Abstract
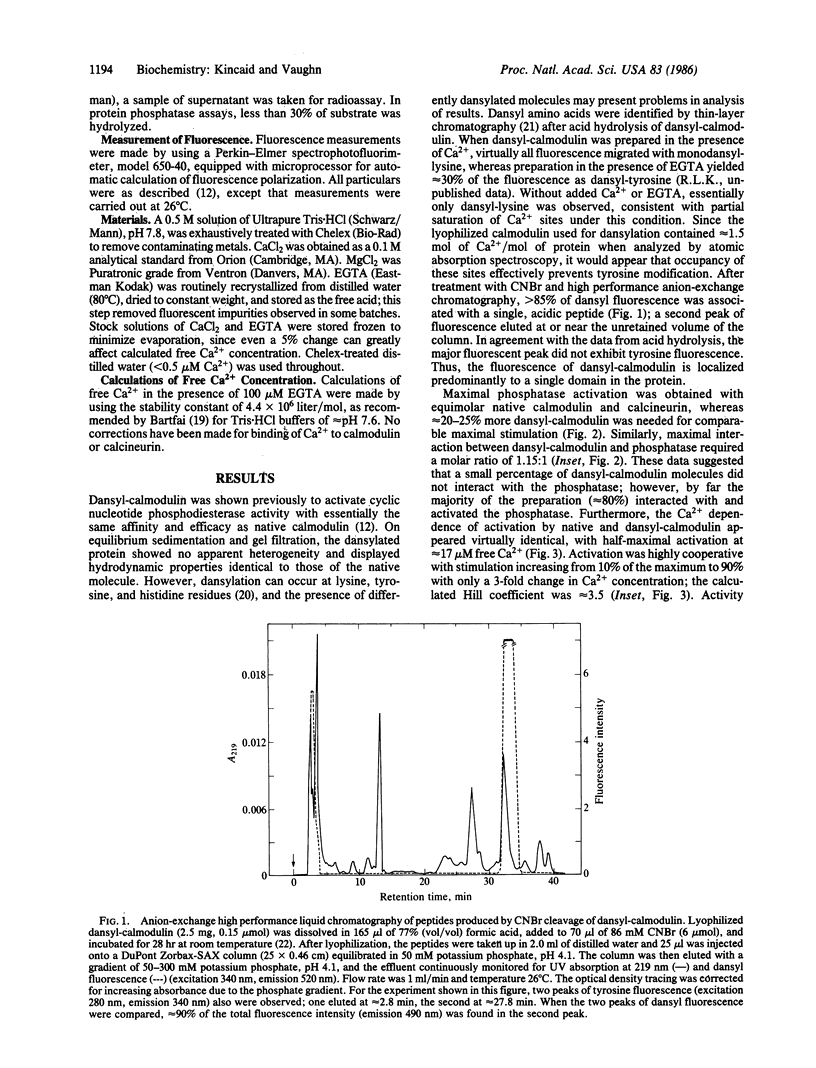
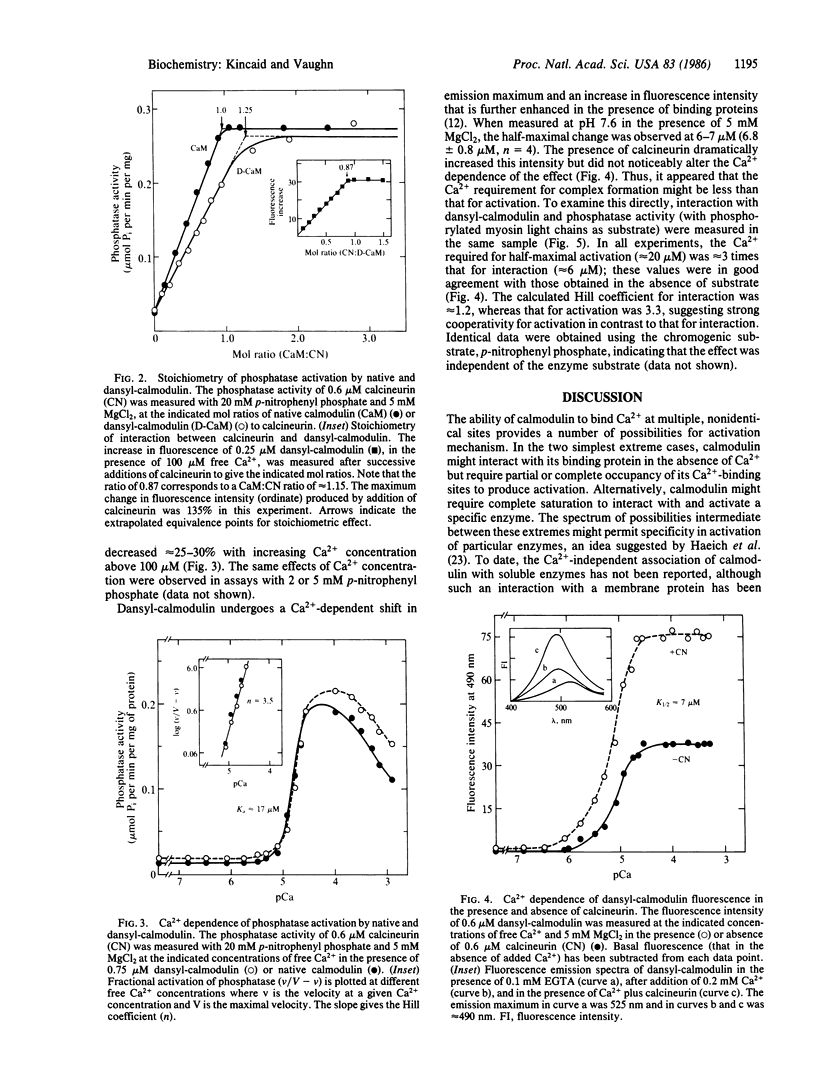
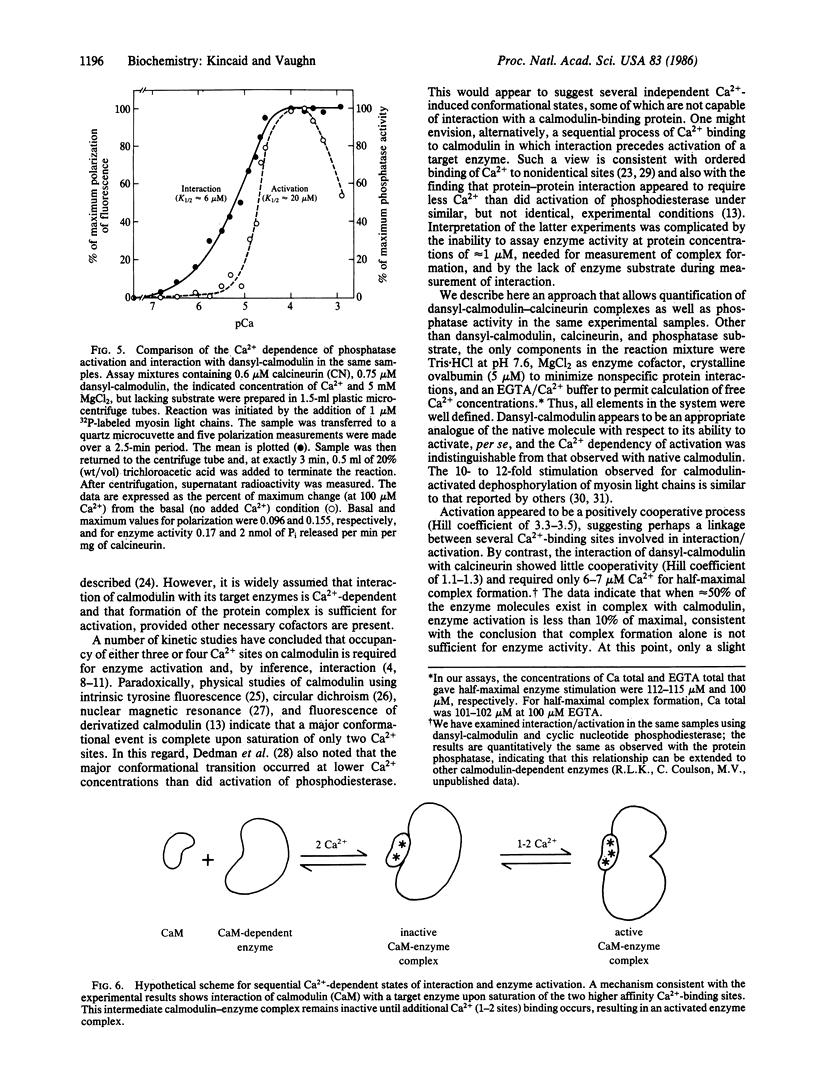
The mechanism of Ca2+-dependent protein-protein interaction and enzyme activation by calmodulin was investigated with the phosphoprotein phosphatase, calcineurin. Dimethylaminonaphthalene (dansyl)-calmodulin, a fluorescent derivative used to monitor complex formation, produced similar maximal activation (10- to 12-fold) with a Ca2+ dependence (Ka = 17 microM) identical to that of native calmodulin. The Ca2+-dependent increase in fluorescence intensity of dansyl-calmodulin was enhanced 100-150% by calcineurin, indicating complex formation; the concentration of Ca2+ required for a half-maximal increase in fluorescence was the same (K1/2 approximately equal to 7 microM) with and without calcineurin. Since the Ca2+ concentration required for activation appeared to differ from that necessary for protein-protein interaction, a method was devised to measure both the formation of complexes between dansyl-calmodulin and calcineurin and enzyme activity in the same samples. Direct comparison of interaction (measured by polarization of fluorescence) and enzyme activity demonstrated different Ca2+ requirements for the two events. Whereas dansyl-calmodulin-calcineurin interaction, measured in the presence of phosphoprotein substrate, exhibited very little cooperativity (Hill coefficient = 1.2, Ca2+ concentration required for the half-maximal increase in fluorescence, K1/2, approximately equal to 6 microM), phosphatase activation was highly cooperative (Hill coefficient = 3.5) and required 3 times higher Ca2+ concentration for half-maximal stimulation. Equivalent results were obtained with p-nitrophenyl phosphate as substrate. These data are consistent with a sequential mechanism for interaction and activation wherein filling of perhaps two Ca2+ sites permits calmodulin interaction with the phosphatase; this complex is inactive, requiring further binding of Ca2+ for activation. Such a scheme would provide a sensitive switch for control of enzyme activity within a narrow range of free Ca2+ concentration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen T. J., Luetje C. W., Heideman W., Storm D. R. Purification of a novel calmodulin binding protein from bovine cerebral cortex membranes. Biochemistry. 1983 Sep 27;22(20):4615–4618. doi: 10.1021/bi00289a001. [DOI] [PubMed] [Google Scholar]
- Bartfai T. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:219–242. [PubMed] [Google Scholar]
- Blumenthal D. K., Stull J. T. Effects of pH, ionic strength, and temperature on activation by calmodulin an catalytic activity of myosin light chain kinase. Biochemistry. 1982 May 11;21(10):2386–2391. doi: 10.1021/bi00539a017. [DOI] [PubMed] [Google Scholar]
- Bruton C. J., Hartley B. S. Chemical studies on methionyl-tRNA synthetase from Escherichia coli. J Mol Biol. 1970 Sep 14;52(2):165–178. doi: 10.1016/0022-2836(70)90023-9. [DOI] [PubMed] [Google Scholar]
- Burger D., Stein E. A., Cox J. A. Free energy coupling in the interactions between Ca2+, calmodulin, and phosphorylase kinase. J Biol Chem. 1983 Dec 10;258(23):14733–14739. [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cox J. A., Comte M., Stein E. A. Activation of human erythrocyte Ca2+-dependent Mg2+-activated ATPase by calmodulin and calcium: quantitative analysis. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4265–4269. doi: 10.1073/pnas.79.14.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. A., Malnoë A., Stein E. A. Regulation of brain cyclic nucleotide phosphodiesterase by calmodulin. A quantitative analysis. J Biol Chem. 1981 Apr 10;256(7):3218–3222. [PubMed] [Google Scholar]
- Crouch T. H., Klee C. B. Positive cooperative binding of calcium to bovine brain calmodulin. Biochemistry. 1980 Aug 5;19(16):3692–3698. doi: 10.1021/bi00557a009. [DOI] [PubMed] [Google Scholar]
- Dedman J. R., Potter J. D., Jackson R. L., Johnson J. D., Means A. R. Physicochemical properties of rat testis Ca2+-dependent regulator protein of cyclic nucleotide phosphodiesterase. Relationship of Ca2+-binding, conformational changes, and phosphodiesterase activity. J Biol Chem. 1977 Dec 10;252(23):8415–8422. [PubMed] [Google Scholar]
- HARTLEY B. S., MASSEY V. The active centre of chymotrypsin. I. Labelling with a fluorescent dye. Biochim Biophys Acta. 1956 Jul;21(1):58–70. doi: 10.1016/0006-3002(56)90093-2. [DOI] [PubMed] [Google Scholar]
- Haiech J., Klee C. B., Demaille J. G. Effects of cations on affinity of calmodulin for calcium: ordered binding of calcium ions allows the specific activation of calmodulin-stimulated enzymes. Biochemistry. 1981 Jun 23;20(13):3890–3897. doi: 10.1021/bi00516a035. [DOI] [PubMed] [Google Scholar]
- Huang C. Y., Chau V., Chock P. B., Wang J. H., Sharma R. K. Mechanism of activation of cyclic nucleotide phosphodiesterase: requirement of the binding of four Ca2+ to calmodulin for activation. Proc Natl Acad Sci U S A. 1981 Feb;78(2):871–874. doi: 10.1073/pnas.78.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson G. A., Jr, Bronson D. D., Schachat F. H., Vanaman T. C. Structure and function relationships among calmodulins and troponin C-like proteins from divergent eukaryotic organisms. Ann N Y Acad Sci. 1980;356:1–13. doi: 10.1111/j.1749-6632.1980.tb29593.x. [DOI] [PubMed] [Google Scholar]
- Keller C. H., Olwin B. B., LaPorte D. C., Storm D. R. Determination of the free-energy coupling for binding of calcium ions and troponin I to calmodulin. Biochemistry. 1982 Jan 5;21(1):156–162. doi: 10.1021/bi00530a027. [DOI] [PubMed] [Google Scholar]
- Kilhoffer M. C., Demaille J. G., Gérard D. Tyrosine fluorescence of ram testis and octopus calmodulins. Effects of calcium, magnesium, and ionic strength. Biochemistry. 1981 Jul 21;20(15):4407–4414. doi: 10.1021/bi00518a027. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L., Danello M. A., Osborne J. C., Jr, Tkachuk V. A., Vaughan M. Calcium-dependent interaction of dansyl-calmodulin and phosphodiesterase: relationship to Ca2+ requirement for enzyme activation. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:77–87. [PubMed] [Google Scholar]
- Kincaid R. L., Manganiello V. C., Odya C. E., Osborne J. C., Jr, Stith-Coleman I. E., Danello M. A., Vaughan M. Purification and properties of calmodulin-stimulated phosphodiesterase from mammalian brain. J Biol Chem. 1984 Apr 25;259(8):5158–5166. [PubMed] [Google Scholar]
- Kincaid R. L., Vaughan M., Osborne J. C., Jr, Tkachuk V. A. Ca2+-dependent interaction of 5-dimethylaminonaphthalene-1-sulfonyl-calmodulin with cyclic nucleotide phosphodiesterase, calcineurin, and troponin I. J Biol Chem. 1982 Sep 25;257(18):10638–10643. [PubMed] [Google Scholar]
- Klee C. B. Conformational transition accompanying the binding of Ca2+ to the protein activator of 3',5'-cyclic adenosine monophosphate phosphodiesterase. Biochemistry. 1977 Mar 8;16(5):1017–1024. doi: 10.1021/bi00624a033. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Binding of simple peptides, hormones, and neurotransmitters by calmodulin. Biochemistry. 1982 Jul 6;21(14):3480–3486. doi: 10.1021/bi00257a035. [DOI] [PubMed] [Google Scholar]
- Manalan A. S., Klee C. B. Activation of calcineurin by limited proteolysis. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4291–4295. doi: 10.1073/pnas.80.14.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallen C. J., Wang J. H. Calmodulin-stimulated dephosphorylation of p-nitrophenyl phosphate and free phosphotyrosine by calcineurin. J Biol Chem. 1983 Jul 25;258(14):8550–8553. [PubMed] [Google Scholar]
- Pato M. D., Adelstein R. S. Dephosphorylation of the 20,000-dalton light chain of myosin by two different phosphatases from smooth muscle. J Biol Chem. 1980 Jul 25;255(14):6535–6538. [PubMed] [Google Scholar]
- Seamon K. B. Calcium- and magnesium-dependent conformational states of calmodulin as determined by nuclear magnetic resonance. Biochemistry. 1980 Jan 8;19(1):207–215. doi: 10.1021/bi00542a031. [DOI] [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Manalan A., Klee C. B., Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80). FEBS Lett. 1982 Jan 11;137(1):80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A., Johnson E. A. Thermodynamic and kinetic cooperativity in ligand binding to multiple sites on a protein: Ca2+ activation of an ATP-driven Ca pump. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4688–4692. doi: 10.1073/pnas.82.14.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaman T. C., Wakil S. J., Hill R. L. The preparation of tryptic, peptic, thermolysin, and cyanogen bromide peptides from the acyl carrier protein of Escherichia coli. J Biol Chem. 1968 Dec 25;243(24):6409–6419. [PubMed] [Google Scholar]
- Wallace R. W., Tallant E. A., Dockter M. E., Cheung W. Y. Calcium binding domains of calmodulin. Sequence of fill as determined with terbium luminescence. J Biol Chem. 1982 Feb 25;257(4):1845–1854. [PubMed] [Google Scholar]



