Abstract
EMBO J 30 21, 4479–4488 (2011); published online September 23 2011
NetrinG proteins and NetrinG ligands (NGLs) are adhesive ligand/receptor pairs that influence axon outgrowth and synaptogenesis. In humans, NetrinG mutations are correlated with developmental disorders of cognition, including schizophrenia and Rett syndrome. In this issue of The EMBO Journal, Seiradake et al (2011) report the crystal structures of NetrinGs and NGLs separately and in complex, revealing a remarkable modularity underlying the molecular specificity of NetrinG/NGL interactions.
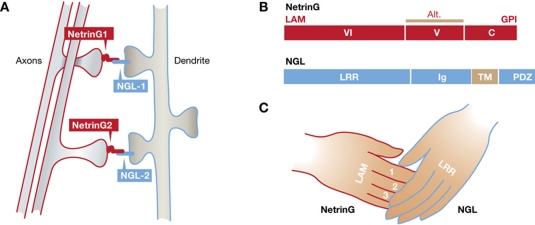
NetrinGs are a specialized subset of laminin-related proteins. Unlike the phylogenetically older secreted netrin chemotropic guidance cues that include UNC6 in C. elegans and netrins 1–4 in vertebrates, NetrinG proteins appear to be exclusive to vertebrates. They are highly expressed in the developing and mature mammalian central nervous system. Two NetrinG genes have been described, netrinG1 and netrinG2. In common with all netrins, the N-terminal sequence of NetrinG proteins encodes a secretory signal peptide followed by domains VI and V. Unlike the secreted netrins, the C terminus of the NetrinGs ends in a GPI lipid linker that tethers the protein to the extracellular surface of the plasma membrane (see Figure 1). NetrinG proteins do not bind the receptors for the secreted netrins, such as DCC and the UNC5 homologues, but instead bind a family of vertebrate-specific proteins named NGLs.
Figure 1.
Molecular hand-clasp interaction between NetrinGs and cognate NGLs. (A) NetrinG1 expressed by specific populations of axons interacts with and aggregates NGL1 present on target dendrites, whereas axonal netrinG2 interacts with and aggregates dendritic NGL2. (B) Illustration of the domain architecture of netrinG and NGL proteins. Domains VI and V of netrinGs are homologous to domains VI and V found in laminins. Domain C terminates in a GPI anchor that links the NetrinG to the plasma membrane. Substantial sequence diversity is generated in domain V through alternative mRNA splicing. Single nucleotide polymorphisms (SNPs) in human netrinG1 and netrinG2 genes have been identified, some of which are associated with Schizophrenia, but how these influence expression or NGL binding remains to be determined. NGLs are single pass transmembrane LRR proteins composed of a series of extracellular leucine-rich repeat domains followed by an Ig domain, a transmembrane sequence, and an intracellular PDZ-binding motif. (C) In this issue of The EMBO Journal, Seiradake et al report that the concave surface of the NGL LRR domain cradles three loops contained within the netrinG LAM domain, described as a ‘hand-clasp’ model of binding.
NGLs are single pass transmembrane members of the leucine-rich repeat (LRR) superfamily of proteins. The NGLs are composed of an extracellular domain that includes nine LRRs and an immunoglobulin (Ig) domain, a transmembrane sequence, and a cytoplasmic domain that includes a PDZ-binding motif (Woo et al, 2009), typical of proteins that bind intracellular synaptic adaptors such as PSD95. The three NGL family members, NGL1, NGL2, and NGL3, exhibit substantial binding specificity. NGL1 selectively binds netrinG1, whereas NGL2 binds netrinG2 (Kim et al, 2006; Nishimura-Akiyoshi et al, 2007). In contrast, NGL3 does not interact with either netrinG1 or G2, but binds to the receptor protein tyrosine phosphatase LAR family of transmembrane proteins (Woo et al, 2009).
NGL1 and NGL2 proteins as well as NetrinG1 and G2 exhibit complimentary patterns of expression in the developing and mature brain (Nakashiba et al, 2002; Meerabux et al, 2005). These observations raised the possibility that their differential expression and selective binding might form a molecular code contributing to the specificity of neuronal circuits. Functional analyses carried out in vitro reinforced these ideas by demonstrating that NGL2 expressed by non-neuronal cells in a co-culture assay is sufficient to promote presynaptic differentiation of a contacting axon. Furthermore, clustering endogenous NGL2 on the dendritic surface triggered the clustering of both post-synaptic intracellular adaptor proteins and NMDA glutamate receptors (Kim et al, 2006). These findings provide evidence that NGL2 regulates excitatory synapse formation by locally recruiting post-synaptic proteins, while also signalling across the synaptic cleft to promote presynaptic specialization.
In a key in-vivo study (Nishimura-Akiyoshi et al, 2007), NGL1 and NGL2 proteins were shown to be compartmentalized to the distal and proximal portions of hippocampal CA1 dendrites. Knockout of netrinG1 or netrinG2 genes demonstrated that dendritic segmentation of NGL1 and NGL2 requires the axon to present the specific netrinG partner. These findings identify the NGL–NetrinG interaction to be a trans-neuronal mechanism, whereby an innervating axon directs the lamina-specific segregation of dendritic proteins (see Figure 1). Although a tantalizing finding, the netrinG1 and G2 knockouts have thus far only revealed changes in the distribution of NGL proteins, and not other synaptic proteins, suggesting that the contribution made by the NGL–NetrinG interaction in vivo is not fully understood, and perhaps redundant with other molecular mechanisms that direct synaptogenesis.
Behavioural studies have demonstrated that animals deficient in NetrinG1, NetrinG2 or NGL2 exhibit altered startle responses to auditory stimuli, though the animals are not deaf and the acoustic neural circuitry appears normal (Zhang et al, 2008). An altered startle response is a characteristic associated with schizophrenia and polymorphisms in netrinG genes in humans have been associated with development of schizophrenia (Aoki-Suzuki et al, 2005; Ohtsuki et al, 2008). Altered expression of NetrinGs has also been linked to bipolar disorder (Eastwood and Harrison, 2008) and rare forms of Rett syndrome (Archer et al, 2006). This conjunction of findings identifying NetrinGs and NGLs as vertebrate-specific gene products that regulate synaptogenesis provides a tantalizing glimpse towards our future understanding of the cell and molecular mechanisms underlying disorders of cognition.
In this issue of The EMBO Journal, Seiradake et al report the crystal structures of netrinG and NGL interaction domains, and show that netrinG and NGL cognate pairs interact in a high-affinity hand-clasp mode that is mechanistically similar, but mutually exclusive between the netrinG1/NGL1 and netrinG2/NGL2 complexes. Specifically, the report describes the interaction domains for the amino terminal laminin-related domains of netrinG1 or netrinG2 in complex with the extracellular LRR and Ig domains of NGL1 or NGL2, respectively. Seiradake et al conclude that the specificity of interaction between cognate netrinG/NGL pairs is conferred by three relatively short loops of amino-acid sequence found in domain VI of NetrinG1 and G2, interacting with the concave surface provided by the LRR domain of the cognate NGL. The netrinG/NGL interaction takes place in a 1:1 stoichiometry, with a geometry that is consistent with netrinG/NGL binding in trans, tethering opposing cell membranes. Based on these structural insights, Seiradake et al then swapped the loops of amino-acid sequence that mediate binding of NGLs to the three identified netrinG loops, and remarkably, demonstrate that this is sufficient to switch the affinity between the cognate NGLs. These amino-acid sequence switches now cause the modified NGL1 to cluster netrinG2, and modified NGL2 to cluster netrinG1, when expressed in heterologous cells. These findings demonstrate the molecular basis of the binding selectivity exhibited by netrinG/NGL pairs.
Conclusions and open questions
Seiradake and colleagues have provided a high-resolution characterization of the molecular mechanism underlying the exclusive affinity between netrinG and NGL, with the structural information obtained providing direct insight into the function of the protein domains responsible for the binding mediated by these vertebrate-specific, synapse-enriched proteins. This substantial step forward sets the stage for further analysis into how the structure of these proteins influences function. For example, both netrinG1 and netrinG2 exhibit substantial alternative mRNA splicing, but how the diversity exhibited by NetrinGs influences NGL binding and synaptic partnering remains to be determined. Additionally, these new findings provide a foundation for considering the structural significance of the nucleotide polymorphisms identified in NetrinG genes and how these differences may potentially influence the maintenance of neural circuitry and human disease.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aoki-Suzuki M, Yamada K, Meerabux J, Iwayama-Shigeno Y, Ohba H, Iwamoto K, Takao H, Toyota T, Suto Y, Nakatani N, Dean B, Nishimura S, Seki K, Kato T, Itohara S, Nishikawa T, Yoshikawa T (2005) A family-based association study and gene expression analyses of netrin-G1 and -G2 genes in schizophrenia. Biol Psychiatry 57: 382–393 [DOI] [PubMed] [Google Scholar]
- Archer HL, Evans JC, Millar DS, Thompson PW, Kerr AM, Leonard H, Christodoulou J, Ravine D, Lazarou L, Grove L, Verity C, Whatley SD, Pilz DT, Sampson JR, Clarke AJ (2006) NTNG1 mutations are a rare cause of Rett syndrome. Am J Med Genet A 140: 691–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ (2008) Decreased mRNA expression of netrin-G1 and netrin-G2 in the temporal lobe in schizophrenia and bipolar disorder. Neuropsychopharmacology 33: 933–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Burette A, Chung HS, Kwon SK, Woo J, Lee HW, Kim K, Kim H, Weinberg RJ, Kim E (2006) NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat Neurosci 9: 1294–1301 [DOI] [PubMed] [Google Scholar]
- Meerabux JM, Ohba H, Fukasawa M, Suto Y, Aoki-Suzuki M, Nakashiba T, Nishimura S, Itohara S, Yoshikawa T (2005) Human netrin-G1 isoforms show evidence of differential expression. Genomics 86: 112–116 [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Nishimura S, Ikeda T, Itohara S (2002) Complementary expression and neurite outgrowth activity of netrin-G subfamily members. Mech Dev 111: 47–60 [DOI] [PubMed] [Google Scholar]
- Nishimura-Akiyoshi S, Niimi K, Nakashiba T, Itohara S (2007) Axonal netrin-Gs transneuronally determine lamina-specific subdendritic segments. Proc Natl Acad Sci USA 104: 14801–14806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki T, Horiuchi Y, Koga M, Ishiguro H, Inada T, Iwata N, Ozaki N, Ujike H, Watanabe Y, Someya T, Arinami T (2008) Association of polymorphisms in the haplotype block spanning the alternatively spliced exons of the NTNG1 gene at 1p13.3 with schizophrenia in Japanese populations. Neurosci Lett 435: 194–197 [DOI] [PubMed] [Google Scholar]
- Seiradake E, Coles CH, Perestenko PV, Harlos K, McIlhinney RAJ, Aricescu AR, Jones EY (2011) Structural basis for cell surface patterning through NetrinG-NGL interactions. EMBO J 30: 4479–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Kwon SK, Kim E (2009) The NGL family of leucine-rich repeat-containing synaptic adhesion molecules. Mol Cell Neurosci 42: 1–10 [DOI] [PubMed] [Google Scholar]
- Zhang W, Rajan I, Savelieva KV, Wang CY, Vogel P, Kelly M, Xu N, Hasson B, Jarman W, Lanthorn TH (2008) Netrin-G2 and netrin-G2 ligand are both required for normal auditory responsiveness. Genes Brain Behav 7: 385–392 [DOI] [PubMed] [Google Scholar]