signalling by pharmacological inhibitors. Along these lines, while inhibition of EGFR signalling clearly induces apoptosis and growth arrest of the cells, it does not completely abrogate their growth, again arguing for additional signalling pathways being active.
A comparable functional interaction between Dlx2 and EGF signalling has been previously shown in neuronal transit amplifying cells, where loss of Dlx2 function dramatically reduces their responsiveness towards EGF (Doetsch et al, 2002; Suh et al, 2009). Consistent with the regulation of Dlx2 expression and its activation of EGFR signalling, EGFR and TGFβ signalling pathways have been previously reported to influence each other's activities in both positive and negative ways; however, the molecular details of such interactions have not been delineated (Assoian et al, 1984; Kizaka-Kondoh et al, 2000; Song et al, 2006; Semlali et al, 2008).
Together the data presented here identify Dlx2 as a novel TGFβ-inducible transcription factor, which plays a critical role in balancing cell survival over cell death during TGFβ treatment of NMuMG cells. We demonstrate that Dlx2, by repressing TGFβRII expression and by inducing betacellulin expression, attenuates canonical TGFβ signalling, and activates the EGFR signalling pathway, thus shifting TGFβ's tumour-suppressive functions to tumour progressive functions and favouring cell survival and proliferation. The finding that the loss of Dlx2 function in mouse retina cells results in increased apoptosis is consistent with its anti-apoptotic activity in another cellular context (de Melo et al, 2005). The protective function of Dlx2 is also utilized by cancer cells, since we show that ablation of Dlx2 expression in B16 melanoma cells significantly decreases their growth as primary tumours and as metastasis upon transplantation into syngeneic C57/Bl6 mice. The fact that Dlx2 expression shows a significant and positive correlation with increased invasiveness of human melanomas and several other cancer types underscores its relevance in human disease (Table I; Javelaud et al, 2008; Boone et al, 2009). The finding that Dlx2 exerts a critical switch function during TGFβ treatment and tumour progression makes it an attractive subject matter for further investigations.
Materials and methods
Reagents and antibodies
Human TGFβ and mouse betacellulin include R&D Systems (Abingdon, UK, R&D, #240-B and #1025-CE-025, respectively). Antibodies include Vinculin (#V9131, Sigma-Aldrich), GAPDH (#ab9485, Abcam), TGFβRII (#sc-220, Santa Cruz), Smad4 (#sc-7154, Santa Cruz), Smad2 (#3103, Cell Signaling), pSmad2 (#3101, Cell Signaling), p21CIP1(#556430, Pharmingen), c-myc (#06-340, Upstate Biotechnology), total-PKB (gift from E Hirsch, Torino), p-PKB Serine (#9271, Cell Signaling), p-Erk (#M-8159, Sigma-Aldrich), total-Erk (#M7927, Sigma-Aldrich), EGFR (#2232 Cell Signaling), p-Tyr1173EGFR (#sc-12351, Santa Cruz), HA (ab9110, Abcam), BrdU-FITC (#347583, Becton&Dickinson), Dlx2 (AB5726, Millipore for immunostainings, sc-18140x, Santa Cruz for immunoblotting), Ki-67 (clone Tec3, DAKO), cleaved Caspase 3 Asp175 (5A1, Cell Signaling) . Inhibitors include MEK1/2 Inhibitor PD98059 (#ALX-385-023, Alexis Biochemicals), TGFβRI inhibitor SB431542 hydrate (#S4317, Sigma-Aldrich), PI3K inhibitor ZSTK474 (#ALX-270-454, Alexis Biochemicals), EGFR Inhibitor AG1478 (#ALX-270-036, Alexis Biochemicals), PDGFR inhibitor Tyrphostin AG1296 (#ALX-270-037, Alexis Biochemicals), VEGFR inhibitor PTK787 (provided by Novartis Pharma), IGF1R inhibitor AEW541 (provided by Novartis Pharma).
Primers
For quantitative RT–PCR, the following primers were used: murine Dlx2 fwd: 5′-GGCCTCACCCAAACTCAGGT-3′, rev: 5′-GTATCTCGCCGCTTTTCCAC-3′; murine TGFβRII fwd: 5′-GGCTCTGGTACTCTGGGAAA-3′, rev: 5′-AATGGGGGCTCGTAATCCT-3′; murine betacellulin: fwd: 5′-ACC AATGGCTCTCTTTGTGG-3′, rev: 5′-CCGAGAGAAGTGGGTTTTCA-3′; murine EGFR fwd: 5′- GCCACGCCAACTGTACCTAT-3′, rev: 5′-GCCACACTTCACATC CTTGA-3′; murine RPL19 fwd: 5′-ATCCGCAAGCCTGTGACTGT-3′, rev: 5′-TCGGGCCAGGGTGTTTTT-3′. For ChIP, ChIP-quantitative PCR was performed by using the following primers: murine TGFβR2 promoter fwd: 5′-GCCCCTGGGAGTAATGCC-3′, rev: 5′-CTTTTAGCTGCCCACTCC-3′; murine betacellulin promoter fwd: 5′-CTGCGTCAACTGTCAAATGC-3′, rev: 5′-AAGAGGACCTGGTCATGTGG-3′; murine intergenic region: fwd: 5′-GCTCCGGGTCCTATTCTTGT-3′, rev: 5′-TCTTGGTTTCCAGGAGATGC-3′.
Cells and cell lines
A subclone of NMuMG cells (NMuMG/E9; hereafter NMuMG and B16-F1 melanoma cells have been previously described (Fidler, 1975; Maeda et al, 2005; Lehembre et al, 2008). Cells were cultured in DMEM supplemented with glutamine, penicillin, streptomycin, and 10% FCS (Sigma). NMuMG-shSmad4 and NMuMG-shControl were obtained from P ten Dijke (Leiden University Medical Center, The Netherlands) (Deckers et al, 2006). NMuMG cells were treated with TGFβ in normal growth medium every 2 days (2 ng/ml). Murine Dlx2 siRNA was purchased from Dharmacon (ON-TARGET plus, SmartPool, L-043273-01-005), murine EGFR siRNA was from Sigma (SASI_Mm02_01_00101666 and SASI_Mm02_01_00101666_AS), and murine betacellulin siRNA was from Sigma (SASI_Mm02_00311942, 44, 45, and SASI_Mm02_00311942_AS, 44_AS, 45_AS). Transfections with LipofectAMINE RNAiMAX (Invitrogen) were performed according to the manufacturer's instructions.
To determine growth curves, 1 × 104 cells were seeded in each well of 24-well plate and cell numbers were assessed every second day by using a Neubauer counting chamber.
Stable, tetracyclin-inducible HEK293 cells expressing either GFP or N-terminal HA-tagged murine Dlx2 were generated via site-directed recombination into the Flp-In T-Rex HEK293 cell system (Invitrogen, #K6500-01, #R750-07). Subsequently, cells were selected using hygromycin and individual clones were used for further experiments. Protein expression was induced upon treatment with 1 μg/ml doxycycline.
Total cell lysates, immunoblots, and immunofluorescence experiments were performed as previously described (Lehembre et al, 2008). Proteins of interest were either visualized by chemoluminescence sequentially or on multiple membranes, and Adobe Photoshop was used to crop the relevant portions of the original scans of X-ray films, as indicated by black frames.
Generation of lentivirus
Murine Dlx2 shRNAs (shDlx2 #1–3, TRCN0000070598-600) and control shRNA (shCTR, SHC002, Mission Non-Target shRNA Control Vector) were purchased from Sigma-Aldrich. A cDNA encoding Dlx2 (kindly provided by P Farlie, University of Melbourne) was tagged N-terminally with HA-tag and cloned into the lentiviral expression vector pWPXL. Lentiviral particles were produced by transfecting HEK293T cells with the lentiviral expression vectors in combination with the packing vector pR8.91 and the envelope encoding vector pVSV using Fugene HD. After 2 days of virus production, lentivirus-containing supernatants were harvested, filtered (0.45 μm), and added to target cells in the presence of polybrene (8 ng/ml). Infections were performed twice a day for 2 consecutive days.
Quantitative RT–PCR
Total RNA was prepared using Trizol (Invitrogen), reverse transcribed with M-MLV reverse transcriptase RNAse (H-) (Promega, Wallisellen, Switzerland), and transcripts were quantified by PCR using SYBR-green PCR MasterMix (Applied Biosystems, Rotkreuz, Switzerland). Human or mouse riboprotein L19 primers were used for normalization (see Supplementary data for primer sequences). PCR assays were performed in triplicates, and fold induction was calculated against control-treated cell lines using the comparative Ct method (ΔΔCt).
Reporter assays
NMuMG and HEK293 FlpIN-Dlx2 and FlpIN-GFP cells were transfected with 200 ng reporter and 5 ng Renilla encoding plasmids using Lipofectamine 2000. After 2 days of transfection, cells were analysed using the Dual-Luciferase Reporter Assay System (#E1960, Promega) and a Berthold Luminometer LB960. HEK cells were induced for 1 day with 1 μg/ml doxycycline to express Dlx2 or GFP and then assayed for reporter activity. Measured luciferase values were normalized to internal Renilla control. Smad4 promoter-reporter, TGFβRII promoter-reporter, and E-cadherin promoter-reporter constructs were kindly provided by P ten Dijke (Leiden University) (Dennler et al, 1998), SJ Kim (National Cancer Institute, Bethesda) (Hahm et al, 1999), and K Verschueren (VIB and University of Leuven; van Grunsven et al, 2003).
Chromatin immunoprecipitation
ChIP experiments were performed as previously described (Weber et al, 2007). In brief, crosslinked chromatin was sonicated to achieve an average fragment size of 500 bp. Starting with 100 μg of chromatin and 5 μg of anti-Dlx2 antibody (Abcam-ab18188), 1 μl of ChIP material and 1 μl of input material were used for quantitative real-time PCR using specific primers covering the TGFβRII gene promoter region from basepair −386 to −204, covering the betacellulin gene promoter region from basepair −450 to −253, and primers covering an intergenic region as control. The efficiencies of PCR amplification were normalized for between the primer pairs.
Proliferation assay
Cells were incubated with BrdU (10 μM) for 2 h at 37°C. Fixed in 70% ice-cold ethanol, permeabilized in 2 N HCL/0.5% Triton X-100 solution for 30 min at RT, resuspended in 0.1 M Na2B4O7 pH 8.5 for 2 min at RT, washed twice with 0.5% Tween-20/1% BSA/PBS, and then incubated with FITC labelled anti-BrdU antibody (#347583, BD) for 30 min at RT. After washing twice with 0.5% Tween-20/1% BSA/PBS and resuspending in PBS with 5 μg/ml PI for at least 1 h at RT, the stained cells were analysed on a FACSCanto II using DIVA software (BD).
Apoptosis assay
Cells were washed twice with cold PBS and resuspended in 1 × binding buffer at a concentration of 1 × 105 cells/ml. In all, 5 μl of Cy5 Annexin V was added to the cells and incubated for 15 min at RT (25°C) in the dark. After incubation, cells were analysed on a FACSCanto II using DIVA software.
ELISA
Cell lysates were prepared using RIPA buffer complemented with a protease inhibitor cocktail. The amount of betacellulin in 100 μl of undiluted lysate was analysed in triplicates with the mouse Betacellulin DuoSet ELISA Kit from R&D (DY1025) as suggested by the manufacturer's instructions. Total protein concentrations were determined by Pierce BCA Protein Assay Kit from Thermo Scientific (23225).
B16 melanoma syngeneic transplantation
In all, 6 week-old female C57/Bl6 mice were injected subcutaneously with 4 × 105 B16-F1 melanomas cells in PBS into both flanks (9–10 mice per individual cell pool). After 2 weeks incubation, mice were sacrificed and tumour and lungs were isolated and weighed. Metastatic nodules in lungs were counted by histological sectioning of the entire lungs (five lungs per individual cell pool). Immunohistochemical and immunofluorescence analysis was performed as described previously (Lehembre et al, 2008). Paraffin sections were deparaffinized and antigen retrieval was performed by autoclaving the samples in 10 mM citrate buffer pH 6.0. Sections were stained with 10 μg/ml anti-Dlx2 antibody (Ab 5726, Millipore) using the Perkin-Elmer TSA amplification system according to the manufacturer's instructions. Stainings were evaluated on an AxioVert microscope and on a LSM 510 META confocal microscope (Zeiss, Oberkochen, Germany).
Statistical analysis
Statistical analysis and graphs were generated using the GraphPad Prism software (GraphPad Software Inc., San Diego, CA). All statistical analysis was performed by unpaired, two-sided t-test. Normality testing was performed using the Kolmogorov–Smirnov test with Dallal–Wilkinson–Lillie for P-values.
Supplementary Material
Acknowledgments
We thank P ten Dijke, P Farlie, K Verschueren, SJ Kim, E Hirsch, and U Cavallaro for sharing cell lines and important reagents. We are grateful to K Strittmatter, H Antoniadis, and R Jost for technical support and M Wymann and EF Wagner for helpful scientific discussions. This work was supported by EU-FP6 BRECOSM LSHC-CT-2004-503224, EU-FP7 TuMIC HEALTH-F2-2008-201662, the Swiss Bridge Award, Oncosuisse, and the Krebsliga Beider Basel.
Footnotes
The authors declare that they have no conflict of interest.
References
- Assoian RK, Frolik CA, Roberts AB, Miller DM, Sporn MB (1984) Transforming growth factor-beta controls receptor levels for epidermal growth factor in NRK fibroblasts. Cell 36: 35–41 [DOI] [PubMed] [Google Scholar]
- Bignell GR, Greenman CD, Davies H, Butler AP, Edkins S, Andrews JM, Buck G, Chen L, Beare D, Latimer C, Widaa S, Hinton J, Fahey C, Fu B, Swamy S, Dalgliesh GL, Teh BT, Deloukas P, Yang F, Campbell PJ et al. (2010) Signatures of mutation and selection in the cancer genome. Nature 463: 893–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone B, Haspeslagh M, Brochez L (2009) Clinical significance of the expression of c-Ski and SnoN, possible mediators in TGF-beta resistance, in primary cutaneous melanoma. J Dermatol Sci 53: 26–33 [DOI] [PubMed] [Google Scholar]
- Bruna A, Darken RS, Rojo F, Ocana A, Penuelas S, Arias A, Paris R, Tortosa A, Mora J, Baselga J, Seoane J (2007) High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell 11: 147–160 [DOI] [PubMed] [Google Scholar]
- Chiba S, Takeshita K, Imai Y, Kumano K, Kurokawa M, Masuda S, Shimizu K, Nakamura S, Ruddle FH, Hirai H (2003) Homeoprotein DLX-1 interacts with Smad4 and blocks a signaling pathway from activin A in hematopoietic cells. Proc Natl Acad Sci USA 100: 15577–15582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Fowlis DJ, Bryson S, Duffie E, Ireland H, Balmain A, Akhurst RJ (1996) TGFbeta1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell 86: 531–542 [DOI] [PubMed] [Google Scholar]
- de Melo J, Du G, Fonseca M, Gillespie LA, Turk WJ, Rubenstein JL, Eisenstat DD (2005) Dlx1 and Dlx2 function is necessary for terminal differentiation and survival of late-born retinal ganglion cells in the developing mouse retina. Development 132: 311–322 [DOI] [PubMed] [Google Scholar]
- Deckers M, van Dinther M, Buijs J, Que I, Lowik C, van der Pluijm G, ten Dijke P (2006) The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res 66: 2202–2209 [DOI] [PubMed] [Google Scholar]
- Del Castillo G, Murillo MM, Alvarez-Barrientos A, Bertran E, Fernandez M, Sanchez A, Fabregat I (2006) Autocrine production of TGF-beta confers resistance to apoptosis after an epithelial-mesenchymal transition process in hepatocytes: role of EGF receptor ligands. Exp Cell Res 312: 2860–2871 [DOI] [PubMed] [Google Scholar]
- Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM (1998) Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J 17: 3091–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL (2003) Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol 5: 410–421 [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A (2002) EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron 36: 1021–1034 [DOI] [PubMed] [Google Scholar]
- Dunbar AJ, Goddard C (2000) Structure-function and biological role of betacellulin. Int J Biochem Cell Biol 32: 805–815 [DOI] [PubMed] [Google Scholar]
- Fabregat I, Herrera B, Fernandez M, Alvarez AM, Sanchez A, Roncero C, Ventura JJ, Valverde AM, Benito M (2000) Epidermal growth factor impairs the cytochrome C/caspase-3 apoptotic pathway induced by transforming growth factor beta in rat fetal hepatocytes via a phosphoinositide 3-kinase-dependent pathway. Hepatology 32: 528–535 [DOI] [PubMed] [Google Scholar]
- Fabregat I, Sanchez A, Alvarez AM, Nakamura T, Benito M (1996) Epidermal growth factor, but not hepatocyte growth factor, suppresses the apoptosis induced by transforming growth factor-beta in fetal hepatocytes in primary culture. FEBS Lett 384: 14–18 [DOI] [PubMed] [Google Scholar]
- Fidler IJ (1975) Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res 35: 218–224 [PubMed] [Google Scholar]
- Gal A, Sjoblom T, Fedorova L, Imreh S, Beug H, Moustakas A (2008) Sustained TGFbeta exposure suppresses Smad and non-Smad signalling in mammary epithelial cells, leading to EMT and inhibition of growth arrest and apoptosis. Oncogene 27: 1218–1230 [DOI] [PubMed] [Google Scholar]
- Gazit D, Ebner R, Kahn AJ, Derynck R (1993) Modulation of expression and cell surface binding of members of the transforming growth factor-beta superfamily during retinoic acid-induced osteoblastic differentiation of multipotential mesenchymal cells. Mol Endocrinol 7: 189–198 [DOI] [PubMed] [Google Scholar]
- Gotzmann J, Huber H, Thallinger C, Wolschek M, Jansen B, Schulte-Hermann R, Beug H, Mikulits W (2002) Hepatocytes convert to a fibroblastoid phenotype through the cooperation of TGF-beta1 and Ha-Ras: steps towards invasiveness. J Cell Sci 115: 1189–1202 [DOI] [PubMed] [Google Scholar]
- Hahm KB, Cho K, Lee C, Im YH, Chang J, Choi SG, Sorensen PH, Thiele CJ, Kim SJ (1999) Repression of the gene encoding the TGF-beta type II receptor is a major target of the EWS-FLI1 oncoprotein. Nat Genet 23: 222–227 [DOI] [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 17: 548–558 [DOI] [PubMed] [Google Scholar]
- Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H, Grunert S (2002) Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol 156: 299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javelaud D, Alexaki VI, Mauviel A (2008) Transforming growth factor-beta in cutaneous melanoma. Pigment Cell Melanoma Res 21: 123–132 [DOI] [PubMed] [Google Scholar]
- Jones JA, Barbour JR, Stroud RE, Bouges S, Stephens SL, Spinale FG, Ikonomidis JS (2008) Altered transforming growth factor-beta signaling in a murine model of thoracic aortic aneurysm. J Vasc Res 45: 457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Bang YJ, Im YH, Yang HK, Lee DA, Lee HY, Lee HS, Kim NK, Kim SJ (1999) Transcriptional repression of the transforming growth factor-beta type I receptor gene by DNA methylation results in the development of TGF-beta resistance in human gastric cancer. Oncogene 18: 7280–7286 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Im YH, Markowitz SD, Bang YJ (2000) Molecular mechanisms of inactivation of TGF-beta receptors during carcinogenesis. Cytokine Growth Factor Rev 11: 159–168 [DOI] [PubMed] [Google Scholar]
- Kizaka-Kondoh S, Akiyama N, Okayama H (2000) Role of TGF-beta in EGF-induced transformation of NRK cells is sustaining high-level EGF-signaling. FEBS Lett 466: 160–164 [DOI] [PubMed] [Google Scholar]
- Lee EK, Lee YS, Han IO, Park SH (2007a) Expression of Caveolin-1 reduces cellular responses to TGF-beta1 through down-regulating the expression of TGF-beta type II receptor gene in NIH3T3 fibroblast cells. Biochem Biophys Res Commun 359: 385–390 [DOI] [PubMed] [Google Scholar]
- Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R (2007b) TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J 26: 3957–3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehembre F, Yilmaz M, Wicki A, Schomber T, Strittmatter K, Ziegler D, Kren A, Went P, Derksen PW, Berns A, Jonkers J, Christofori G (2008) NCAM-induced focal adhesion assembly: a functional switch upon loss of E-cadherin. EMBO J 27: 2603–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M, Johnson KR, Wheelock MJ (2005) Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci 118: 873–887 [DOI] [PubMed] [Google Scholar]
- Maira M, Long JE, Lee AY, Rubenstein JL, Stifani S (2010) Role for TGF-beta superfamily signaling in telencephalic GABAergic neuron development. J Neurodev Disord 2: 48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J (2004) G1 cell-cycle control and cancer. Nature 432: 298–306 [DOI] [PubMed] [Google Scholar]
- Massague J (2008) TGFbeta in cancer. Cell 134: 215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH (2005) Non-Smad TGF-beta signals. J Cell Sci 118(Part 16): 3573–3584 [DOI] [PubMed] [Google Scholar]
- Murillo MM, del Castillo G, Sanchez A, Fernandez M, Fabregat I (2005) Involvement of EGF receptor and c-Src in the survival signals induced by TGF-beta1 in hepatocytes. Oncogene 24: 4580–4587 [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Wang M, Carr BI (1998) Changes in TGF-beta receptors of rat hepatocytes during primary culture and liver regeneration: increased expression of TGF-beta receptors associated with increased sensitivity to TGF-beta-mediated growth inhibition. J Cell Physiol 176: 612–623 [DOI] [PubMed] [Google Scholar]
- Oft M, Heider KH, Beug H (1998) TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol 8: 1243–1252 [DOI] [PubMed] [Google Scholar]
- Pardali K, Moustakas A (2007) Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta 1775: 21–62 [DOI] [PubMed] [Google Scholar]
- Rhodes DR, Ateeq B, Cao Q, Tomlins SA, Mehra R, Laxman B, Kalyana-Sundaram S, Lonigro RJ, Helgeson BE, Bhojani MS, Rehemtulla A, Kleer CG, Hayes DF, Lucas PC, Varambally S, Chinnaiyan AM (2009) AGTR1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an AGTR1 antagonist. Proc Natl Acad Sci USA 106: 10284–10289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlali A, Jacques E, Plante S, Biardel S, Milot J, Laviolette M, Boulet LP, Chakir J (2008) TGF-beta suppresses EGF-induced MAPK signaling and proliferation in asthmatic epithelial cells. Am J Respir Cell Mol Biol 38: 202–208 [DOI] [PubMed] [Google Scholar]
- Shing Y, Christofori G, Hanahan D, Ono Y, Sasada R, Igarashi K, Folkman J (1993) Betacellulin: a mitogen from pancreatic beta cell tumors. Science 259: 1604–1607 [DOI] [PubMed] [Google Scholar]
- Siegel PM, Massagué J (2003) Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 3: 807–821 [DOI] [PubMed] [Google Scholar]
- Song K, Krebs TL, Danielpour D (2006) Novel permissive role of epidermal growth factor in transforming growth factor beta (TGF-beta) signaling and growth suppression. Mediation by stabilization of TGF-beta receptor type II. J Biol Chem 281: 7765–7774 [DOI] [PubMed] [Google Scholar]
- Suh Y, Obernier K, Holzl-Wenig G, Mandl C, Herrmann A, Worner K, Eckstein V, Ciccolini F (2009) Interaction between DLX2 and EGFR in the regulation of proliferation and neurogenesis of SVZ precursors. Mol Cell Neurosci 42: 308–314 [DOI] [PubMed] [Google Scholar]
- Teicher BA (2001) Malignant cells, directors of the malignant process: role of transforming growth factor-beta. Cancer Metastasis Rev 20: 133–143 [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7: 131–142 [DOI] [PubMed] [Google Scholar]
- Trinh BQ, Barengo N, Naora H (2011) Homeodomain protein DLX4 counteracts key transcriptional control mechanisms of the TGF-beta cytostatic program and blocks the antiproliferative effect of TGF-beta. Oncogene 30: 2718–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truty MJ, Lomberk G, Fernandez-Zapico ME, Urrutia R (2009) Silencing of the transforming growth factor-beta (TGFbeta) receptor II by Kruppel-like factor 14 underscores the importance of a negative feedback mechanism in TGFbeta signaling. J Biol Chem 284: 6291–6300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Grunsven LA, Michiels C, Van de Putte T, Nelles L, Wuytens G, Verschueren K, Huylebroeck D (2003) Interaction between Smad-interacting protein-1 and the corepressor C-terminal binding protein is dispensable for transcriptional repression of E-cadherin. J Biol Chem 278: 26135–26145 [DOI] [PubMed] [Google Scholar]
- Wakefield LM, Roberts AB (2002) TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev 12: 22–29 [DOI] [PubMed] [Google Scholar]
- Weber M, Hellmann I, Stadler MB, Ramos L, Pääbo S, Rebhan M, Schübeler D (2007) Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet 39: 457–466 [DOI] [PubMed] [Google Scholar]
- Woodward TL, Dumont N, O’Connor-McCourt M, Turner JD, Philip A (1995) Characterization of transforming growth factor-beta growth regulatory effects and receptors on bovine mammary cells. J Cell Physiol 165: 339–348 [DOI] [PubMed] [Google Scholar]
- Yang J, Weinberg RA (2008) Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14: 818–829 [DOI] [PubMed] [Google Scholar]
- Zhang M, Behbod F, Atkinson RL, Landis MD, Kittrell F, Edwards D, Medina D, Tsimelzon A, Hilsenbeck S, Green JE, Michalowska AM, Rosen JM (2008) Identification of tumor-initiating cells in a p53-null mouse model of breast cancer. Cancer Res 68: 4674–4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, Han Y, Feng XH, Meng A, Chen YG (2007) Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol 27: 4488–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.