Abstract
EMBO J 30 21, 4371–4386 (2011); published online October 07 2011
In response to adrenergic stimulation, adipocytes undergo protein kinase A (PKA)-stimulated lipolysis. A key PKA target in this context is perilipin 1, a major regulator of lipolysis on lipid droplets (LDs). A study published in this issue of The EMBO Journal (Pidoux et al, 2011) identifies optic atrophy 1 (OPA1), a protein that regulates mitochondrial dynamics, as perilipin 1 interaction partner and the A-kinase anchoring protein (AKAP) on LDs that is involved in the induction of stimulated lipolysis.
The discovery of perilipin 1 (plin1, formerly called perilipin) in adipocytes (Greenberg et al, 1991) marked the first description of an LD-associated protein and advanced the concept that neutral lipids are stored within well-delineated intracellular organelles. Our understanding of these organelles has developed and, importantly, the critical role of LD metabolism in normal and pathological states has become increasingly evident (Greenberg et al, 2011). Adipocytes robustly respond to catecholamines by increasing intracellular cAMP levels and activating cyclic-AMP-dependent PKA to stimulate lipolysis. Plin1 is dramatically hyperphosphorylated by PKA activation in parallel with lipolytic stimulation (Greenberg et al, 1991) and is localized at the surface of intracellular LDs.
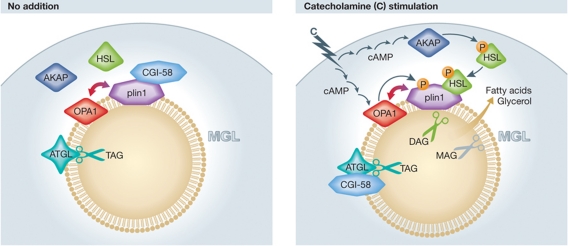
Maximal activation of adipocyte lipolysis (Figure 1) requires the protein CGI-58 (ABHD-5) and three lipases, adipose tissue triglyceride lipase (ATGL), hormone-sensitive lipase (HSL) and monoglyceride lipase (MGL). In the absence of PKA activation, CGI-58 binds to plin1 and is sequestered at the surface of the LD. Activation of PKA promotes the release of CGI-58 from plin1, allowing it to bind ATGL and increase ATGL's activity to hydrolyze triacylglycerol (Lass et al, 2006; Granneman et al, 2007). The majority of HSL is located in the cytoplasm. With PKA activation HSL is phosphorylated, translocates to the LD and binds to phosphorylated plin1 where it hydrolyzes primarily diacylglycerol (DAG) produced by ATGL's actions (Miyoshi et al, 2006). MGL is thought to be constitutively active and completes the lipolytic reaction. In this scenario, plin1 acts as a relay station/scaffold to orchestrate the trafficking of proteins and regulate lipolysis.
Figure 1.
Acting via OPA1, catecholamines phosphorylate plin1, releasing CGI-58, which binds and activates ATGL, hydrolyzing triacylglycerol to DAG. Catecholamines also phosphorylate HSL, leading to its translocation and binding to phosphorylated plin1 and hydrolysis of DAG. MGL hydrolyzes and releases the remaining fatty acid and glycerol.
The different subcellular localizations of HSL and plin1 raise the question of how PKA activation can regulate proteins in different subcellular compartments. A likely explanation lies in separate AKAPs in different subcellular compartments. AKAPs act to tether and regulate activation of PKA subunits. Depending upon the AKAP, they can bind to a single or to both types of PKA R subunit pools, type I or type II. It was suggested that there is a cytoplasmic AKAP that regulates HSL activation (Nomura et al, 2002); however, the presence of a specific AKAP on LDs has not been convincingly demonstrated until now.
In an accompanying article, Pidoux et al (2011) employed an elegant strategy to discover the AKAP present on LDs in adipocytes. Putative AKAP proteins were screened by overlaying labelled RII onto a blot of purified LD proteins in the presence and absence of a blocking peptide to AKAPs to identify potential candidate bands by proteomic analysis. In silico review of the proteomic screen yielded AKAP sequences that identified the likely candidate protein as OPA1. The observation that OPA1 was localized on the LD was somewhat surprising as a mutated OPA protein was identified as the major cause of autosomal dominant OPA, a disease that results in blindness with loss of retinal ganglion cells and atrophy of the optic nerve (Delettre et al, 2000), and previously localized to the inner membrane of mitochondria where it has an important role in mitochondrial fusion and apoptosis (Frezza et al, 2006). However, modelling of the OPA1 protein revealed a signature conformation of AKAPs, containing an amphipathic helix with a clearly defined hydrophobic and a separate negatively charged polar face characteristic of AKAPs. Pidoux et al performed a series of experiments that identified OPA1 as the putative LD AKAP.
OPA1 expression increased with adipocyte differentiation and confocal analysis revealed that most of the OPA1 isoforms were located on the LD with a lesser amount in mitochondria. OPA1 was expressed in liver, skeletal muscle, brown and white adipose tissue with several isoforms noted in the different tissues. Of importance to the proposed role for OPA1, the authors found that OPA1 co-immunoprecipitated with plin1. Knockdown of OPA1 in adipocytes also reduced plin1 expression and further studies suggested that the two proteins exist in a multimolecular complex. OPA1 knockdown decreased stimulated lipolysis but these results were confounded by the reduction in plin1 expression. To overcome this issue, the authors used shRNAi to reduce OPA1 in cultured adipocytes and then replaced the endogenous OPA1 mRNA with OPA1 constructs that were not sensitive to the actions of the shRNAi. A wild-type and mutated OPA1, which could not bind the RI and RII subunits, were introduced into adipocytes in which the endogenous protein had been reduced by shRNAi. Adipocytes expressing the wild-type OPA1 demonstrated normal PKA-stimulated lipolysis and plin1 hyperphosphorylation. Importantly, the mutated OPA1 blocked the actions of PKA to stimulate lipolysis, without affecting cAMP levels. HSL was phosphorylated by PKA but there was no hyperphosphorylation of plin1. In additional studies, the authors were able to demonstrate that OPA1 could bind both type I and type II PKA subunits using specific inhibitors, indicating that it is a dual-specific AKAP.
The observation of a specific AKAP on the LD surface provides an important step in understanding the regulation of LD biology. The observations that a protein previously identified in mitochondria was localized to the LD was surprising and intriguing. Prior proteomic studies have found mitochondrial proteins expressed on the LD, and, in general, these proteins were thought to be contaminants. We now need to reconsider the possible roles of such proteins. An intriguing question is how do mitochondria and LDs interact to regulate lipid metabolism. A recent paper suggests that another member of the plin family, plin5, which is found in oxidative tissue such as brown adipose tissue and skeletal, and cardiac muscle, may recruit mitochondria to the LD (Wang et al, 2011). The identification of OPA1 initiates a new frontier in LD biology and also raises new questions. For example, what happens when plin1 is ectopically expressed in non-adipocytes where it has been found to regulate lipolysis? Does ectopically expressed plin1 then recruit OPA1 to the LD? Are proteins recruited from the mitochondria to the developing, nascent LD? What is the AKAP that regulates PKA-stimulated phosphorylation of HSL? How many AKAPs are there that influence LD homeostasis? What is OPA1's role in mitochondrial biology now that we know it is an AKAP? The burgeoning field of LD biology still has many unanswered questions; nonetheless, the identification of the AKAP OPA1 on the LD surface enhances our understanding of the spatial and subcellular regulation of triacylglycerol hydrolysis.
Acknowledgments
ASG's work was supported by funding from American Diabetes Association (7-08-RA-57), the USDA Agricultural Research Service (58-1950-7-707), NIH DK082574, 1RC2ES01871 and R24DK0867669. CEG was supported by T32 HL069770-07. FBK was supported by the Department of Veterans Affairs (Office of Research and Development, Medical Research Service) and NIH Grant AG028098.
Footnotes
The authors declare that they have no conflict of interest.
References
- Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP (2000) Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 26: 207–210 [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L (2006) OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126: 177–189 [DOI] [PubMed] [Google Scholar]
- Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z (2007) Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem 282: 5726–5735 [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG (2011) The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest 121: 2102–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C (1991) Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 266: 11341–11346 [PubMed] [Google Scholar]
- Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R (2006) Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab 3: 309–319 [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Souza SC, Zhang HH, Strissel KJ, Christoffolete MA, Kovsan J, Rudich A, Kraemer FB, Bianco AC, Obin MS, Greenberg AS (2006) Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem 281: 15837–15844 [DOI] [PubMed] [Google Scholar]
- Nomura S, Kawanami H, Ueda H, Kizaki T, Ohno H, Izawa T (2002) Possible mechanisms by which adipocyte lipolysis is enhanced in exercise-trained rats. Biochem Biophys Res Commun 295: 236–242 [DOI] [PubMed] [Google Scholar]
- Pidoux G, Witczak O, Jarnæss E, Myrvold L, Urlaub H, Stokka AJ, Küntziger T, Taskén K (2011) Optic Atrophy 1 is an A-kinase anchoring protein on lipid droplets that mediates adrenergic control of lipolysis. EMBO J 30: 4371–4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sreenevasan U, Hu H, Saladino A, Polster BM, Lund LM, Gong DW, Stanley WC, Sztalryd C (2011) Perilipin 5, lipid droplet associated protein provides physical and metabolic linkage to mitochondria. J Lipid Res (advance online publication 31 August 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]