Abstract
Background: Exposure to ambient air particulate matter (PM) has been shown to increase rates of cardiopulmonary morbidity and mortality, but the underlying mechanisms are still not well understood.
Objective: We examined signaling events involved in the expression of the inflammatory gene interleukin-8 (IL-8) in human airway epithelial cells (HAECs) exposed to ambient PM collected in an urban area of Mexicali, Mexico.
Methods: We studied IL-8 expression and regulatory signaling pathways in cultured HAECs exposed to Mexicali PM suspended in media for 0–4 hr.
Results: Exposure resulted in a dose-dependent, 2- to 8-fold increase in IL-8 mRNA expression relative to controls. PM exposure induced IL-8 transcriptional activity in BEAS-2B cells that was dependent on the nuclear factor-kappa B (NF-κB) response element in the IL-8 promoter. Chromatin immunoprecipitation (ChIP) assays showed a 3-fold increase in binding of the p65 (RelA) NF-κB isoform to the IL-8 promoter sequence in HAECs exposed to PM. Western blot analyses showed elevated levels of phosphorylation of p65 but no changes in IκBα phosphorylation or degradation. IL-8 expression was blunted in a dose-dependent manner in BEAS-2B cells transduced with a lentivirus encoding a dominant negative p65 mutant in which phosphorylation sites were inactivated.
Conclusion: Taken together, these findings show that the increase in IL-8 mRNA expression in HAECs exposed to PM10 (PM ≤ 10 μm in aerodynamic diameter) is mediated through an NF-κB–dependent signaling mechanism that occurs through a pathway involving direct phosphorylation of the transcription factor p65 in the absence of IκBα degradation. These data show that exposure to PM10 in ambient air can induce inflammatory responses by activating specific signaling mechanisms in HAECs.
Keywords: human airway epithelium, interleukin-8, NF-κB, particulate matter, signal transduction
Epidemiological studies have demonstrated a positive association between levels of ambient particulate matter (PM) and mortality and morbidity (Dockery et al. 1993; Samet et al. 2000). Health effects associated with PM exposure include decreased pulmonary function, exacerbation of respiratory disease, and increased acute cardiovascular mortality (Pope 2000). In addition to PM concentration on a mass and number basis, physicochemical properties of the particles (e.g., chemical composition, surface area, size) have been proposed as determinants of the adverse effects associated with PM inhalation (Schwarze et al. 2006). However, despite being the subject of more than a decade of study, the toxicological mechanisms underlying the health effects of PM inhalation are not well understood.
Human airway epithelial cells (HAECs) are a primary target for inhaled PM as well as a major source of inflammatory mediators in the lung, including the potent neutrophil chemoattractant interleukin-8 (IL-8) (Strieter 2002), which plays an important role in pathogenic responses in the human lung. Elevated levels of IL-8 in the lung are a feature of a number of respiratory diseases, including cystic fibrosis, asthma, and chronic obstructive pulmonary disease (Bonfield et al. 1995; Gibson et al. 2001; Yamamoto et al. 1997). Increased expression of IL-8, therefore, is a pivotal event in the pulmonary inflammatory response associated with disease states (Harada et al. 1994; Zwahlen et al. 1993) and inhalation of ambient air contaminants (Ghio and Devlin 2001; Krishna et al. 1998; Stenfors et al. 2004).
IL-8 expression is regulated by transcriptional and posttranscriptional mechanisms under the control of multiple signaling pathways. In addition to a CCAAT box, regulatory elements have been identified in the 5´-flanking region of the IL-8 gene for multiple transcription factors, including glucocorticoid receptor, hepatocyte nuclear factor-1, interferon regulatory factor-1, activator protein-1 (AP-1), CCAAT/enhancer binding protein β (C/EBPβ), and nuclear factor-kappa B (NF-κB) (Jung et al. 2002; Mukaida et al. 1994). In addition, mRNA stabilization can play a role in increased IL-8 expression (Silbajoris et al. 2009; Sparkman and Boggaram 2004).
The transcription factor NF-κB is the best-characterized regulator of IL-8 transcription in HAECs. NF-κB functions as a heterogeneous collection of dimers composed of various combinations of five members of the NF-κB family. Classical NF-κB activation involves phosphorylation of the inhibitory subunit IκBα on two N-terminal serine residues by IκB kinases, rapidly followed by ubiquitination and proteosomal degradation of IκBα. The p50–p65 NF-κB heterodimer is thereby released from the complex and translocates to the nucleus, where it binds to the NF-κB response element, such as that found in the promoter region of the IL-8 gene (Karin and Ben-Neriah 2000). NF-κB–dependent gene transcription is also regulated by serine or threonine phosphorylations or other modifications of the Rel subunits that affect the transcription-activating function of NF-κB (Neumann and Naumann 2007). Thus, NF-κB–dependent gene transcription can be regulated by mechanisms that involve only NF-κB transactivation in the absence of IκB degradation and NF-κB nuclear translocation (Bohuslav et al. 2004; Neumann and Naumann 2007; Zhang et al. 2005). These IκBα-independent pathways can involve posttranslational modifications of NF-κB subunits, including site-specific phosphorylation of p65 (Neumann and Naumann 2007).
To characterize relevant mechanisms that lead to inflammatory responses to PM inhalation, we examined the activation of signaling events involved in IL-8 expression in a model of human airway epithelium exposed to PM obtained from Mexicali, a city in Mexico that experiences some of the highest PM levels in North America (Osornio-Vargas et al. 2010). Exposure of cultured HAECs to PM ≤ 10 μm in aerodynamic diameter (PM10) collected from Mexicali ambient air induced NF-κB–dependent transcriptional expression of IL-8 through an alternate signaling pathway involving direct phosphorylation of p65.
Materials and Methods
Reagents and supplies. We obtained tissue culture media and supplements from Lonza (Walkersville, MD, USA), Costar tissue culture plates from Corning (Corning, NY, USA), human recombinant tumor necrosis factor-α (TNFα) from Peprotech (Rocky Hill, NJ, USA), and MG-132 from Calbiochem (La Jolla, CA, USA). Western blotting supplies were obtained from Bio-Rad Laboratories (Hercules, CA, USA). Common laboratory reagents and supplies were obtained from Sigma (St. Louis, MO, USA) and Fisher Scientific (Raleigh, NC, USA).
Cell culture. After receiving informed consent from volunteers, we obtained HAECs from normal adult human volunteers by brush biopsy of the mainstem bronchus during fiberoptic bronchoscopy; this procedure was conducted under a protocol approved by the Committee on the Protection of the Rights of Human Subjects at the University of North Carolina at Chapel Hill. HAECs were cultured as previously described (Silbajoris et al. 2009). Transformed human airway epithelial cells (BEAS-2B subclone S6, passages 60–80) were grown on tissue culture plates in supplemented keratinocyte growth medium. HAECs and BEAS-2B cells were growth factor–starved in unsupplemented media for 15 hr before PM exposure.
PM. PM2.5 (PM ≤ 2.5 µm in aerodynamic diameter) and PM10 size fractions were collected between October 2005 and March 2006 at urban and semiurban locations in Mexicali, Mexico (Osornio-Vargas et al. 2010). For additional methodological details and PM10 elemental composition and size, see Supplemental Material, p. 2 and Table 1 (http://dx.doi.org/10.1289/ehp.1103594). PM suspensions were prepared by sonicating the recovered particles in supplement-free basal media in an ultrasonic water bath immediately before cell exposure.
Plasmid construction and lentiviral vector production. Lentiviral transfer plasmids for human IL-8 wild-type (IL-8wt) and NF-κB–site mutant (IL-8m– NF-κB) luciferase reporters, as well as the NF-κB tandem repeat (NF-κBtr) luciferase reporter, have been previously described (Tal et al. 2010). For additional methodological details, see Supplemental Material, pp. 2–3 (http://dx.doi.org/10.1289/ehp.1103594).
Reverse-transcription polymerase chain reaction (RT-PCR). We quantified relative gene expression in HAECs and BEAS-2B cells using RT-PCR as previously described (Tal et al. 2010). For additional methodological details, see Supplemental Material, pp. 3–4 and Table 2 (http://dx.doi.org/10.1289/ehp.1103594).
IL-8 promoter–reporter activity assay. BEAS-2B cells grown to 50% confluency were transduced with lentiviral IL-8wt, IL-8m– NF-κB, or NF-κBtr for 72 hr with a multiplicity of infection (MOI; number of viral particles per cell) of 5. Cells were cotransduced with EF1a_EGFP (elongation factor 1-alpha–enhanced green fluorescent protein) to control for transduction efficiency. Transduced cells were changed to supplement-free media overnight and then exposed to Mexicali urban PM10 at 40 μg/cm2 for 4 hr or to TNFα (20 ng/mL) for 15 min. Because preliminary experiments showed that the presence of particles interfered with optical measurements of luminescence and fluorescence, promoter activity was assessed by measuring firefly luciferase (fLuc) mRNA levels in cDNA generated from cell lysates.
ChIP (chromatin immunoprecipitation) assay. HAECs were changed to supplement-free media overnight and then exposed to Mexicali urban PM10 at 40 μg/cm2 for 4 hr or to TNFα (20 ng/mL) for 15 min. We then performed a ChIP assay using the Chromatin Immunoprecipitation Assay kit (Upstate, Charlottesville, VA, USA) according to the manufacturer’s instructions. Briefly, protein–DNA complexes were cross-linked with formaldehyde, cells were lysed and pelleted, and chromatin was sheared by sonication. Equal amounts of DNA were precleared with salmon sperm DNA/protein A agarose, and NF-κB–bound DNA fragments were immunoprecipitated with an antibody against NF-κBp65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). A sham immunoprecipitation using no antibody was used as a control. The antibody–DNA complex was captured with salmon sperm DNA/protein A agarose, pelleted, and washed. DNA was eluted with TE (Tris–EDTA) buffer, and cross-linking was reversed with 5 M NaCl at 65°C. RT-PCR, employing primers and a fluorescent probe directed against the NF-κB sequence in the IL-8 gene promoter region, was used to measure percentage of input, by comparing signals of ChIP samples and total input (DNA signal obtained by PCR amplification of samples taken before immunoprecipitation).
Western blotting. HAECs were changed to supplement-free media overnight and then exposed to PM10 (40 μg/cm2), TNFα (20 ng/mL), or media alone. To measure expression levels of phosphorylated IκBα (p-IκBα) we pretreated HAECs with the proteosomal inhibitor MG-132 (20 μM). Protein extracts were subjected to SDS-PAGE. Each resulting blot was blocked with 5% casein or bovine serum albumin and incubated with one of the following antibodies: anti-phosphorylated-NF-κBp65 (anti-p-NF-κBp65; Ser 536), anti-NF-κBp65 (Ser 536), anti-p-IκBα (Cell Signaling, Beverly, MA, USA), anti-IκBα, or β-actin (Santa Cruz Biotechnology). After incubation with horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG and HRP conjugated goat anti-mouse IgG secondary antibodies (Santa Cruz Biotechnology), bands were detected using ECL chemiluminescence reagents (Amersham, Piscataway, NJ, USA) and visualized using a Fujifilm LAS-3000 imaging system (Fujifilm, Stamford, CT, USA).
Inhibition of IL-8 transcription using pTRED-CMV_p65 triple mutant. BEAS-2B cells grown to 50% confluency were transduced with the lentiviral pTRED-CMV_p65 triple mutant for 72 hr with an MOI of 5 or 10. Cells were changed to supplement-free media overnight and then exposed to Mexicali urban PM10 at 40 μg/cm2 or media alone for 4 hr. Levels of IL-8 mRNA in cell lysates were measured using RT-PCR, normalized to levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA, and are expressed as fold increases over media control.
Statistics. Data are expressed as mean ± SE from at least three independent experiments. We performed data comparisons using one-way analysis of variance followed by Dunnett’s post hoc test for multigroup comparisons. Two-tailed paired or unpaired Student’s t-tests were used to evaluate differences between control and treated groups. Where results are presented as relative fold increases, statistics were performed using absolute data. Differences were assessed as significant when p ≤ 0.05.
Results
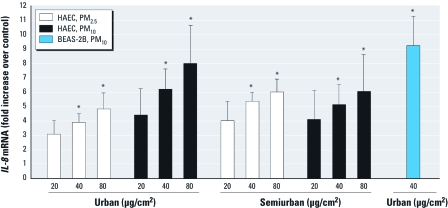
Exposure to PM induces IL-8 gene expression in HAECs. To determine the relative inflammatory potency of PM, we measured the expression of the proinflammatory gene IL-8 in HAECs exposed to a range of concentrations of PM10 and PM2.5 collected from urban and semiurban locations in Mexicali, Mexico (Osornio-Vargas et al. 2010). Levels of IL-8 mRNA increased in a dose-dependent manner after exposure to each particle type (Figure 1). Exposure to 40 or 80 μg/cm2 of all PM types for 4 hr resulted in 3.9- to 8-fold increases in IL-8 mRNA that were statistically significantly different from untreated controls. Incubation of PM10 with the lipopolysaccharide (LPS)-binding polypeptide polymyxin B did not change its potency in inducing IL-8 expression. Moreover, addition of LPS up to 1 μg/mL had no effect on IL-8 mRNA levels in HAECs (data not shown). We observed no statistically significant differences in potency between the various types/sources of PM; thus, we selected PM10 from an urban location for further study. A comparison of HAECs and the BEAS-2B human bronchial epithelial cell line showed similar responses to PM10 exposure and established the suitability of BEAS-2B cells as a surrogate for HAECs in this study (Figure 1).
Figure 1.
Exposure to PM increases levels of IL‑8 mRNA. HAECs and BEAS-2B cells were exposed to the indicated size fractions and concentrations of freshly suspended Mexicali PM or to media alone for 4 hr. Levels of IL‑8 mRNA in cell lysates were measured using RT-PCR (TaqMan; Applied Biosystems, Foster City, CA, USA), normalized to levels of GAPDH mRNA, and are expressed as fold increases over media control. Data are mean ± SE (n = > 3). *p < 0.05.
PM10-induced IL-8 expression involves NF-κB–dependent transcriptional activity. To identify the molecular mechanism responsible for PM-induced IL-8 expression, we measured the transcriptional activity of IL-8 wild-type (IL-8wt-fLuc) and NF-κB mutant (IL-8m–NF-κB–fLuc) reporter constructs expressed in BEAS-2B cells exposed to PM10 using TNFα as a positive control for comparison. Preliminary experiments showed that the presence of particles interfered with optical measurements of luminescence and fluorescence. Therefore, we used fLuc and EGFP mRNA levels measured by RT-PCR as readouts of the promoter activity. Exposures to 40 μg/cm2 PM10 for 4 hr resulted in statistically significant increases in promoter reporter activity in BEAS-2B cells expressing the human wild-type IL-8 promoter. In contrast, BEAS-2B cells expressing an IL-8 promoter with a mutated NF-κB response element showed no response to the same exposures (Figure 2).
Figure 2.
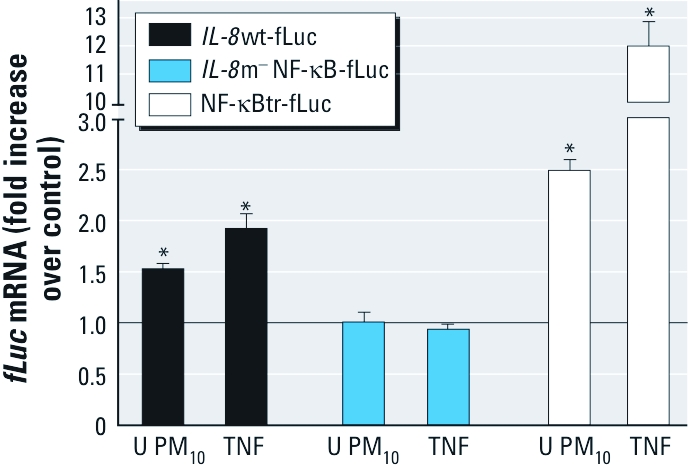
PM-induced IL‑8 transcription is NF-κB dependent. BEAS-2B cells were transduced with lentiviral vectors encoding IL‑8wt-fLuc, IL‑8m–NF-κB-fLuc, or NF-κBtr-fLuc before exposure to urban (U) PM10 at 40 μg/cm2 for 4 hr, TNFα (20 ng/mL) for 15 min, or media alone. Promoter activity was assessed by measuring levels of fLuc mRNA by RT-PCR. fLuc mRNA levels were normalized to EGFP mRNA levels, and results are expressed as fold change over untreated controls. Data are mean ± SE (n = 3). *p < 0.05.
As an independent measurement of NF-κB–dependent transcriptional activity, we next used PM10 to stimulate BEAS-2B cells transduced with a lentiviral vector encoding a luciferase reporter gene driven by a concatenated NF-κB consensus sequence (NF-κBtr-fLuc). Exposure to PM10 stimulated a statistically significant increase in NF-κB–dependent transcriptional activity in BEAS-2B cells (Figure 2).
Exposure to PM10 induces p65 binding to the NF-κB response element in the IL-8 promoter. Next we investigated whether PM10-induced IL-8 transcriptional activity is accompanied by a corresponding increase in transcription factor binding to NF-κB response elements in the native IL-8 promoter in HAECs exposed to PM10. ChIP analysis using an antibody directed to p65 followed by RT-PCR amplification of immunoprecipitated IL-8 promoter DNA sequences containing the NF-κB response elements showed that PM10 exposure resulted in a marked increase in p65 binding to the genomic IL-8 promoter in HAEC relative to media-exposed controls (Figure 3). As expected, treatment with TNFα induced a comparatively large increase in immunoprecipitated IL-8 promoter DNA in HAECs.
Figure 3.
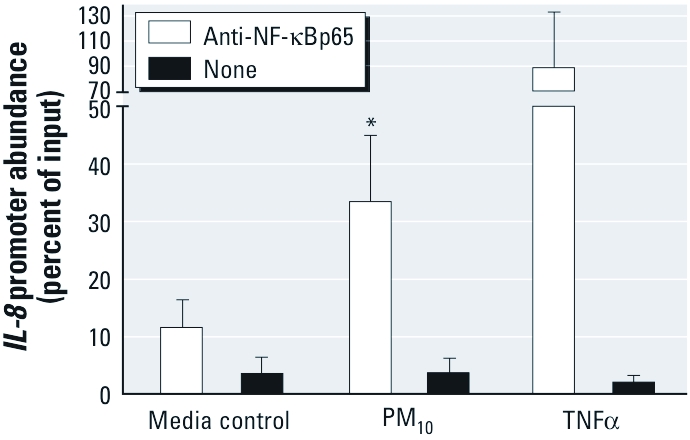
PM10 induces NF-κB binding to the IL‑8 promoter in HAECs. HAECs were exposed to PM10 (40 μg/cm2) for 4 hr, TNFα (20 ng/mL) for 15 min, or media alone. ChIP followed by RT-PCR was used to measure p65 binding to genomic IL‑8 promoter sequences. Data are mean ± SE (n = 3). *p < 0.05.
Exposure to PM10 induces NF-κB–dependent transcriptional activity that does not involve IκBα phosphorylation and degradation. Phosphorylation and degradation of IκBα are hallmark events in the activation of the canonical NF-κB pathway. Therefore, we examined the effect of PM10 exposure on the phosphorylation and abundance of IκBα in HAECs. Western blotting analysis showed that acute challenge with TNFα induced a significant and rapid decrease in the intracellular concentration of IκBα in HAECs (Figure 4A). In contrast, we observed no changes in levels of IκBα in HAECs exposed to 40 μg/cm2 PM10 for up to 1 hr. Similarly, we detected an increase in the level of p-IκBα in response to TNFα treatment, yet exposure to PM10 did not induce a change in p-IκBα levels in HAECs at any of the exposure time points examined (Figure 4B). These results indicated that PM10-induced NF-κB transcriptional activity does not involve the canonical activation pathway.
Figure 4.

PM-induced NF-κB activation does not involve the canonical pathway in HAECs. (A) HAECs were exposed to PM10 (40 μg/cm2), TNFα (20 ng/mL), or media alone for 10–60 min, and lysates were subjected to Western blotting using anti-IκBα antibody. (B) HAECs were pretreated for 30 min with 20 μM of proteosomal inhibitor MG-132 and exposed to PM10 (40 μg/cm2) or media alone for 30–240 min or TNFα (20 ng/mL) for 30 min; cell lysates were immunoblotted using anti-p‑IκBα and anti-IκBα antibodies. Data are representative of three or more experiments.
PM10-induced transcriptional expression of IL-8 is dependent on phosphorylation of p65. Phosphorylation of the NF-κB subunit p65 at multiple serine sites has been reported to increase NF-κB–dependent transcriptional activity in an IκBα-independent manner in a number of cell types (Neumann and Naumann 2007). Therefore, we investigated whether NF-κB activation in HAECs exposed to PM10 occurs through phosphorylation of p65. Densitometry of Western blots showed a statistically significant 2-fold increase in levels of phosphorylated p65 (serine 536) after 30 min of exposure to 40 μg/mL PM10 that remained elevated for up to 4 hr of exposure (Figure 5).
Figure 5.
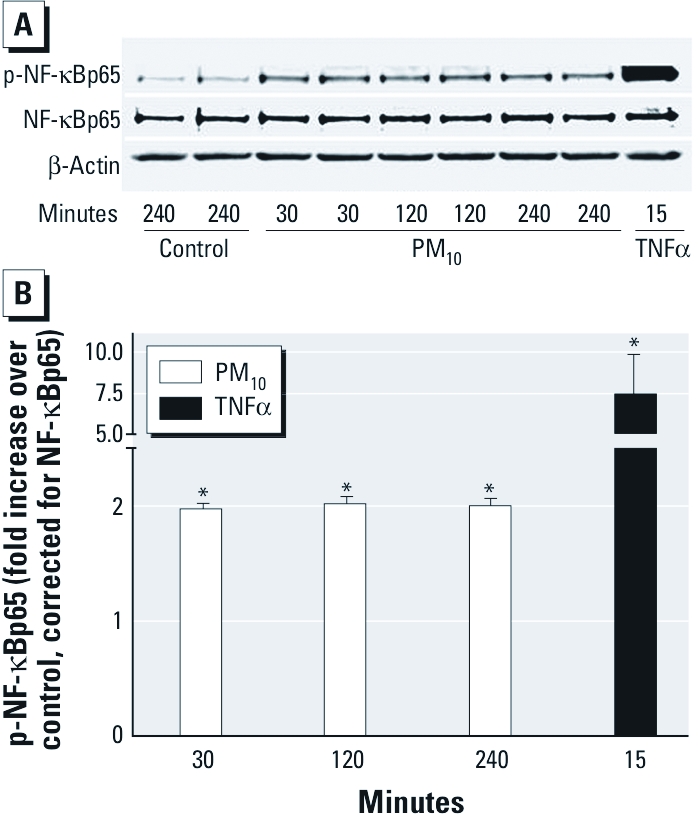
Exposure to PM10 induces NF-κB activation through an alternate pathway in HAECs. (A) Cell lysates from HAECs exposed to PM10 (40 μg/cm2) for 30 min to 4 hr or TNFα (20 ng/mL) for 15 min were immunoblotted using antibodies to p-NF-κBp65 (Ser 536), NF-κBp65 (Ser 536), and β-actin. Results are representative of three separate experiments. (B) Densitometric quantitation of p-p65 band optical density. Data are mean ± SE (n = 3). *p < 0.05.
We next investigated the functional link between p65 phosphorylation and the induction of IL-8 transcription in HAECs exposed to PM10. BEAS-2B cells were transduced with a retrovirus expressing a dominant negative p65 in which three major phosphorylation sites were eliminated by substituting alanines for serines 276, 529, and 536. RT-PCR analyses showed that, relative to control cells, PM10-induced IL-8 expression was blunted in BEAS-2B cells in which p65 serine phosphorylation was prevented (Figure 6).
Figure 6.
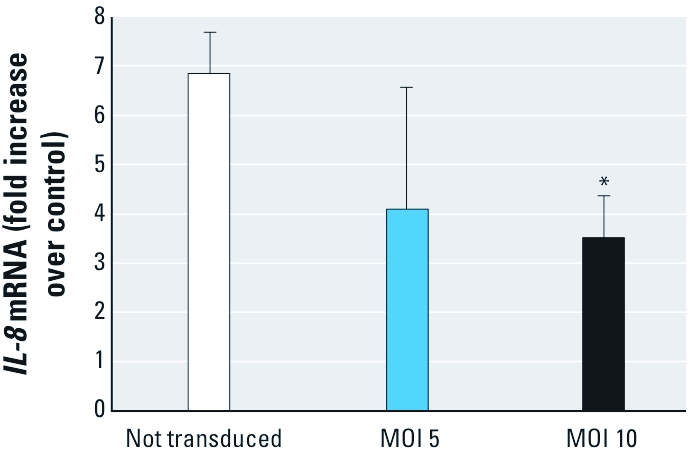
IL‑8 transcription induced by PM10 requires p65 phosphorylation. BEAS-2B cells transduced with a lentiviral vector encoding pTRED-CMV_p65 at an MOI of 5 or 10, in which serines 276, 529, and 536 were mutated to alanines, were exposed to PM10 (40 μg/cm2) or media alone for 4 hr. Levels of IL‑8 mRNA in lysates were measured using RT-PCR, normalized to levels of GAPDH mRNA, and expressed as fold increases over media control. Data are mean ± SE (n = 3). *p < 0.05, compared with the untransduced control.
Discussion
Although the association of ambient air PM exposure with cardiopulmonary health effects is well established (Peters et al. 2001; Wellenius et al. 2005), the mechanisms by which inhaled PM exerts such deleterious effects remain unclear. As a primary target of inhaled pollutants, the airway epithelium is capable of initiating or supplementing pulmonary inflammatory defenses by synthesizing a number of mediators that can recruit and activate inflammatory cells, thereby promoting inflammation that is thought to culminate in cardiovascular dysfunction (Bai et al. 2007). Our group has reported inflammatory mediator expression by HAECs exposed to a variety of PM components, including diesel exhaust (Tal et al. 2010), zinc ions (Kim et al. 2006; Wu et al. 2010), and ultrafine elemental carbon (Kim et al. 2005). In the present study, we enhance the environmental relevance of these observations by showing that a “real-world” PM sample induces IL-8 expression through activation of a specific physiological pathway involving an alternate NF-κB activation mechanism.
Several studies have established phosphorylation of p65 as an alternate mechanism leading to the activation of NF-κB–dependent transcriptional activity in response to physiological stimuli such as growth factors, gasotransmitters, and cytokines (Bijli et al. 2008; Chantôme et al. 2004; Kai et al. 2009; Reber et al. 2009; Zhang et al. 2005). p65 phosphorylation is also involved in NF-κB activation induced by LPS, oncogenic viral infection, and cellular transformation (Chen and Harrison 2005; Doyle et al. 2005; Hu et al. 2004). The mechanisms of action of silica (Kang et al. 2006) and the peroxisome proliferator-activated receptor-γ ligand ciglitazone (Chen and Harrison 2005), both xenobiotics that induce NF-κB activation, have been shown to include p65 phosphorylation. In addition, we previously reported that divalent zinc induces IL-8 expression through an NF-κB–dependent mechanism that involves serine-specific phosphorylation of p65 in BEAS-2B cells (Kim et al. 2007). However, in contrast to the data we show in the present study, zinc also induced IκBα phosphorylation, albeit without inducing its degradation (Kim et al. 2007). We have also reported that ultrafine carbon particles induce IL-8 expression in HAECs through a mechanism that involves p38 activation, without involvement of NF-κB (Kim et al. 2005). It is possible that the signaling difference seen between ultrafine carbon particles and Mexicali PM is evidence that multiple mechanisms exist in HAECs for the induction of IL-8 expression in response to a broad range of inhaled xenobiotic materials. It is noteworthy that oxidant stress is a critical event in the toxicity of each of these agents (Atarod and Kehrer 2004; Beck-Speier et al. 2005; Ghio et al. 1992).
Oxidant-induced loss of signaling quiescence, possibly involving inhibition of phosphatases that oppose the activating effect of kinases that phosphorylate p65, is a common feature in the toxicity of diverse groups of chemical agents (Samet and Tal 2010). Therefore, although the upstream mechanisms that initiate p65 phosphorylation induced by PM10 have not been identified, it is possible to hypothesize that an oxidant-dependent event is involved. Moreover, although a ligand-mediated activation of a receptor-initiated signaling event cannot be ruled out as a contributing mechanism, the prolonged time course of Mexicali-induced NF-κB activation and IL-8 expression is consistent with a permissive process such as phosphatase inhibition as an initiating signaling event.
A diverse array of kinases has been implicated in the phosphorylation of p65, including IKKβ (IκB kinase β) (Kang et al. 2006), PKCζ (protein kinase Cζ) (Kai et al. 2009), Btk (Bruton’s tyrosine kinase) (Doyle et al. 2005), CKII (casein kinase II) (Chantôme et al. 2004), Rsk1 (ribosomal S6 kinase 1) (Bohuslav et al. 2004), Syk (spleen tyrosine kinase) (Bijli et al. 2008), MSK1 (mitogen- and stress-activated protein kinase 1) (Reber et al. 2009), and PI3K (phosphoinositide 3-kinase) (Liu et al. 2008). We have observed that pharmacological inhibition of PI3K failed to block PM10-induced IL-8 expression, implying that the PI3K-phosphatase and tensin homolog (PTEN) pathway is not involved. Separately, we also determined that inhibition of MEK (MAP kinase-ERK kinase), JNK (Jun-N-terminal kinase), p38 kinase, and the src tyrosine kinases p56lck, p59fynT, and Hck failed to block PM10-induced IL-8 expression (Silbajoris R, unpublished data). Given the number of pathways that are known to lead to p65 phosphorylation, our finding that PM10-induced gene expression does not involve canonical activation of NF-κB will require detailed follow-up study in order to identify the upstream kinase(s) involved, as well as the proximal event that leads to p65 phosphorylation in response to PM10. Such studies will expand the results presented here and further elucidate the mechanisms through which inflammatory responses are regulated in the airway epithelium after inhalation of PM10.
The PM10 sample we used in this study was obtained from Mexicali, Mexico, a semirural border city afflicted with high levels of inhalable PM derived from both combustion and geological sources. Previous studies on Mexicali PM have associated particle size with specific chemical composition, source, and biological effects (Mendoza et al. 2010; Osornio-Vargas et al. 2010). However, in the present study, PM2.5 and PM10 collected in urban and semiurban locations in Mexicali were of comparable potency in inducing IL-8 expression in HAECs. Further, pharmacological inhibition of NF-κB activation abrogated IL-8 expression induced by each of the Mexicali particles used in this study (Silbajoris R, unpublished data). These results suggest that a property these particles have in common is responsible for their inflammogenicity.
Considering that these materials were collected from ambient air, it is not surprising that analyses showed significant endotoxin levels associated with each of the Mexicali particles, raising the possibility that LPS contributes to their bioactivity. However, endotoxin activates the canonical NF-κB pathway, and we did not observe such activation with the Mexicali particles. Moreover, HAECs are known to express very low levels of endotoxin receptors (Becker et al. 2000), and we have previously reported that endotoxin levels far in excess of those presented to the cells in this study resulted in only minimal induction of IL-8 expression by HAECs (Silbajoris et al. 2009). In the present study, we confirmed those findings and determined that an endotoxin-binding polypeptide (polymyxin B) does not affect the potency of PM10 in inducing HAEC IL-8 gene expression. Thus, the toxicity of Mexicali PM is attributable to other chemical components associated with these particles. Of the metals that are present in Mexicali PM10 [see Supplemental Material, Table 1 (http://dx.doi.org/10.1289/ehp.1103594)], only zinc has been reported to induce NF-κB activation that involves p65 phosphorylation (Kim et al. 2007), albeit at concentrations considerably higher than the submicromolar concentrations used in the present study.
Although the considerable chemical and physical complexity that characterizes ambient PM prevents a ready identification of bioactive components and sources in this mechanistic study, the results presented here nonetheless provide insight into the contribution of proinflammatory processes in the lung to the toxicity of PM inhalation. Further, these findings lend biological plausibility in support of epidemiological reports of the adverse public health impact of PM exposure.
Supplemental Material
Footnotes
This study was supported by InterGen-LASPAU (Border Ozone Reduction and Air Quality Improvement Program).
The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. The contents of this article should not be construed to represent agency policy, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
The authors declare they have no actual or potential competing financial interests.
References
- Atarod EB, Kehrer JP. Dissociation of oxidant production by peroxisome proliferator-activated receptor ligands from cell death in human cell lines. Free Radic Biol Med. 2004;37:36–47. doi: 10.1016/j.freeradbiomed.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Bai N, Khazaei M, van Eeden SF, Laher I. The pharmacology of particulate matter air pollution-induced cardiovascular dysfunction. Pharmacol Ther. 2007;113(1):16–29. doi: 10.1016/j.pharmthera.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Becker MN, Diamond G, Verghese MW, Randell SH. CD14-dependent lipopolysaccharide-induced β-defensin-2 expression in human tracheobronchial epithelium. J Biol Chem. 2000;275(38):29731–29736. doi: 10.1074/jbc.M000184200. [DOI] [PubMed] [Google Scholar]
- Beck-Speier I, Dayal N, Karg E, Maier KL, Schumann G, Schulz H, et al. Oxidative stress and lipid mediators induced in alveolar macrophages by ultrafine particles. Free Radic Biol Med. 2005;38:1080–1092. doi: 10.1016/j.freeradbiomed.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Bijli KM, Fazal F, Minhajuddin M, Rahman A. Activation of Syk by protein kinase C-δ regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via tyrosine phosphorylation of RelA/p65. J Biol Chem. 2008;283(21):14674–14684. doi: 10.1074/jbc.M802094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohuslav J, Chen LF, Kwon H, Mu Y, Greene WC. p53 induces NF-κB activation by an IκB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J Biol Chem. 2004;279(25):26115–26125. doi: 10.1074/jbc.M313509200. [DOI] [PubMed] [Google Scholar]
- Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, et al. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152(6 pt 1):2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- Chantôme A, Pance A, Gauthier N, Vandroux D, Chenu J, Solary E, et al. Casein kinase II-mediated phosphorylation of NF-κB p65 subunit enhances inducible nitric-oxide synthase gene transcription in vivo. J Biol Chem. 2004;279(23):23953–23960. doi: 10.1074/jbc.M313731200. [DOI] [PubMed] [Google Scholar]
- Chen F, Harrison LE. Ciglitazone induces early cellular proliferation and NF-κB transcriptional activity in colon cancer cells through p65 phosphorylation. Int J Biochem Cell Biol. 2005;37(3):645–654. doi: 10.1016/j.biocel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, III, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Doyle SL, Jefferies CA, O’Neill LA. Bruton’s tyrosine kinase is involved in p65-mediated transactivation and phosphorylation of p65 on serine 536 during NFκB activation by lipopolysaccharide. J Biol Chem. 2005;280(25):23496–23501. doi: 10.1074/jbc.C500053200. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Devlin RB. Inflammatory lung injury after bronchial instillation of air pollution particles. Am J Respir Crit Care Med. 2001;164(4):704–708. doi: 10.1164/ajrccm.164.4.2011089. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Kennedy TP, Whorton AR, Crumbliss AL, Hatch GE, Hoidal JR. Role of surface complexed iron in oxidant generation and lung inflammation induced by silicates. Am J Physiol. 1992;263:L511–L518. doi: 10.1152/ajplung.1992.263.5.L511. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119(5):1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56(5):559–564. [PubMed] [Google Scholar]
- Hu J, Nakano H, Sakurai H, Colburn NH. Insufficient p65 phosphorylation at S536 specifically contributes to the lack of NF-κB activation and transformation in resistant JB6 cells. Carcinogenesis. 2004;25(10):1991–2003. doi: 10.1093/carcin/bgh198. [DOI] [PubMed] [Google Scholar]
- Jung YD, Fan F, McConkey DJ, Jean ME, Liu W, Reinmuth N, et al. Role of P38 MAPK, AP-1, and NF-κB in interleukin-1beta-induced IL-8 expression in human vascular smooth muscle cells. Cytokine. 2002;18(4):206–213. doi: 10.1006/cyto.2002.1034. [DOI] [PubMed] [Google Scholar]
- Kai M, Yasuda S, Imai S, Toyota M, Kanoh H, Sakane F. Diacylglycerol kinase α enhances protein kinase Cζ-dependent phosphorylation at Ser311 of p65/RelA subunit of nuclear factor-κB. FEBS Lett. 2009;583(19):3265–3268. doi: 10.1016/j.febslet.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Kang JL, Jung HJ, Lee K, Kim HR. Src tyrosine kinases mediate crystalline silica-induced NF-κB activation through tyrosine phosphorylation of IκB-α and p65 NF-κB in RAW 264.7 macrophages. Toxicol Sci. 2006;90(2):470–477. doi: 10.1093/toxsci/kfj096. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Kim YM, Cao D, Reed W, Wu W, Jaspers I, Tal T, et al. Zn2+-induced NF-κB-dependent transcriptional activity involves site-specific p65/RelA phosphorylation. Cell Signal. 2007;19(3):538–546. doi: 10.1016/j.cellsig.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kim YM, Reed W, Lenz AG, Jaspers I, Silbajoris R, Nick HS, et al. Ultrafine carbon particles induce interleukin-8 gene transcription and p38 MAPK activation in normal human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288(3):L432–L441. doi: 10.1152/ajplung.00285.2004. [DOI] [PubMed] [Google Scholar]
- Kim YM, Reed W, Wu W, Bromberg PA, Graves LM, Samet JM. Zn2+-induced IL-8 expression involves AP-1, JNK, and ERK activities in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L1028–L1035. doi: 10.1152/ajplung.00479.2005. [DOI] [PubMed] [Google Scholar]
- Krishna MT, Chauhan AJ, Frew AJ, Holgate ST. Toxicological mechanisms underlying oxidant pollutant-induced airway injury. Rev Environ Health. 1998;13(1–2):59–71. [PubMed] [Google Scholar]
- Liu J, Yoshida Y, Yamashita U. DNA-binding activity of NF-κB and phosphorylation of p65 are induced by N-acetylcysteine through phosphatidylinositol (PI) 3-kinase. Mol Immunol. 2008;45(15):3984–3989. doi: 10.1016/j.molimm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Mendoza A, Pardo EI, Gutierrez AA. Chemical characterization and preliminary source contribution of fine particulate matter in the Mexicali/Imperial Valley border area. J Air Waste Manag Assoc. 2010;60(3):258–270. doi: 10.3155/1047-3289.60.3.258. [DOI] [PubMed] [Google Scholar]
- Mukaida N, Morita M, Ishikawa Y, Rice N, Okamoto S, Kasahara T, et al. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-κB is target for glucocorticoid-mediated interleukin 8 gene repression. J Biol Chem. 1994;269(18):13289–13295. [PubMed] [Google Scholar]
- Neumann M, Naumann M. Beyond IκBs: alternative regulation of NF-κB activity. FASEB J. 2007;21(11):2642–2654. doi: 10.1096/fj.06-7615rev. [DOI] [PubMed] [Google Scholar]
- Osornio-Vargas AR, Serrano J, Rojas-Bracho L, Miranda J, Garcia-Cuellar C, Reyna MA, et al. 2010In vitro biological effects of airborne PM2.5 and PM10 from a semi-desert city on the Mexico–US border. Chemosphere:618–626. [DOI] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103(23):2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Pope CA., III Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who’s at risk? Environ Health Perspect. 2000;108(suppl 4):713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber L, Vermeulen L, Haegeman G, Frossard N.2009Ser276 phosphorylation of NF-kB p65 by MSK1 controls SCF expression in inflammation. PLoS One 42e4393; doi: 10.1371/journal.pone.0004393[Online 6 February 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, Tal TL. Toxicological disruption of signaling homeostasis: tyrosine phosphatases as targets. Annu Rev Pharmacol Toxicol. 2010;50:5–79. doi: 10.1146/annurev.pharmtox.010909.105841. [DOI] [PubMed] [Google Scholar]
- Samet JM, Zeger SL, Dominici F, Curriero F, Coursac I, Dockery DW, et al. The National Morbidity, Mortality, and Air Pollution Study. Part II: morbidity and mortality from air pollution in the United States. Res Rep Health Eff Inst. 2000;94(pt 2):5–70. [PubMed] [Google Scholar]
- Schwarze PE, Ovrevik J, Lag M, Refsnes M, Nafstad P, Hetland RB, et al. Particulate matter properties and health effects: consistency of epidemiological and toxicological studies. Hum Exp Toxicol. 2006;25(10):559–579. doi: 10.1177/096032706072520. [DOI] [PubMed] [Google Scholar]
- Silbajoris R, Huang JM, Cheng WY, Dailey L, Tal TL, Jaspers I, et al. Nanodiamond particles induce IL-8 expression through a transcript stabilization mechanism in human airway epithelial cells. Nanotoxicology. 2009;3(2):152–160. [Google Scholar]
- Sparkman L, Boggaram V. Nitric oxide increases IL-8 gene transcription and mRNA stability to enhance IL-8 gene expression in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287(4):L764–L773. doi: 10.1152/ajplung.00165.2004. [DOI] [PubMed] [Google Scholar]
- Stenfors N, Nordenhall C, Salvi SS, Mudway I, Soderberg M, Blomberg A, et al. Different airway inflammatory responses in asthmatic and healthy humans exposed to diesel. Eur Respir J. 2004;23(1):82–86. doi: 10.1183/09031936.03.00004603. [DOI] [PubMed] [Google Scholar]
- Strieter RM. Interleukin-8: a very important chemokine of the human airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2002;283(4):L688–L689. doi: 10.1152/ajplung.00146.2002. [DOI] [PubMed] [Google Scholar]
- Tal TL, Simmons SO, Silbajoris R, Dailey L, Cho SH, Ramabhadran R, et al. Differential transcriptional regulation of IL-8 expression by human airway epithelial cells exposed to diesel exhaust particles. Toxicol Appl Pharmacol. 2010;243(1):46–54. doi: 10.1016/j.taap.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Bateson TF, Mittleman MA, Schwartz J. Particulate air pollution and the rate of hospitalization for congestive heart failure among Medicare beneficiaries in Pittsburgh, Pennsylvania. Am J Epidemiol. 2005;161(11):1030–1036. doi: 10.1093/aje/kwi135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Samet JM, Peden DB, Bromberg PA. Phosphorylation of p65 is required for zinc oxide nanoparticle-induced interleukin 8 expression in human bronchial epithelial cells. Environ Health Perspect. 2010;118:982–987. doi: 10.1289/ehp.0901635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto C, Yoneda T, Yoshikawa M, Fu A, Tokuyama T, Tsukaguchi K, et al. Airway inflammation in COPD assessed by sputum levels of interleukin-8. Chest. 1997;112(2):505–510. doi: 10.1378/chest.112.2.505. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma Y, Zhang J, Cheng J, Du J. A new cellular signaling mechanism for angiotensin II activation of NF-κB: an IκB-independent, RSK-mediated phosphorylation of p65. Arterioscler Thromb Vasc Biol. 2005;25(6):1148–1153. doi: 10.1161/01.ATV.0000164624.00099.e7. [DOI] [PubMed] [Google Scholar]
- Zwahlen R, Walz A, Rot A. 1993. In vitro and in vivo activity and pathophysiology of human interleukin-8 and related peptides. Int Rev Exp Pathol 34(pt B):27–42.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.