Abstract
Background: Both coplanar and noncoplanar polychlorinated biphenyls (PCBs) exhibit neurotoxic effects in animal studies, but individual congeners do not always produce the same effects as PCB mixtures. Humans genetically have > 60-fold differences in hepatic cytochrome P450 1A2 (CYP1A2)-uninduced basal levels and > 12-fold variability in aryl hydrocarbon receptor (AHR)affinity; because CYP1A2 is known to sequester coplanar PCBs and because AHR ligands include coplanar PCBs, both genotypes can affect PCB response.
Objectives: We aimed to develop a mouse paradigm with extremes in Cyp1a2 and Ahr genotypes to explore genetic susceptibility to PCB-induced developmental neurotoxicity using an environmentally relevant mixture of PCBs.
Methods: We developed a mixture of eight PCBs to simulate human exposures based on their reported concentrations in human tissue, breast milk, and food supply. We previously characterized specific differences in PCB congener pharmacokinetics and toxicity, comparing high-affinity–AHR Cyp1a2 wild-type [Ahrb1_Cyp1a2(+/+)], poor-affinity–AHR Cyp1a2 wild-type [Ahrd_Cyp1a2(+/+)], and high-affinity–AHR Cyp1a2 knockout [Ahrb1_Cyp1a2(–/–)] mouse lines [Curran CP, Vorhees CV, Williams MT, Genter MB, Miller ML, Nebert DW. 2011. In utero and lactational exposure to a complex mixture of polychlorinated biphenyls: toxicity in pups dependent on the Cyp1a2 and Ahr genotypes. Toxicol Sci 119:189–208]. Dams received a mixture of three coplanar and five noncoplanar PCBs on gestational day 10.5 and postnatal day (PND) 5. In the present study we conducted behavioral phenotyping of exposed offspring at PND60, examining multiple measures of learning, memory, and other behaviors.
Results: We observed the most significant deficits in response to PCB treatment in Ahrb1_Cyp1a2(–/–) mice, including impaired novel object recognition and increased failure rate in the Morris water maze. However, all PCB-treated genotypes showed significant differences on at least one measure of learning or behavior.
Conclusions: High levels of maternal hepatic CYP1A2 offer the most important protection against deficits in learning and memory in offspring exposed to a mixture of coplanar and noncoplanar PCBs. High-affinity AHR is the next most important factor in protection of offspring.
Keywords: acoustic startle response, aryl hydrocarbon receptor (AHR), coplanar PCBs, cytochrome P450 1A2 (CYP1A2), developmental neurotoxicity, locomotor activity, long-term potentiation, Morris water maze, noncoplanar PCBs, novel object recognition, PCB exposure in utero, PCB exposure via breast milk, polychlorinated biphenyls (PCBs), prepulse inhibition
Polychlorinated biphenyls (PCBs) are among the top five priority pollutants (Agency for Toxic Substances and Disease Registry 2007). The primary route of human exposure is consumption of contaminated foods (Huwe and Larsen 2005); in the past, occupational exposures were significant (Gustavsson and Hogstedt 1997). Populations near polluted toxic waste dump sites have demonstrated learning, memory, and behavioral abnormalities in children exposed in utero and via breast milk (Schantz et al. 2003). Therefore, previous studies have defined at-risk populations, primarily based on their exposure to PCB-contaminated foods or their proximity to PCB-contaminated sites.
Evidence for PCB-induced neurotoxicity includes studies of exposed human populations worldwide (Grandjean et al. 2001; Guo et al. 1997; Gustavsson and Hogstedt 1997; Jacobson and Jacobson 1997, 2003; Nakai et al. 2004). These studies consistently show learning, memory, and behavioral deficits that extend into school age (Jacobson and Jacobson 2003; Vreugdenhil et al. 2004) and increased neurodegenerative diseases (Petersen et al. 2008; Schantz et al. 2001). The greatest risk is to children exposed in utero and through consumption of contaminated breast milk (Guo et al. 2004; Schantz et al. 2003). Studies in nonhuman primates (Rice 2000; Schantz et al. 1989) and rodents (Gilbert et al. 2000; Roegge and Schantz 2006) have confirmed the unique susceptibility of the developing central nervous system (CNS) to PCBs.
In the present study we used a previously developed mixture of eight PCBs that included coplanar and noncoplanar PCBs prevalent in food, human tissue, and breast milk (Curran et al. 2011); these PCBs were chosen because they have previously been implicated in developmental neurotoxicity. Single-congener studies offer utility when searching for mechanisms, but they are less satisfactory at modeling human exposures.
Coplanar PCBs are aryl hydrocarbon receptor (AHR) ligands (Poland and Glover 1977), and maternal levels of hepatic cytochrome P450 1A2 (CYP1A2) influence the amount of AHR ligand reaching the embryo or fetus (see Dragin et al. 2006 and references therein). Moreover, humans are known genetically to exhibit > 12-fold variability in AHR affinity and > 60-fold differences in hepatic CYP1A2 basal uninduced levels (Nebert et al. 2004). Thus, we administered the PCB mixture to mice representing extremes for variation in high- versus poor-affinity AHR and high versus absent CYP1A2 basal levels.
In characterizing these mice (Curran et al. 2011), we examined effects of the PCB mixture [given on gestational day (GD) 10.5 and postnatal day (PND) 5] on three genotypes: wild-type having high-affinity AHR [Ahrb1_Cyp1a2(+/+)], wild-type having poor-affinity AHR [Ahrd_Cyp1a2(+/+)], and knockout having high-affinity AHR [Ahrb1_Cyp1a2(–/–)]. These lines were evaluated for PCB effects on birth weight, growth, immunosuppression, AHR activation, and CYP1A1 and CYP1A2 mRNA levels in tissues of the mother, embryo, fetus, and pup; the concentrations of each of the PCB congeners in these tissues were measured at five time points. We also confirmed important genetic differences in the above-mentioned parameters (Curran et al. 2011). In that study (Curran et al. 2011), administration of the mixture to the mother at GD10.5 and PND5 resulted in continuous AHR activation in the high-affinity–Ahrb1 embryo, fetus, and weanling. GD10.5 to PND20 is the period of rodent brain development that most closely matches brain development in the second to third trimesters of human development (Clancy et al. 2007).
Materials and Methods
Chemicals. Noncoplanar PCB congeners 105, 118, 138, 153, and 180 and coplanar PCB congeners 77, 126, and 169 [see Supplemental Material, Table S1 (http://dx.doi.org/10.1289/ehp.1002965)] were purchased from ULTRA Scientific (North Kingstown, RI) and dissolved in acetone and corn oil (acetone removed under argon). Other reagents were from Fisher (Fairlawn, NJ) or Sigma (St. Louis, MO). Personnel were instructed in safe handling and disposal of PCBs.
Animals. Mice (Table 1) included C57BL/6J (B6) and B6.D2-Ahrd (congenic having poor-affinity Ahrd allele from DBA/2J) from Jackson Laboratory (Bar Harbor, ME); both are Cyp1a2(+/+) wild-type. The Ahrb1_Cyp1a2(–/–) knockout mouse is an in-house line (Liang et al. 1996). Backcrossing produced genotypes that express > 99.8% B6. Animals were housed in a vivarium accredited by the Association for Assessment and Accreditation of Laboratory Animal Care; the animals were treated humanely and with regard for alleviation of suffering.
Breeding. Nulliparous females 3–5 months of age (body weight, 20–25 g) were used for all matings. The morning when a vaginal plug was found was considered GD0.5, and plug-positive females were removed from the breeding cages. Pregnant females were housed individually with pups until weaning on PND28.
Dosing of animals. Pregnant females were given the PCB mixture by gavage on GD10.5 and PND5; these time points were chosen to ensure continual AHR activation throughout lactation and were based on our previous study (Curran et al. 2011). Controls were gavaged with an equivalent volume of corn oil vehicle (15 mL/kg). Dosing was delayed until GD10.5 to avoid interfering with implantation and to minimize neonatal lethality (Curran et al. 2006).
Behavior. Animals were tested in groups from all three genotypes (PCB-treated vs. corn-oil–treated controls). One male and one female per litter were tested (16–20/group) beginning on PND60: week 1, elevated zero maze, locomotor activity, and acoustic startle response (ASR) with prepulse inhibition (PPI); week 2, novel-object recognition; week 3, Morris water maze (MWM) cued; week 4, MWM hidden acquisition; week 5, MWM hidden reversal; week 6, MWM hidden shift; week 7, locomotor activity with (+) methamphetamine (1 mg/kg) challenge. Mice were placed in the apparatus for 30 min to habituate them to the environment; they were then removed, injected with methamphetamine, and returned to the apparatus for an additional 120 min. All tests were performed during the light portion of the light:dark cycle.
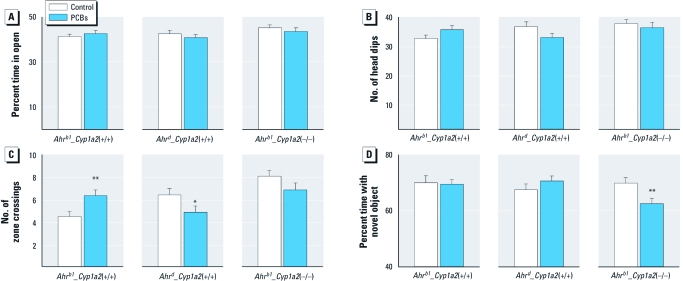
Elevated zero maze. The apparatus for this test is a circular runway (105-cm diameter), 72-cm above the floor with a 10-cm path divided into equal quadrants; two opposite quadrants have 28-cm walls, and two remaining opposite quadrants have 1.3-cm acrylic curbs. Mice were videotaped for 5 min. Time in open and numbers of head dips and zone crossings were scored (Shepherd et al. 1994).
Locomotor activity. For evaluation of locomotor activity, mice were tested for 1 hr in arenas that measured 41 × 41 cm and had 16 LED photocells in the x- and y-planes (Accuscan Instruments, Columbus, OH).
ASR-PPI. For this test, we used an SR-LAB apparatus (San Diego Instruments, San Diego, CA) with 5-min acclimation, followed by a 4 × 4 Latin square of four trial types repeated three times: no stimulus, startle signal (SS), 74-dB prepulse + SS, or 76-dB prepulse + SS. The intertrial interval was 8 sec, and the interstimulus interval was 70 msec. The signal was a mixed-frequency white noise burst (120 dB sound pressure level for 20 msec). Peak response amplitudes (Vmax) were analyzed.
Novel object recognition. For evaluation of novel object recognition, mice were habituated to arenas (91-cm diameter) for 2 days, followed by 2 days of exposure to two objects (10 min/day). On the test day new objects were presented until 30 sec of observation accrued; 1 hr later, the familiar (copy) and novel object were both presented, until 30 sec of observation accrued (up to 10 min).
MWM. The tank for the MWM was 122 cm in diameter (Vorhees and Williams 2006). Testing was as follows: day 1 consisted of six cued trials with the start and platform fixed; for days 2–6, there were two trials per day with random start and finish positions (curtains were closed to block visual cues). The 10-cm platform contained an orange ball 10 cm above the surface. Mice received three phases of hidden-platform testing, four trials per day for 6 days, with 30-sec probe trial on day 7 [see Supplemental Material, Table S2 (http://dx.doi.org/10.1289/ehp.1002965)]. Each phase used a smaller platform (10, 7, or 5 cm). Data were analyzed for latency, cumulative distance, path length, speed on platform trials and crossovers, average distance, and quadrant preference on probe. For additional information, see Supplemental Material, p. 2.
Table 1.
Mouse lines and hypotheses regarding susceptibility or resistance to PCB-induced developmental neurotoxicity.
| Genotype | AHR ligand | CYP1A2 | Common name | Hypothesis | ||||
|---|---|---|---|---|---|---|---|---|
| Ahrd_Cyp1a2(+/+) | Poor affinity | Present | B6.D2-Ahrd | Most resistant | ||||
| Ahrb1_Cyp1a2(+/+) | High affinity | Present | C57BL/6J (B6) | Intermediate | ||||
| Ahrb1_Cyp1a2(–/–) | High affinity | Absent | Cyp1a2 knockout | Most susceptible |
Long-term potentiation (LTP). We measured LTP using a MED64 multielectrode array (Alpha Med Sciences, Kadoma, Japan) (Shimono et al. 2002) on parasagittal hippocampal sections (350 μm) of PND30–35 mice. Paired pulses were delivered to CA1, and excitatory postsynaptic potentials (EPSPs) were recorded until stable. Slope of EPSPs was recorded for 90 min after a theta burst [tetanus = 100 Hz in 10 bursts (4 pulses/burst) delivered at a burst frequency of 5 Hz for 2 sec]. Sections were analyzed in duplicate for each animal.
Monoamine neurotransmitter assay. Neurotransmitters [dopamine (DA) and its metabolite 3,4-dihydroxyphenylacetic acid, and serotonin and its metabolite 5-hydroxyindoleacetic acid] were analyzed as previously described (Graham et al. 2011). See Supplemental Material, p. 4 (http://dx.doi.org/10.1289/ehp.1002965) for details.
Corticosterone assay. Corticosterone levels were measured using an immunoassay kit (Octeia Corticosterone EIA kit AC-14F1; IDS Inc., Fountain Hills, AZ) following the manufacturer’s protocol. Blood was collected in heparinized tubes and centrifuged at 2,500 relative centrifugal force for 5 min at 4°C, and plasma was stored at –80°C. All samples were run in duplicate, and corticosterone levels were calculated by comparison with a standard curve ranging from 0 to 133 ng/mL.
Statistical analyses. Behavioral data were analyzed using mixed-linear analysis of variance (ANOVA) with repeated measures or by analysis of covariance (ANCOVA) using SAS software (version 9.2; SAS Institute Inc., Cary, NC). Results were considered statistically significant if p < 0.05, as analyzed by slice-effect ANOVAs and biochemical data by two-way ANOVA followed by Holm-Sidak post hoc comparisons.
Results
Body weight. Mice were weighed on PND60 and PND100; we observed no differences in body weight among treatment groups or genotypes.
Elevated zero maze. PCB-treated Ahrd_Cyp1a2(+/+) mice exhibited fewer head dips and zone crossings (p = 0.06), indicating increased anxiety (Figure 1), whereas PCB-treated Ahrb1_Cyp1a2(+/+) mice showed more head dips (p = 0.09) and significantly more zone crossings than controls, suggesting a mild anxiety effect of PCBs but no differences in time in open (the principal index of anxiety in this test, which is fear of open spaces). Increased time in open therefore indicates decreased anxiety. The AHR phenotype caused opposite effects in response to PCBs, slightly increasing anxiety in poor-affinity–AHR mice (fewer head dips) and slightly decreasing anxiety in high-affinity–AHR mice. PCB decreased zone crossings in poor-affinity–AHR mice and increased them in high-affinity–AHR mice, suggesting that PCB causes increased anxiety in high-affinity–AHR mice. We cannot exclude that these differences contribute to variations in head dips; however, absence of difference in time in open (Figure 1A) argues against this interpretation.
Figure 1.
Results of elevated zero maze (5 min). (A) Percent time in open. (B) Number of head dips. (C) Number of zone crossings. (D) Percent time exploring novel object. Data shown are least-squares mean ± SE. *p < 0.05, and **p < 0.01, compared with untreated controls of the same genotype.
Novel object recognition. PCB-treated Ahrb1_Cyp1a2(–/–) was the only group showing significant deficits in novel object recognition (Figure 1D). PCB-treated Ahrb1_Cyp1a2(–/–) mice spent a lower percentage of time exploring the novel object compared with controls, suggesting that PCB-treated Ahrb1_Cyp1a2(–/–) mice are less able to remember the familiar object and distinguish it from the new object.
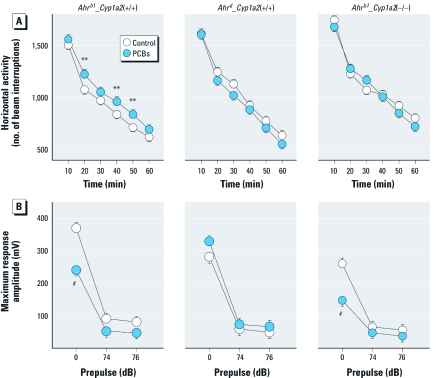
Locomotor activity. Decreases in PCB-treated rodent locomotor habituation have been reported (Eriksson 1997). Regardless of treatment, Ahrb1_Cyp1a2(–/–) mice were more active than the other genotypes (Figure 2A). Treatment differences were significant only for PCB-treated Ahrb1_Cyp1a2(+/+) mice, compared with controls during the middle (20-, 40-, and 50-min) intervals.
Figure 2.
Results of locomotor activity (60 min; A) and ASR-PPI (B) tests (mean ± SE). **p < 0.01, and #p < 0.001 compared with controls of the same genotype.
ASR with PPI. The ASR-PPI test measures baseline startle response and its attenuation when preceded by a lower-decibel tone preceding it (prepulse). We found no differences in PPI in PCB-treated mice compared with controls, regardless of AHR genotype. Untreated Ahrb1_Cyp1a2(–/–) mice had significantly reduced amplitude in ASR than did untreated controls of either of the other two genotypes. PCB-treated Ahrb1_Cyp1a2(–/–) mice exhibited decreased ASR (Figure 2B) compared with controls, as did the PCB-treated Ahrb1_Cyp1a2(+/+) mice. In contrast, PCB-treated Ahrd_Cyp1a2(+/+) mice showed a trend (p = 0.08) toward increased ASR. PCB exposure has been linked to hearing loss; however, hearing deficits cannot explain these results because all mice exhibited ASR after the 74- and 76-dB prepulses.
MWM, cued platform. We observed no differences among the groups on day 1 (data not shown). Data for days 2 and 3 showed that PCB-treated Ahrb1_Cyp1a2(–/–) mice took longer to reach the platform (greater latency) than did controls (Figure 3A). Further analysis revealed that this difference was attributable to slower swimming in PCB-treated Ahrb1_Cyp1a2(–/–) mice on days 2 and 3 (Figure 3B), with no significant differences thereafter.
Figure 3.
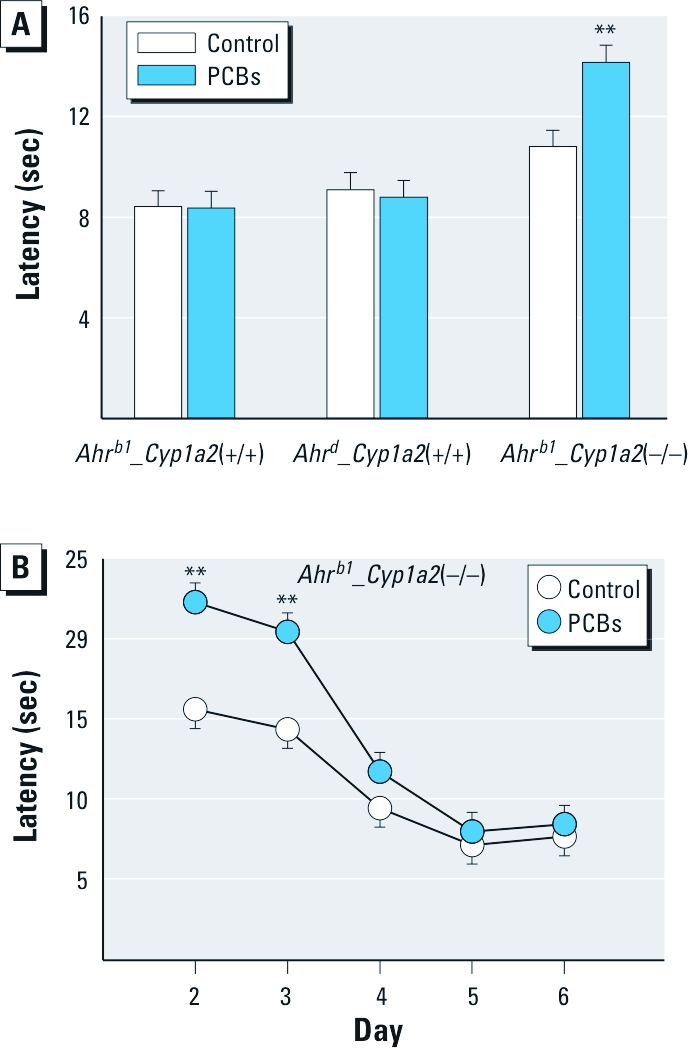
Results of the MWM (cued) test (mean ± SE). (A) Latency to reach goal. (B) Latency in the Ahrb1_Cyp1a2(–/–) group on days 2–6 (two trials per day). No differences were seen on day 1 (data not shown). **p < 0.01 compared with untreated control.
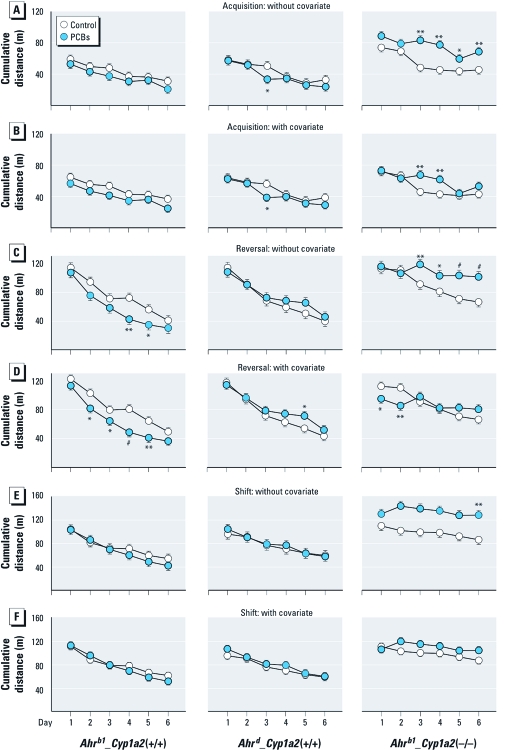
MWM, hidden platform. Latency, path length, and cumulative distance showed the same pattern; therefore, only cumulative distance is shown (Figure 4). During all phases (acquisition, reversal, and shift), PCB-treated mice swam more slowly than did controls regardless of genotype. Therefore, we analyzed the data without and with adjustment for swim speed. Among the Ahrb1_Cyp1a2(+/+) mice, PCB treatment affected performance during reversal learning (Figure 4C,D). With and without adjustment for speed, the PCB-treated mice showed improved performance (i.e., shorter cumulative distances to reach the goal), compared with controls. Among Ahrd_Cyp1a2(+/+) mice, PCB treatment was associated with minor differences: on day 3 of acquisition, PCB-treated mice showed shorter cumulative distance than did controls, and on day 5 PCB-treated mice had increased cumulative distance, which is significant with covariate adjustment (Figure 4A–D).
Figure 4.
Results of MWM (hidden) test presented as cumulative distance to the platform (mean ± SE). During each phase there were genotype × treatment effects on speed (all p < 0.01); therefore, data were analyzed using without (A,C,E) and with (B,D,F) speed as covariates. (A and B) Acquisition (southwest platform). (C and D) Reversal (northeast platform). (E and F) Shift (northwest platform). See Supplemental Material, Table S2 (http://dx.doi.org/10.1289/ehp.1002965). *p < 0.05, **p < 0.01, and #p < 0.001 compared with control.
The most striking effects occurred among Ahrb1_Cyp1a2(–/–) mice (Figure 4): The PCB-treated group showed impaired acquisition, reversal, and shift learning without adjustment for speed (Figure 4A,C,E). During acquisition, adjustment for speed reduced the magnitude and number of days that were significant but did not eliminate the deficit (Figure 4B). During reversal, adjustment for speed eliminated and reversed the deficit on days 1 and 2 (Figure 4D), indicating that swimming ability of the PCB-treated group might account for this effect. Similarly, during the shift, impairment in the PCB-treated group was eliminated after adjustment for swim speed (Figure 4F).
Variations were seen between day and sex when they were included in the analyses along with genotype and treatment. PCB treatment was associated with better performance in both Cyp1a2(+/+) lines but with poorer performance in the Ahrb1_Cyp1a2(–/–) line. Analysis showed that PCB treatment primarily caused differences among females [see Supplemental Material, Figure S1 (http://dx.doi.org/10.1289/ehp.1002965)].
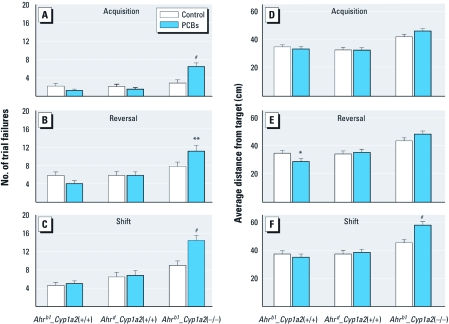
MWM trial failure. We also analyzed trial failure, which represents the proportion of trials that the mouse reached the 60-sec time limit and had to be removed. Analyses of these data (Figure 5A–C) demonstrate that only PCB-treated Ahrb1_Cyp1a2(–/–) mice showed increased rates of failure on all three phases (acquisition, reversal, and shift), compared with controls.
Figure 5.
MWM trial failure (trials during which animals reached the time limit; A–C) and probe trials (average distance from the platform site 24 hr after the last platform trial of each phase; D–F). (A and D) Acquisition. (B and E) Reversal. (E and F) Shift. Values shown are (mean ± SE). *p < 0.05, **p < 0.01, and #p < 0.001 compared with control.
MWM memory. All measures of probe-trial performance (a measure of spatial memory because the platform has been removed) showed similar patterns; therefore, only average distance to target is presented in Figure 5D–F. We observed no differences on the acquisition probe (Figure 5D). On reversal probe, PCB-treated Ahrb1_Cyp1a2(+/+) mice had significantly shorter distances to the platform site than did controls (Figure 5E). On shift probe, PCB-treated Ahrb1_Cyp1a2(–/–) mice had significantly longer distances to the platform site than did controls, consistent with the trial failure data for this group (Figure 5F).
Methamphetamine challenge. We retested locomotor activity after a dose of the positive entantiomer (+) of methamphetamine (an indirect dopaminergic agonist). Before the challenge, we observed no differences among Ahrd_Cyp1a2(+/+) or Ahrb1_Cyp1a2(–/–) mice [see Supplemental Material, Figure S2B,C (http://dx.doi.org/10.1289/ehp.1002965)]; however, among Ahrb1_Cyp1a2(+/+) mice, PCB-treated animals were again significantly more active than controls (see Supplemental Material, Figure S2A). After methamphetamine, mice in all groups showed the typical pattern of hyperactivity. No significant differences as a function of PCB treatment were seen among the Ahrd_Cyp1a2(+/+) mice (see Supplemental Material, Figure S2B). Among Ahrb1_Cyp1a2(–/–) mice (see Supplemental Material, Figure S2C), we observed small differences in the PCB-treated group compared with controls. Because of differences in predrug activity in PCB-treated Ahrb1_Cyp1a2(+/+) mice (see Supplemental Material, Figure S2A, left), the postchallenge analysis used the last 10 min of the prechallenge data as a covariate; ANCOVA indicated only one significant PCB-related difference (during the 80-min test interval; data not shown).
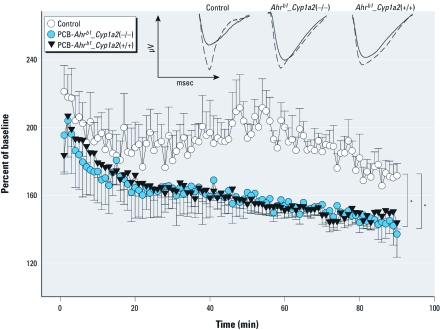
LTP. Another way to examine whether PCB treatment alters neuroplasticity is LTP induction. LTP in the CA1 region of the hippocampus is a cellular correlate of spatial learning and memory (Pavlides et al. 1991). We restricted our analysis to Ahrb1_Cyp1a2(+/+) vehicle-treated controls and the two PCB-treated groups that showed the greatest difference in MWM tests compared with control wild-type, PCB-treated Ahrb1_Cyp1a2(–/–) and PCB-treated Ahrb1_Cyp1a2(+/+) mice. In the presence or absence of CYP1A2, PCB treatment significantly impaired LTP in the CA1 region compared with Ahrb1_Cyp1a2(+/+) controls (Figure 6), confirming that PCB treatment early in life alters neuroplasticity irrespective of the Cyp1a2 genotype.
Figure 6.
LTP results shown as the percentage of baseline (mean ± SE). The inset shows the EPSP slope in CA1 region slices; the solid line represents the baseline response, and the dashed line represents the response 30 min following tetanus [prolonged membrane response to the electrical stimulation; 100 Hz in 10 bursts (four pulses per burst) delivered at a burst frequency of 5 Hz for 2 sec]. No additional units are possible on the axes because the absolute amplitudes and times vary because of the different specific cells being measured (graphs in the inset represent the wave form, not the exact values of any given curve). No differences between the groups were seen within treatments for response to stimulus or baseline response (n = 6, with all mice originating from separate litters). ANOVA showed an effect of group (treatment/genotype) [F(2, 13) = 4.87; p < 0.05] and no group × time interaction. Dunnett tests comparing PCB-treated groups with controls showed that both the Ahrb1_Cyp1a2(+/+) and Ahrb1_Cyp1a2(–/–) PCB-treated groups exhibited significantly decreased LTP induction compared with untreated Ahrb1_Cyp1a2(+/+) controls. *p < 0.05 compared with control averaged across time.
Monoamine neurotransmitter levels. We observed no differences in monoamine levels in hippocampus or prefrontal cortex among genotypes, regardless of treatment. In the neostriatum, DA levels were lower in PCB-treated versus control Ahrd_Cyp1a2(+/+) mice (p < 0.01); DA levels were significantly lower in PCB-treated Ahrb1_Cyp1a2(–/–) than in PCB-treated Ahrb1_Cyp1a2(+/+) mice (p < 0.01). For the latter, DA levels were lower in PCB-treated than in control Ahrd_Cyp1a2(+/+) mice (p < 0.01); DA levels were significantly lower in PCB-treated Ahrb1_Cyp1a2(–/–) than in PCB-treated Ahrb1_Cyp1a2(+/+) mice (p < 0.01; data available upon request).
We observed no structural abnormalities or differences in size, shape, or appearance of the hippocampus, prefrontal cortex, or neostriatum in hematoxylin-and-eosin–stained sections (data not shown).
Plasma corticosterone levels. No PCB-related differences were found in plasma corticosterone levels (data not shown).
Discussion
Since the earliest report of cognitive dysfunction in Yusho and Yu-Cheng PCB poison victims (Abe et al. 1975), there have been attempts to identify the individual congeners responsible for various toxic end points reported, including neurotoxicity (Ryan et al. 1990). The controversy remains unsettled: Some argue that only noncoplanar PCBs have neurotoxic effects (Rice 1999), whereas others report neurotoxic effects after exposure to coplanar PCBs, dioxins, and related AHR ligands (Amin et al. 2000; Seegal et al. 2005).
Others have used commercial mixtures (Branchi et al. 2005; Chishti et al. 1996) or laboratory-developed mixtures (Hamm et al. 2003; Kostyniak et al. 2005). However, even a mixture having the same name (e.g., Aroclor 1254) and expected chemical composition can vary from batch to batch (Kodavanti et al. 2001). The controversy is best summed up in a review by Ulbrich and Stahlmann (2004), in which the authors documented attempts to model PCB-induced neurotoxicity in rodents. Wide variation in dosing concentrations, routes of administration, and animal models has resulted in variability and results that are not always reproducible.
Data from the present study suggest that both AHR and CYP1A2 play important roles in response to an environmentally relevant mixture of PCB congeners (three coplanar and five noncoplanar). The presence of high-affinity AHR decreases the amount of exposure of offspring to coplanar congeners via inducible P450-mediated detoxication pathways (Nebert et al. 2004), whereas maternal CYP1A2 sequesters coplanar PCBs, thereby diminishing the level of their exposure (Dragin et al. 2006).
Our behavioral phenotyping uncovered significant differences at many levels involving the effects of developmental PCB exposure associated with the Ahr and Cyp1a2 genotypes. Genetic background influences behavior (Crawley et al. 1997; Jacobson and Cryan 2007), but differences in genetic background were decreased as contributing factors in the present study because all lines were backcrossed at least eight generations into B6 mice. Nonetheless, we found significant genotype effects independent of treatment for several tests, including the elevated zero maze, locomotor activity, ASR, and MWM. For example, among controls, Ahrb1_Cyp1a2(+/+) mice showed fewer zone crossings in the elevated zero maze than did mice of the other genotypes (Figure 1C). In addition, Ahrb1_Cyp1a2(–/–) control mice exhibited longer latencies in the MWM (Figure 3) and higher overall levels of locomotor activity (Figure 2A) compared with controls of the other genotypes.
Effects of PCB were evident in all tests, reinforcing what had been previously reported with PCB congeners. Interestingly, PCB-treated Ahrb1_Cyp1a2(+/+) mice showed improved MWM performance in reversal (Figure 4) and reversal probe (Figure 5), suggesting that coplanar-PCB–mediated AHR activation can have beneficial effects on selected aspects of learning. Previous studies have shown that rats exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) or coplanar PCBs make fewer errors in the radial-arm maze (Schantz et al. 1996; Seo et al. 1999; Widholm 2003). The results in the present study support the finding that AHR plays an important role in mammalian CNS development. Indeed, even with no apparent ligand-binding properties, AHR analogs in Caenorhabditis elegans (Qin and Powell-Coffman 2004) and Drosophila (Crews and Brenman 2006) have been demonstrated to be associated with neuronal development.
The behavioral deficits that we observed in the present study are consistent with the hypothesis that maternal hepatic CYP1A2 protects against PCB-induced developmental neurotoxicity in offspring. In a previous study, Dragin et al. (2006) reported that maternal hepatic CYP1A2 and maternal hepatic human CYP1A2 (in place of the mouse analogous protein) provided protection from TCDD-induced cleft palate and hydronephrosis, and that absence of maternal CYP1A2 increased sensitivity to TCDD-induced birth defects. Another study (Curran et al. 2011) showed that maternal hepatic CYP1A2 protected offspring of mothers that received the same PCB mixture used in the present study from PCB-induced toxicity.
Only PCB-treated Ahrb1_Cyp1a2(–/–) mice showed impairment in novel object recognition (Figure 1D). These data are consistent with human studies (Jacobson et al. 1985; Kilburn 2000), one of which showed deficits on the Fagan test of novel object recognition (Jacobson et al. 1985). As noted above, all human populations display a > 60-fold gradient ranging from low to high CYP1A2 basal levels; however, no human study has specifically assessed CYP1A2 phenotype as a risk factor for PCB neurotoxicity. During MWM testing, we also uncovered spatial learning and memory deficits associated with PCB exposure. PCB-treated Ahrb1_Cyp1a2(–/–) mice took longer to learn the cued platform than did untreated controls (Figure 3) but only on days 2 and 3, indicating that effects were not a result of treatment-related visual impairment that might interfere with spatial learning during hidden platform testing. PCB-treated Ahrb1_Cyp1a2(–/–) mice showed deficits in all three phases of hidden-platform testing (Figures 4 and 5). These deficits were more pronounced as the difficulty of the task increased, but some differences between treated and control mice were reduced when we adjusted the cumulative distance parameter for swim speed. This occurred despite the fact that the cumulative distance parameter is less affected by swim speed than is latency (time needed to find platform), suggesting that covariate analysis (with swim speed as the covariate) may have overadjusted this index, perhaps because swim speed and learning were affected simultaneously, making the separation of the two factors imperfect. Most important, the failure rate in PCB-treated Ahrb1_Cyp1a2(–/–) mice was significantly higher than in all other groups, arguing against an effect mediated by swim speed alone.
In addition, relative to controls, the baseline ASR was lower in PCB-exposed Ahrb1_Cyp1a2(–/–) and Ahrb1_Cyp1a2(+/+) mice but not in Ahrd_Cyp1a2(+/+) mice, indicating that both AHR and CYP1A2 are critical for proper development of this defensive reflex. In a study of Long-Evans rats, Goldey et al. (1995) reported that developmental Aroclor 1254 exposure through PND21 reduced ASR at PND24 but not during adulthood. In a follow-up experiment, a decreased ASR at PND23 was replicated, but the ASR was increased in the adult offspring (Goldey and Crofton 1998). In a more recent study, developmental PCB153 exposure did not affect ASR in Wistar rats (Gralewicz et al. 2009).
Our finding that PCB alters LTP is consistent with previous findings. We found impaired LTP in the hippocampus (Figure 6), as have others (Altmann et al. 2001; Carpenter et al. 2002; Gilbert and Crofton 1999; Gilbert et al. 2000), which suggests that this region is particularly vulnerable to developmental PCB exposure.
Alterations in DA in the neostriatum found in the present study have also been reported previously. Coplanar PCB congeners appear to increase DA, whereas noncoplanar congeners lead to decreased DA levels (Seegal et al. 1997).
Conclusion
Developmental exposure to an environmentally relevant mixture of coplanar and noncoplanar PCBs was associated with learning and memory deficits in genetically susceptible Ahrb1_Cyp1a2(–/–) mice; in mice having normal basal and inducible CYP1A2 expression, these effects were significantly decreased. In addition, developmental exposure to AHR agonists appears to improve spatial learning and memory in Ahrb1_Cyp1a2(+/+) mice in the presence of maternal CYP1A2.
We have generated a novel mouse model for studying genetic susceptibility to PCB-induced neurotoxicity, which is relevant to at-risk human populations. As noted above, humans display > 12-fold variability in AHR affinity and > 60-fold differences in hepatic CYP1A2 basal levels (Nebert et al. 2004). This means that a highly exposed mother with genetic resistance (i.e., high levels of hepatic CYP1A2, both basal and PCB induced) might have a normal child, whereas a less exposed mother who is genetically susceptible (with low levels of hepatic CYP1A2, both basal and PCB induced) could have a child with developmental delays despite lower PCB exposure. AHR inducibility influences both hepatic levels of CYP1A2 and clearance of lower-molecular-weight planar and non coplanar PCBs. Therefore, ultimately, the risk of PCB-induced neurotoxicity must account for both CYP1A2 and AHR variability.
Supplemental Material
Acknowledgments
We thank M. Moran for statistical help.
Footnotes
This work was supported by grants P30-ES006096 (D.W.N.), T32-DK059803 (C.P.C.), T32-ES007051 (C.P.C., T.L.S.), R01-ES008147 (D.W.N.), R01-ES014403 (D.W.N.), and R21-ES015335 (D.W.N., C.V.V., M.T.W.) from the National Institutes of Health.
The authors declare they have no actual or potential competing financial interests.
References
- Abe S, Inoue Y, Takamatsu M. Polychlorinated biphenyl residues in plasma of Yusho children born to mothers who had consumed oil contaminated by PCB. Fukuoka Igaku Zasshi. 1975;66:605–609. [in Japanese] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2007. CERCLA Priority List of Hazardous Substances. [Google Scholar]
- Altmann L, Mundy WR, Ward TR, Fastabend A, Lilienthal H. Developmental exposure of rats to a reconstituted PCB mixture or Aroclor 1254: effects on long-term potentiation and [3H]MK-801 binding in occipital cortex and hippocampus. Toxicol Sci. 2001;61:321–330. doi: 10.1093/toxsci/61.2.321. [DOI] [PubMed] [Google Scholar]
- Amin S, Moore RW, Peterson RE, Schantz SL. Gestational and lactational exposure to TCDD or coplanar PCBs alters adult expression of saccharin preference behavior in female rats. Neurotoxicol Teratol. 2000;22:675–682. doi: 10.1016/s0892-0362(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Branchi I, Capone F, Vitalone A, Madia F, Santucci D, Alleva E, et al. Early developmental exposure to BDE 99 or Aroclor 1254 affects neurobehavioural profile: interference from the administration route. Neurotoxicology. 2005;26:183–192. doi: 10.1016/j.neuro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, Hussain RJ, Berger DF, Lombardo JP, Park HY. Electrophysiologic and behavioral effects of perinatal and acute exposure of rats to lead and polychlorinated biphenyls. Environ Health Perspect. 2002;110(suppl 3):377–386. doi: 10.1289/ehp.02110s3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chishti MA, Fisher JP, Seegal RF. Aroclors 1254 and 1260 reduce dopamine concentrations in rat striatal slices. Neurotoxicology. 1996;17:653–660. [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Crews ST, Brenman JE. Spineless provides a little backbone for dendritic morphogenesis. Genes Dev. 2006;20:2773–2778. doi: 10.1101/gad.1487706. [DOI] [PubMed] [Google Scholar]
- Curran CP, Miller KA, Dalton TP, Vorhees CV, Miller ML, Shertzer HG, et al. Genetic differences in lethality of newborn mice treated in utero with coplanar versus non-coplanar hexabromobiphenyl. Toxicol Sci. 2006;89:454–464. doi: 10.1093/toxsci/kfj048. [DOI] [PubMed] [Google Scholar]
- Curran CP, Vorhees CV, Williams MT, Genter MB, Miller ML, Nebert DW. In utero and lactational exposure to a complex mixture of polychlorinated biphenyls: toxicity in pups dependent on the Cyp1a2 and Ahr genotypes. Toxicol Sci. 2011;119:189–208. doi: 10.1093/toxsci/kfq314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragin N, Dalton TP, Miller ML, Shertzer HG, Nebert DW. For dioxin-induced birth defects, mouse or human CYP1A2 in maternal liver protects whereas mouse CYP1A1 and CYP1B1 are inconsequential. J Biol Chem. 2006;281:18591–18600. doi: 10.1074/jbc.M601159200. [DOI] [PubMed] [Google Scholar]
- Eriksson P. Developmental neurotoxicity of environmental agents in the neonate. Neurotoxicology. 1997;18:719–726. [PubMed] [Google Scholar]
- Gilbert ME, Crofton KM. Developmental exposure to a commercial PCB mixture (Aroclor 1254) produces a persistent impairment in long-term potentiation in the rat dentate gyrus in vivo. Brain Res. 1999;850:87–95. doi: 10.1016/s0006-8993(99)02107-1. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mundy WR, Crofton KM. Spatial learning and long-term potentiation in the dentate gyrus of the hippocampus in animals developmentally exposed to Aroclor 1254. Toxicol Sci. 2000;57:102–111. doi: 10.1093/toxsci/57.1.102. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Crofton KM. Thyroxine replacement attenuates hypothyroxemia, hearing loss, and motor deficits following developmental exposure to Aroclor 1254 in rats. Toxicol Sci. 1998;45:94–105. doi: 10.1006/toxs.1998.2495. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol. 1995;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- Graham DL, Grace CE, Braun AA, Schaefer TL, Skelton MR, Tang PH, et al. Effects of developmental stress and lead (Pb) on corticosterone after chronic and acute stress, brain monoamines and blood Pb levels. Int J Dev Neurosci. 2011;29(1):45–55. doi: 10.1016/j.ijdevneu.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralewicz S, Wiaderna D, Lutz P, Sitarek K. Neurobehavioral functions in adult progeny of rat mothers exposed to methylmercury or 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB 153) alone or their combination during gestation and lactation. Int J Occup Med Environ Health. 2009;22:277–291. doi: 10.2478/v10001-009-0020-9. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Burse VW, Needham LL, Storr-Hansen E, Heinzow B, et al. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol Teratol. 2001;23:305–317. doi: 10.1016/s0892-0362(01)00155-6. [DOI] [PubMed] [Google Scholar]
- Guo YL, Lambert GH, Hsu CC, Hsu MM. Yucheng: health effects of prenatal exposure to polychlorinated biphenyls and dibenzofurans. Int Arch Occup Environ Health. 2004;77:153–158. doi: 10.1007/s00420-003-0487-9. [DOI] [PubMed] [Google Scholar]
- Guo YL, Ryan JJ, Lau BP, Yu ML, Hsu CC. Blood serum levels of PCBs and PCDFs in Yucheng women 14 years after exposure to a toxic rice oil. Arch Environ Contam Toxicol. 1997;33:104–108. doi: 10.1007/s002449900230. [DOI] [PubMed] [Google Scholar]
- Gustavsson P, Hogstedt C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs). Am J Ind Med. 1997;32(3):234–239. doi: 10.1002/(sici)1097-0274(199709)32:3<234::aid-ajim8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Hamm JT, Chen CY, Birnbaum LS. A mixture of dioxins, furans, and non-ortho PCBs based upon consensus toxic equivalency factors produces dioxin-like reproductive effects. Toxicol Sci. 2003;74:182–191. doi: 10.1093/toxsci/kfg107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwe JK, Larsen GL. Polychlorinated dioxins, furans, and biphenyls, and polybrominated diphenyl ethers in a U.S. meat market basket and estimates of dietary intake. Environ Sci Technol. 2005;39:5606–5611. doi: 10.1021/es050638g. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Evidence for PCBs as neurodevelopmental toxicants in humans. Neurotoxicology. 1997;18:415–424. [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J Pediatr. 2003;143:780–788. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav Genet. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, Dowler JK. The effect of intrauterine PCB exposure on visual recognition memory. Child Dev. 1985;56:853–860. [PubMed] [Google Scholar]
- Kilburn KH. Visual and neurobehavioral impairment associated with polychlorinated biphenyls. Neurotoxicology. 2000;21:489–499. [PubMed] [Google Scholar]
- Kodavanti PR, Kannan N, Yamashita N, Derr-Yellin EC, Ward TR, Burgin DE, et al. Differential effects of two lots of Aroclor 1254: congener-specific analysis and neurochemical end points. Environ Health Perspect. 2001;109:1153–1161. doi: 10.1289/ehp.011091153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, et al. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol Sci. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Liang HC, Li H, McKinnon RA, Duffy JJ, Potter SS, Puga A, et al. Cyp1a2(–/–) null mutant mice develop normally but show deficient drug metabolism. Proc Natl Acad Sci USA. 1996;93:1671–1676. doi: 10.1073/pnas.93.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K, Suzuki K, Oka T, Murata K, Sakamoto M, Okamura K, et al. The Tohoku Study of Child Development: a cohort study of effects of perinatal exposures to methylmercury and environmentally persistent organic pollutants on neurobehavioral development in Japanese children. Tohoku J Exp Med. 2004;202:227–237. doi: 10.1620/tjem.202.227. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Westlind-Danielsson AI, Nyborg H, McEwen BS. Neonatal hyperthyroidism disrupts hippocampal LTP and spatial learning. Exp Brain Res. 1991;85:559–564. doi: 10.1007/BF00231740. [DOI] [PubMed] [Google Scholar]
- Petersen MS, Halling J, Bech S, Wermuth L, Weihe P, Nielsen F, et al. Impact of dietary exposure to food contaminants on the risk of Parkinson disease. Neurotoxicology. 2008;29:584–590. doi: 10.1016/j.neuro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Poland A, Glover E. Chlorinated biphenyl induction of aryl hydrocarbon hydroxylase activity: a study of the structure-activity relationship. Mol Pharmacol. 1977;13:924–938. [PubMed] [Google Scholar]
- Qin H, Powell-Coffman JA. The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Dev Biol. 2004;270:64–75. doi: 10.1016/j.ydbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Rice DC. Effect of exposure to 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) throughout gestation and lactation on development and spatial delayed alternation performance in rats. Neurotoxicol Teratol. 1999;21:59–69. doi: 10.1016/s0892-0362(98)00031-2. [DOI] [PubMed] [Google Scholar]
- Rice DC. Parallels between attention deficit hyperactivity disorder and behavioral deficits produced by neurotoxic exposure in monkeys. Environ Health Perspect. 2000;108(suppl 3):405–408. doi: 10.1289/ehp.00108s3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegge CS, Schantz SL. Motor function following developmental exposure to PCBS and/or MEHG. Neurotoxicol Teratol. 2006;28:260–277. doi: 10.1016/j.ntt.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Gasiewicz TA, Brown JF., Jr Human body burden of polychlorinated dibenzofurans associated with toxicity based on the Yusho and Yucheng incidents. Fundam Appl Toxicol. 1990;15:722–731. doi: 10.1016/0272-0590(90)90188-p. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Gasior DM, Polverejan E, McCaffrey RJ, Sweeney AM, Humphrey HE, et al. Impairments of memory and learning in older adults exposed to polychlorinated biphenyls via consumption of Great Lakes fish. Environ Health Perspect. 2001;109:605–611. doi: 10.1289/ehp.01109605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Levin ED, Bowman RE, Heironimus MP, Laughlin NK. Effects of perinatal PCB exposure on discrimination-reversal learning in monkeys. Neurotoxicol Teratol. 1989;11:243–250. doi: 10.1016/0892-0362(89)90066-4. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Seo BW, Moshtaghian J, Peterson RE, Moore RW. Effects of gestational and lactational exposure to TCDD or coplanar PCBs on spatial learning. Neurotoxicol Teratol. 1996;18:305–313. doi: 10.1016/s0892-0362(96)90033-1. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect. 2003;111:357–576. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, Brosch KO, Okoniewski RJ. Effects of in utero and lactational exposure of the laboratory rat to 2,4,2’,4’- and 3,4,3’,4’-tetrachlorobiphenyl on dopamine function. Toxicol Appl Pharmacol. 1997;146:95–103. doi: 10.1006/taap.1997.8226. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Brosch KO, Okoniewski RJ. Coplanar PCB congeners increase uterine weight and frontal cortical dopamine in the developing rat: implications for developmental neurotoxicity. Toxicol Sci. 2005;86:125–131. doi: 10.1093/toxsci/kfi174. [DOI] [PubMed] [Google Scholar]
- Seo BW, Sparks AJ, Medora K, Amin S, Schantz SL. Learning and memory in rats gestationally and lactationally exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Neurotoxicol Teratol. 1999;21:231–239. doi: 10.1016/s0892-0362(98)00049-x. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewl SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacol (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Shimono K, Baudry M, Ho L, Taketani M, Lynch G. Long-term recording of LTP in cultured hippocampal slices. Neural Plast. 2002;9:249–254. doi: 10.1155/NP.2002.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich B, Stahlmann R. Developmental toxicity of polychlorinated biphenyls (PCBs): a systematic review of experimental data. Arch Toxicol. 2004;78:252–268. doi: 10.1007/s00204-003-0519-y. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil HJ, Mulder PG, Emmen HH, Weisglas-Kuperus N. Effects of perinatal exposure to PCBs on neuropsychological functions in the Rotterdam cohort at 9 years of age. Neuropsychology. 2004;18:185–193. doi: 10.1037/0894-4105.18.1.185. [DOI] [PubMed] [Google Scholar]
- Widholm JJ. Effects of perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on spatial and visual reversal learning in rats. Neurotoxicol Teratol. 2003;25:459–471. doi: 10.1016/s0892-0362(03)00014-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.