Abstract
EMBO J 30 21, 4414–4422 (2011); published online September 30 2011
Micro RNAs (miRNAs) are 21–23 nucleotide long RNAs that associate with the Argonaute family of proteins and direct interactions of these and RNA-induced silencing complex (RISC) components to the 3′-UTRs of mRNAs that harbour partially complementary sequences. The paradigm for miRNA function has been that they inhibit gene expression by translational inhibition and subsequent mRNA degradation. However, a series of recent studies have revealed that subsets of miRNAs are also localized in the nucleus, suggesting that they perform different functions in this cellular compartment. In this issue of The EMBO Journal, Hansen et al (2011) describe that nuclear localized miRNAs target non-coding RNAs (ncRNAs) revealing an intriguing and novel mechanism for gene regulation.
Recent evidence using genome-wide arrays revealed that the majority of the genome is transcribed. In addition to the abundant, nuclear localized non-coding snRNAs and snoRNAs, there are non-coding RNAs (ncRNAs) that are as long or longer than primary coding RNA transcripts. Many of these undergo nuclear splicing events like their coding pre-mRNA counterparts. To date, there is little known about the functional roles these ncRNAs play in cellular physiology. These ncRNAs can be antisense to coding mRNAs where they may function as antisense inhibitors of sense RNA expression. Clear-cut examples of the mechanisms of action of these ncRNAs are difficult to identify and thus much remains to be learned. Given the recent findings of nuclear localized miRNAs it is of interest to understand the relationship between these small RNAs and other ncRNAs. In this issue of The EMBO Journal, Kjems and colleagues investigated the possibility that nuclear localized miRNAs might target ncRNAs. They identified an miRNA that targets a long ncRNA which is antisense to a coding mRNA. Their findings reveal an intriguing and novel mechanism for gene regulation.
It has been demonstrated that a single miRNA can affect the expression of well over 100 transcripts, thus having global effects on gene expression (Baek et al, 2008). These small RNAs have thus been termed the microregulators of gene expression. MiRNAs primarily function by translational inhibition, which occurs in the cytoplasm, and it has therefore largely been assumed that miRNAs function exclusively in this cellular compartment. Findings that there are subsets of miRNAs that are also localized in the nucleus raises the interesting possibility that they functionally regulate gene expression by a mechanism other than translational inhibition in this compartment (Liao et al, 2010). At least one miRNA has been demonstrated to direct chromatin remodelling of a promoter region (Kim et al, 2008). Other experiments have demonstrated that synthetically produced small RNAs of the same size as miRNAs can effectively target promoter regions and direct transcriptional gene silencing by chromatin remodelling (Morris et al, 2004). Promoter-associated sense or antisense transcripts have been demonstrated to be a requirement for small RNA directed chromatin remodelling (Han et al, 2007). Intriguingly, a large database of non-coding RNAs has been developed, and many ncRNAs are antisense to active genes, and thus have been termed natural antisense transcripts (NATs; Lapidot and Pilpel, 2006). Several NATs have ascribed functions, such as gene silencing, activation and mRNA stabilization (Mattick, 2009). Small interfering RNAs (siRNAs) targeting NATs have been shown to activate the transcription of the corresponding sense gene (Morris et al, 2008; Yue et al, 2010).
Despite studies that demonstrated that siRNAs and an miRNA could trigger gene silencing or activation at the epigenetic level, there have been no examples of miRNAs targeting ncRNAs directly. Hansen et al (2011), therefore, investigated whether or not there are known miRNAs that have extensive complementarity of validated ncRNAs. To do this, they first conducted a bioinformatics scan for miRNA complementary sequences in promoter proximal ncRNAs and identified an antisense transcript to the gene encoding Cerebellar Degeneration-Related protein 1 (CDR1). This ncRNA had near complete complementarity to the miRNA miR-671. These investigators next carried out experiments in which they expressed a tetracycline inducible miR-671 in HEK293 cells. They observed that the majority of the expressed miR-671 was nuclear. They next asked whether or not the induced miR-671 functionally targeted the CDR1 antisense transcript. Their results showed miR-671 directed downregulation of this antisense transcript. Surprisingly, the antisense downregulation was accompanied by a corresponding downregulation of the CDR1 sense transcript (see Figure 1). Since HEK293 cells endogenously produce miR-671, they further investigated this phenomenon by using an antagomir, which specifically blocks the function of miR-671. Blocking miR-671 led to an upregulation of the CDR1 antisense and sense mRNA levels in HEK293. To validate that miR-671 was selectively targeting only the antisense transcript, they made miR-671 mimics in which one of the two strands was segmented into two short fragments to prevent that strand from functioning as an miRNA trigger. When they tested these mimics they observed that only the miR mimic in which the non-segmented strand was complementary to the CDR1 antisense functioned in downregulating this target. These results validated that the target of miR-671 is the CDR1 antisense RNA.
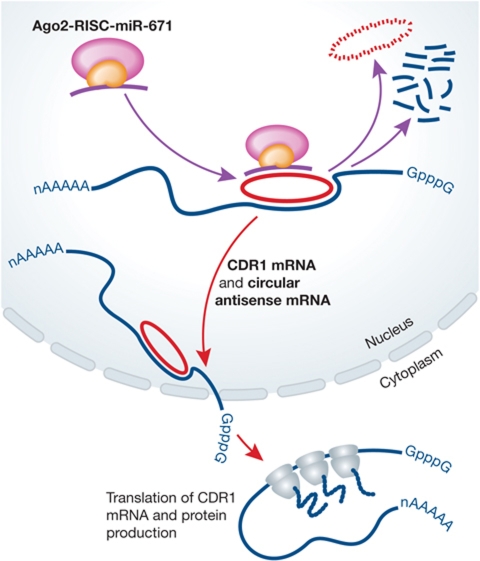
Figure 1.
Model for stabilization–destabilization of CDR1 sense and circular antisense RNAs. Circular CDR1 non-coding antisense pairs with sense CDR1 mRNA in the nucleus and stabilizes it for export to the cytoplasm where the mRNA can be translated. In the presence of miR-671-Ago2-RISC in the nucleus, the antisense is degraded, destabilizing the sense mRNA.
Perhaps the most intriguing aspect of this work is that downregulation of the CDR1 antisense also resulted in downregulation of the sense message for CDR1. Thus, the antisense transcript somehow stabilizes the sense transcript. An unusual feature of the CDR1 antisense transcript is that it is a circular RNA produced by a non-linear splicing event in which the splice acceptor sequence is 5′ of the splice donor. The authors, therefore, investigated the possible mechanisms of miR-671-mediated downregulation of both sense and antisense transcripts. One possibility is epigenetic modification of the genomic DNA, but this was ruled out by experimental results that showed no changes in histone modifications in the presence or absence of miR-671. They also asked if the circular antisense stabilized the sense transcript indirectly via sequestering miRNAs that could target the sense transcript. If such were the case, miR-671-mediated degradation of the circular antisense would result in release of these sequestered miRNAs allowing them to interact with the sense construct and trigger its downregulation. A bioinformatic analysis of both the CDR1 antisense and sense sequences did not reveal any miRNA binding sites in common to the two transcripts. Finally, they validated that the miR-671-mediated downregulation of the antisense was dependent on the ‘slicer’ activity of Ago2, and therefore miR-671 was guiding site directed cleavage of the antisense. This mechanism was further confirmed by sequence analyses of RACE–PCR products which revealed that miR-671 was guiding Ago2 to site specifically cleave the antisense between bases 10 and 11 relative to the 5′-end of the miRNA.
Taken together, this study has revealed novel mechanisms for post-transcriptional gene regulation that involve stabilization of a sense transcript by a circular non-coding antisense RNA and destabilization of the antisense by a nuclear localized miRNA via an Ago2-mediated cleavage. The mechanism by which the CDR1 circular antisense stabilizes the CDR1 mRNA is not understood, but obviously will be an important follow-up for these studies. It is possible that the stabilization is simply by Watson–Crick base pairing of the antisense to the sense, which somehow blocks degradation. Alternatively, the circular antisense could be sequestering proteins that destabilize the sense transcript. Importantly, these studies highlight the fact that miRNAs can function in non-canonical ways to regulate gene expression. It will be of great interest to determine if there are other combinations of ncRNAs and miRNAs that are involved in regulation or modulation of coding mRNA levels.
Footnotes
The author declares that he has no conflict of interest.
References
- Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP (2008) The impact of microRNAs on protein output. Nature 455: 64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Kim D, Morris KV (2007) Promoter-associated RNA is required for RNA-directed transcriptional gene silencing in human cells. Proc Natl Acad Sci USA 104: 12422–12427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, Kjems J (2011) miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J 30: 4414–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Saetrom P, Snove O Jr, Rossi JJ (2008) MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc Natl Acad Sci USA 105: 16230–16235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot M, Pilpel Y (2006) Genome-wide natural antisense transcription: coupling its regulation to its different regulatory mechanisms. EMBO Rep 7: 1216–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JY., Ma LM, Guo YH, Zhang YC, Zhou H, Shao P, Chen YQ, Qu LH (2010) Deep sequencing of human nuclear and cytoplasmic small RNAs reveals an unexpectedly complex subcellular distribution of miRNAs and tRNA 3′ trailers. PLoS One 5: e10563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick JS (2009) The genetic signatures of noncoding RNAs. PLoS Genet 5: e1000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KV, Chan SW, Jacobsen SE, Looney DJ (2004) Small interfering RNA-induced transcriptional gene silencing in human cells. Science 305: 1289–1292 [DOI] [PubMed] [Google Scholar]
- Morris KV, Santoso S, Turner AM, Pastori C, Hawkins PG (2008) Bidirectional transcription directs both transcriptional gene activation and suppression in human cells. PLoS Genet 4: e1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Schwartz JC, Chu Y, Younger ST, Gagnon KT, Elbashir S, Janowski BA, Corey DR (2010) Transcriptional regulation by small RNAs at sequences downstream from 3′ gene termini. Nat Chem Biol 6: 621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]