Abstract
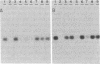
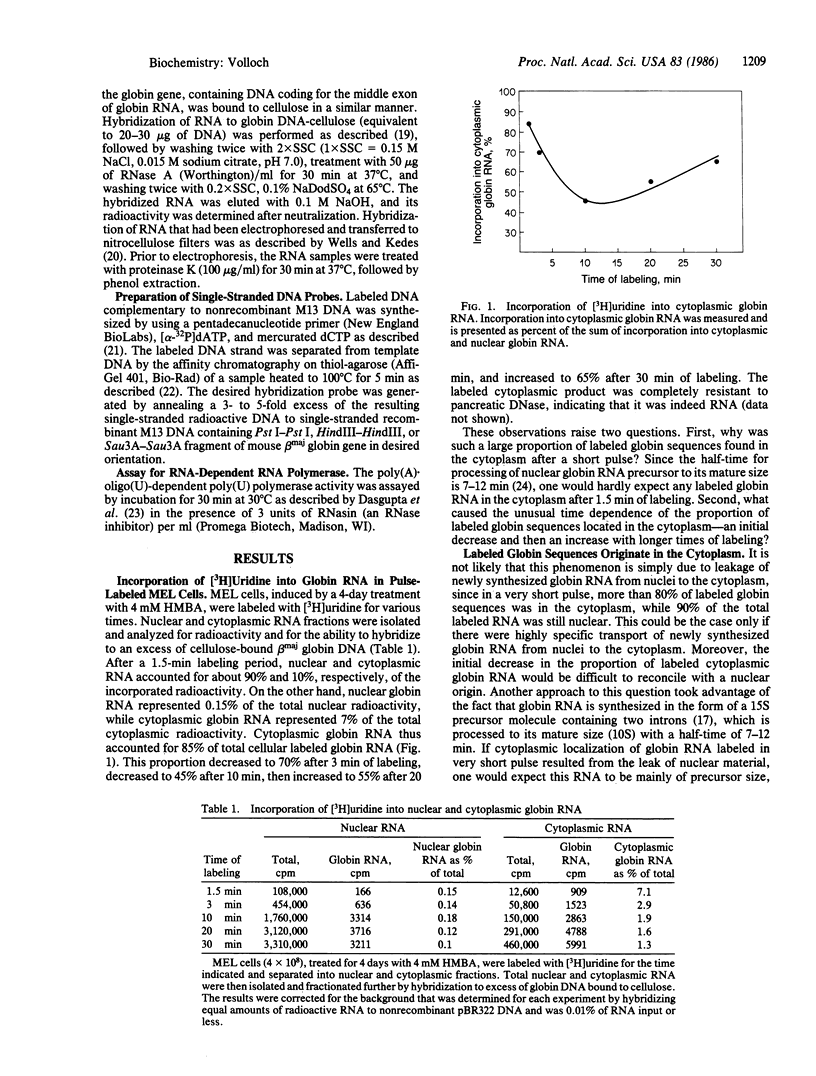
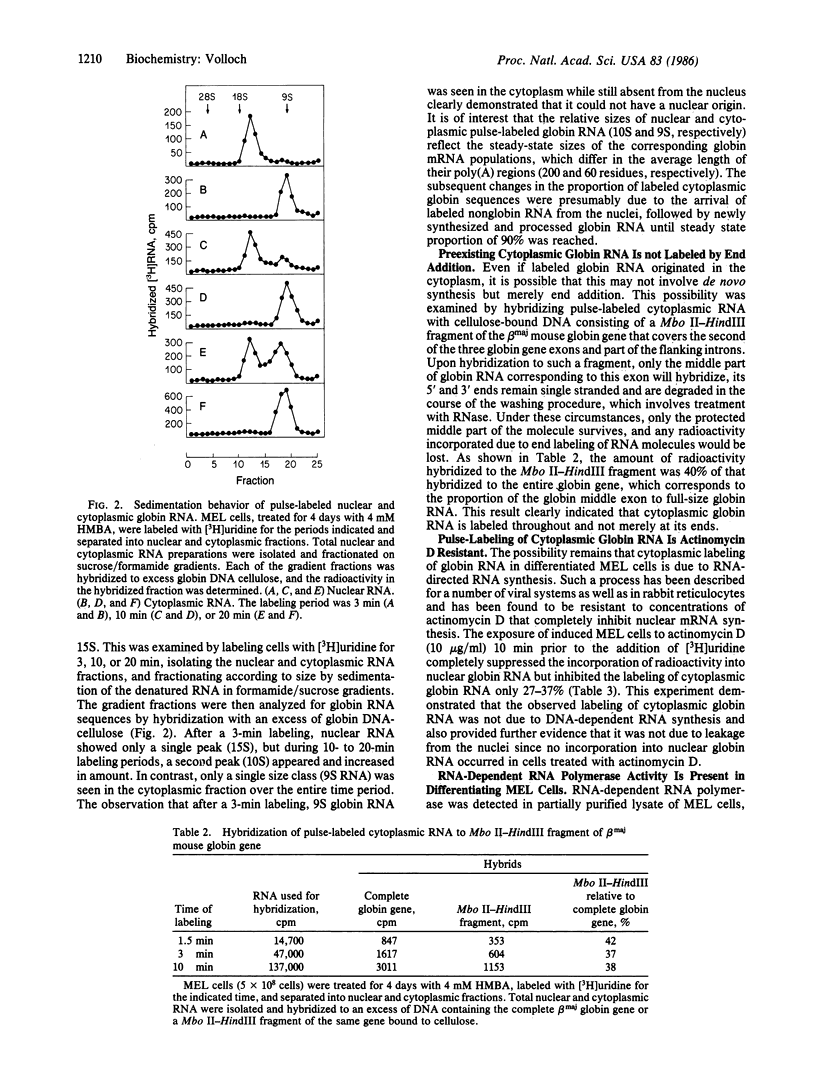
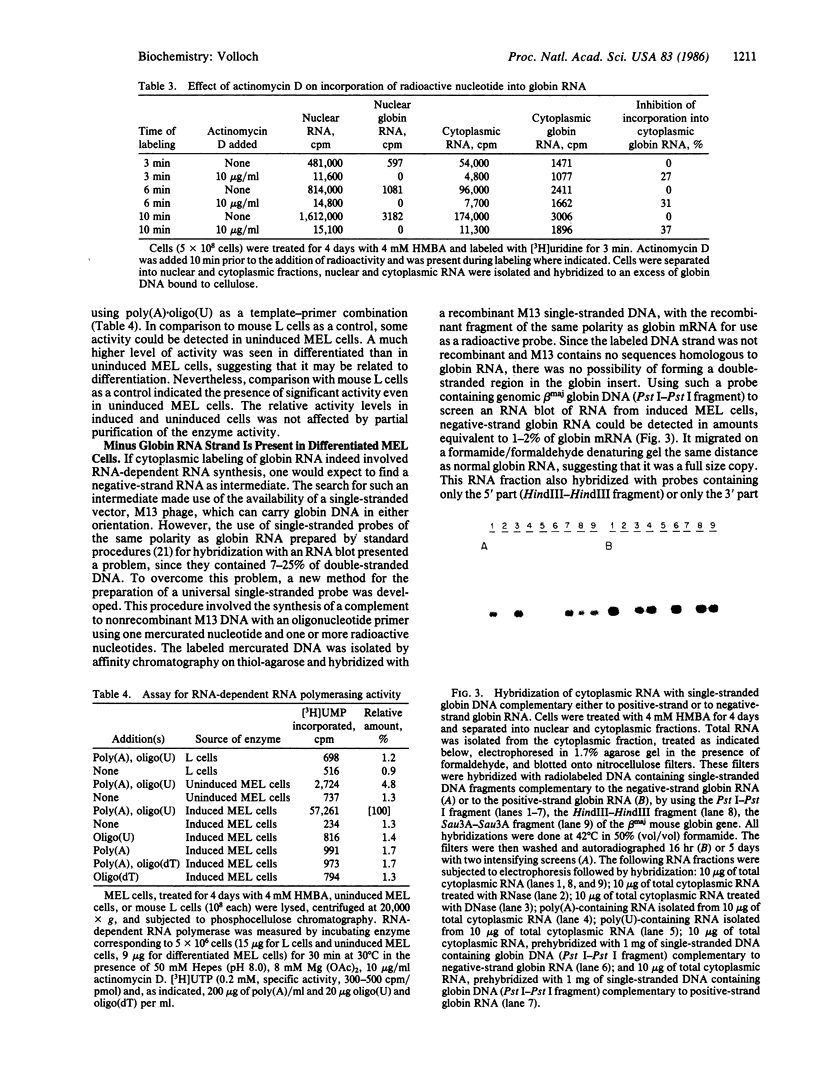
Three lines of evidence indicate that RNA-dependent RNA synthesis occurs in mouse erythroleukemia cells. The first involves labeling studies with [3H]uridine and shows a greater initial labeling rate of globin RNA in the cytoplasm than in the nucleus. Labeled globin RNA found in the cytoplasm after a very short pulse with tritiated uridine is of the "mature" 9S size while labeled globin RNA in the nuclei is exclusively in the form of 15S precursor molecules, suggesting that cytoplasmic globin RNA is not of nuclear origin. A high concentration of actinomycin D has no effect on the initial rate of labeling of cytoplasmic globin RNA, supporting this conclusion. Other experiments showed that the labeling of cytoplasmic globin RNA does not involve end addition to preexisting globin RNA. The second line of evidence is the identification of globin RNA minus strand in the cytoplasm of differentiated murine erythroleukemia cells by hybridization with single-stranded DNA probes containing the strand of the same sense as globin mRNA. This material has the same electrophoretic mobility as globin RNA and hybridizes with probes containing only the 5' part or only the 3' part of the gene suggesting that it is a full size copy of globin RNA. Finally, in murine erythroleukemia cells an RNA-dependent RNA polymerase activity is detected by using poly(A) . oligo(U) as a template-primer combination. This activity increases significantly after induction, suggesting that it is differentiation specific.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., KOCH G., MOUNTAIN I. M., SPRUNT K., VAN DAMME O. Infectivity of ribonucleic acid of poliovirus on HeLa cell mono-layers. Virology. 1958 Feb;5(1):172–173. doi: 10.1016/0042-6822(58)90014-x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos R. N., Aviv H. Globin RNA precursor molecules: biosynthesis and process in erythroid cells. Cell. 1977 Jul;11(3):641–650. doi: 10.1016/0092-8674(77)90081-2. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Carmichael G. G. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- COLTER J. S., BIRD H. H., MOYER A. W., BROWN R. A. Infectivity of ribonucleic acid isolated from virus-infected tissues. Virology. 1957 Dec;4(3):522–532. doi: 10.1016/0042-6822(57)90084-3. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Baron M. H., Baltimore D. Poliovirus replicase: a soluble enzyme able to initiate copying of poliovirus RNA. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2679–2683. doi: 10.1073/pnas.76.6.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A., Zabel P., Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. doi: 10.1016/0092-8674(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Downey K. M., Byrnes J. J., Jurmark B. S., So A. G. Reticulocyte RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3400–3404. doi: 10.1073/pnas.70.12.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granboulan N., Girard M. Molecular weight of poliovirus ribonucleic acid. J Virol. 1969 Oct;4(4):475–479. doi: 10.1128/jvi.4.4.475-479.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross P. R. Biochemistry of differentiation. Annu Rev Biochem. 1968;37:631–660. doi: 10.1146/annurev.bi.37.070168.003215. [DOI] [PubMed] [Google Scholar]
- Konkel D. A., Maizel J. V., Jr, Leder P. The evolution and sequence comparison of two recently diverged mouse chromosomal beta--globin genes. Cell. 1979 Nov;18(3):865–873. doi: 10.1016/0092-8674(79)90138-7. [DOI] [PubMed] [Google Scholar]
- Levy S., Aviv H. Quantitation of labeled globin messenger RNA by hybridization with excess complementary DNA covalently bound to cellulose. Biochemistry. 1976 May 4;15(9):1844–1847. doi: 10.1021/bi00654a009. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Burka E. R., Schlessinger D. PROTEIN SYNTHESIS IN ERYTHROID CELLS, I. RETICULOCYTE RIBOSOMES ACTIVE IN STIMULATING AMINO ACID INCORPORATION. Proc Natl Acad Sci U S A. 1962 Dec;48(12):2163–2171. doi: 10.1073/pnas.48.12.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow C. D., Gibbons G. F., Dasgupta A. The host protein required for in vitro replication of poliovirus is a protein kinase that phosphorylates eukaryotic initiation factor-2. Cell. 1985 Apr;40(4):913–921. doi: 10.1016/0092-8674(85)90351-4. [DOI] [PubMed] [Google Scholar]
- Noyes B. E., Stark G. R. Nucleic acid hybridization using DNA covalently coupled to cellulose. Cell. 1975 Jul;5(3):301–310. doi: 10.1016/0092-8674(75)90105-1. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Polyadenylic acid on poliovirus RNA. II. poly(A) on intracellular RNAs. J Virol. 1975 Jun;15(6):1418–1431. doi: 10.1128/jvi.15.6.1418-1431.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Requirement of 3'-terminal poly(adenylic acid) for the infectivity of poliovirus RNA. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2983–2987. doi: 10.1073/pnas.71.8.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Villa-Komaroff L., Baltimore D. Studies on the function of polyadenylic acid on poliovirus RNA. Cell. 1975 Sep;6(1):41–44. doi: 10.1016/0092-8674(75)90071-9. [DOI] [PubMed] [Google Scholar]
- Wells D., Kedes L. Structure of a human histone cDNA: evidence that basally expressed histone genes have intervening sequences and encode polyadenylylated mRNAs. Proc Natl Acad Sci U S A. 1985 May;82(9):2834–2838. doi: 10.1073/pnas.82.9.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Polyadenylic acid at the 3'-terminus of poliovirus RNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1877–1882. doi: 10.1073/pnas.69.7.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]