Abstract
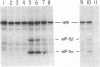
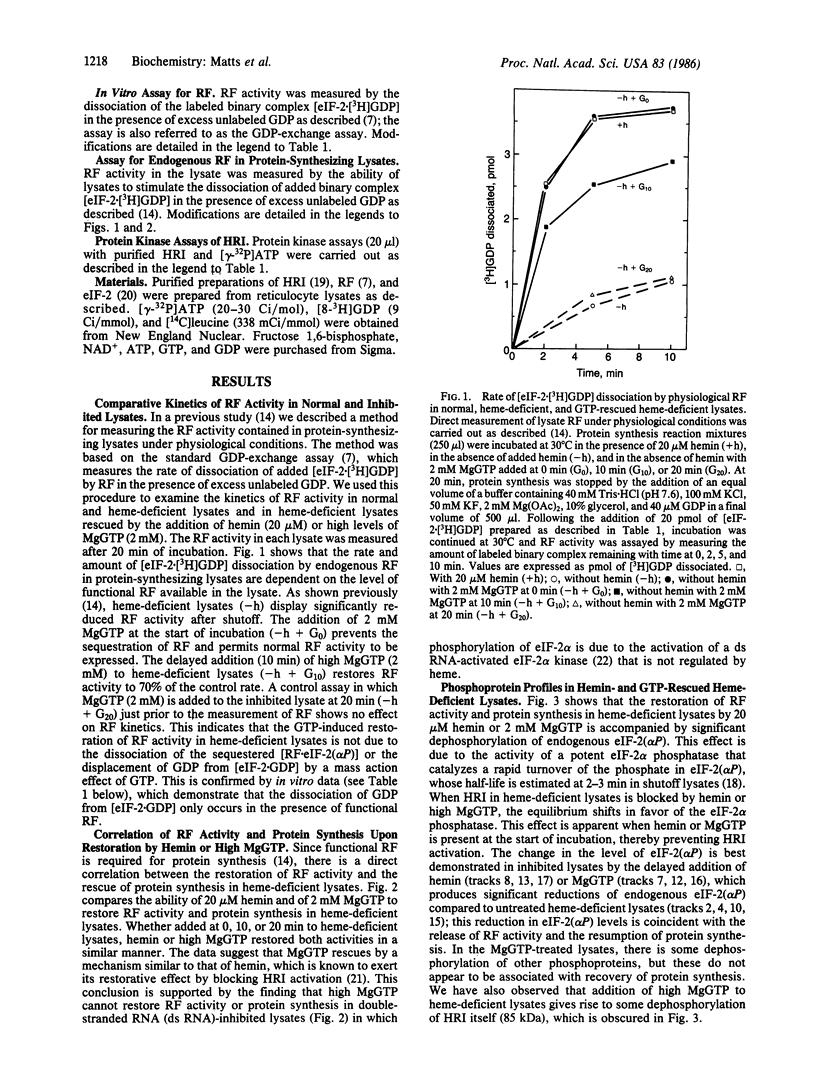
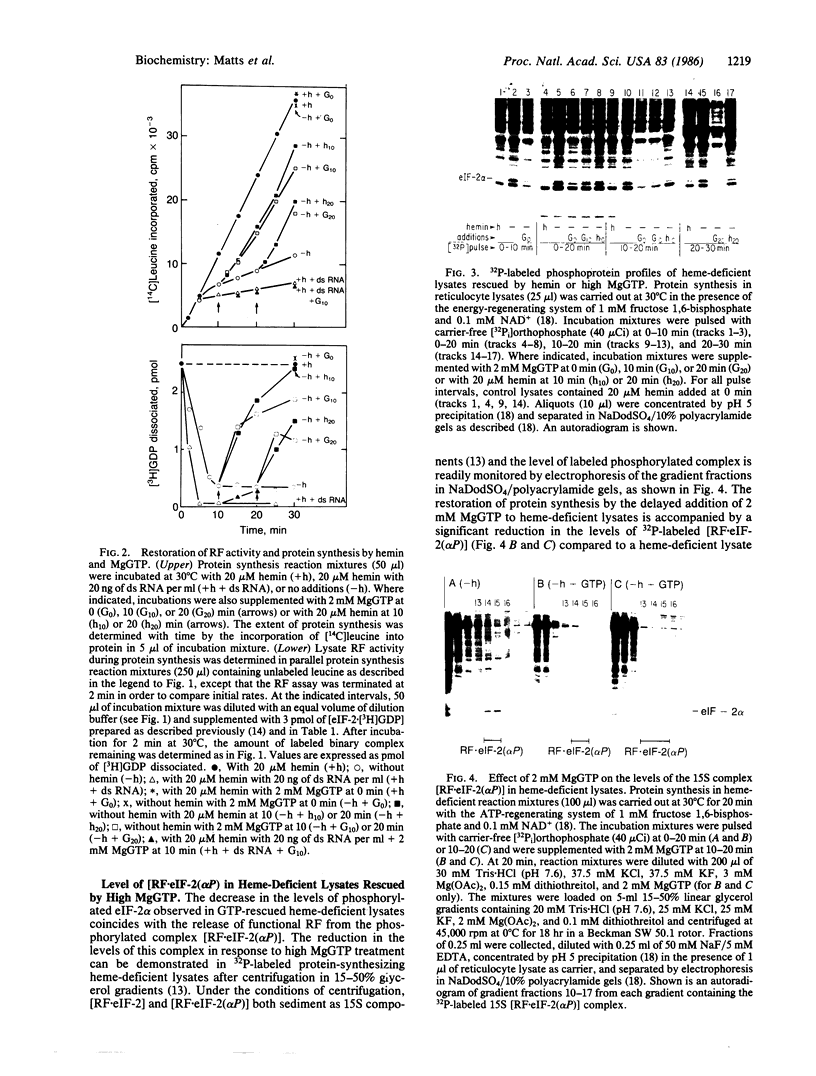
The inhibition of protein synthesis in hemedeficient reticulocyte lysates is reversed by the addition of hemin (20 microM) or MgGTP (2 mM). The rate of recovery is rapid and approaches control kinetics within a few minutes after the addition of either component. The restoration of protein synthesis is dependent upon the availability of functional reversing factor (RF). The fate of RF was monitored during recovery by using a method that measures RF activity in the lysate under physiological conditions. In the fully inhibited lysate, RF is sequestered in a nondissociable 15S [RF . eIF-2(alpha P)] complex (where eIF-2 indicates eukaryotic initiation factor 2) in which RF activity is not functional and cannot be assayed. The first step in the rescue of protein synthesis in inhibited lysates by hemin or MgGTP is the inhibition of heme-regulated eIF-2 alpha kinase, which enables endogenous phosphatase to dephosphorylate eIF-2(alpha P) and [RF . eIF-2(alpha P)]. The release of approximately 50% of the sequestered RF activity is sufficient to support optimal kinetics of recovery. Hemin and MgGTP both reverse inhibition by blocking the activation and/or activity of heme-regulated eIF-2 alpha kinase in the lysate. The conclusion that MgGTP exerts its effect on eIF-2 alpha kinase is supported by several in vitro findings: (i) 2 mM MgGTP inhibits the autophosphorylation of purified heme-regulated eIF-2 alpha kinase and abolishes its ability to phosphorylate eIF-2 alpha; (ii) 2 mM MgGTP cannot displace GDP in the binary complexes [eIF-2 . GDP] or [eIF-2(alpha P) . GDP] by mass action; and (iii) RF in the [RF . eIF-2(alpha P)] complex is not dissociated by 2 mM MgGTP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amesz H., Goumans H., Haubrich-Morree T., Voorma H. O., Benne R. Purification and characterization of a protein factor that reverses the inhibition of protein synthesis by the heme-regulated translational inhibitor in rabbit reticulocyte lysates. Eur J Biochem. 1979 Aug 1;98(2):513–520. doi: 10.1111/j.1432-1033.1979.tb13212.x. [DOI] [PubMed] [Google Scholar]
- Andrews N. C., Levin D., Baltimore D. Poliovirus replicase stimulation by terminal uridylyl transferase. J Biol Chem. 1985 Jun 25;260(12):7628–7635. [PubMed] [Google Scholar]
- Benne R., Hershey J. W. The mechanism of action of protein synthesis initiation factors from rabbit reticulocytes. J Biol Chem. 1978 May 10;253(9):3078–3087. [PubMed] [Google Scholar]
- Ernst V., Levin D. H., Foulkes J. G., London I. M. Effects of skeletal muscle protein phosphatase inhibitor-2 on protein synthesis and protein phosphorylation in rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7092–7096. doi: 10.1073/pnas.79.23.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., London I. M. Evidence that glucose 6-phosphate regulates protein synthesis initiation in reticulocyte lysates. J Biol Chem. 1978 Oct 25;253(20):7163–7172. [PubMed] [Google Scholar]
- Ernst V., Levin D. H., Ranu R. S., London I. M. Control of protein synthesis in reticulocyte lysates: effects of 3':5'-cyclic AMP, ATP, and GTP on inhibitions induced by hemedeficiency, double-stranded RNA, and a reticulocyte translationa inhibitor. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1112–1116. doi: 10.1073/pnas.73.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard R., London I. M. Relationship between phosphorylation and activity of heme-regulated eukaryotic initiation factor 2 alpha kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):866–870. doi: 10.1073/pnas.78.2.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss D. J., Parkhurst L. J., Mehta H. B., Woodley C. L., Wahba A. J. Studies on the role of eukaryotic nucleotide exchange factor in polypeptide chain initiation. J Biol Chem. 1984 Jun 25;259(12):7374–7377. [PubMed] [Google Scholar]
- Gross M., Redman R., Kaplansky D. A. Evidence that the primary effect of phosphorylation of eukaryotic initiation factor 2(alpha) in rabbit reticulocyte lysate is inhibition of the release of eukaryotic initiation factor-2.GDP from 60 S ribosomal subunits. J Biol Chem. 1985 Aug 5;260(16):9491–9500. [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Konieczny A., Safer B. Purification of the eukaryotic initiation factor 2-eukaryotic initiation factor 2B complex and characterization of its guanine nucleotide exchange activity during protein synthesis initiation. J Biol Chem. 1983 Mar 10;258(5):3402–3408. [PubMed] [Google Scholar]
- Levin D., London I. M. Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1121–1125. doi: 10.1073/pnas.75.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matts R. L., Levin D. H., London I. M. Effect of phosphorylation of the alpha-subunit of eukaryotic initiation factor 2 on the function of reversing factor in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1983 May;80(9):2559–2563. doi: 10.1073/pnas.80.9.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matts R. L., London I. M. The regulation of initiation of protein synthesis by phosphorylation of eIF-2(alpha) and the role of reversing factor in the recycling of eIF-2. J Biol Chem. 1984 Jun 10;259(11):6708–6711. [PubMed] [Google Scholar]
- Merrick W. C. Evidence that a single GTP is used in the formation of 80 S initiation complexes. J Biol Chem. 1979 May 25;254(10):3708–3711. [PubMed] [Google Scholar]
- Pain V. M., Clemens M. J. Assembly and breakdown of mammalian protein synthesis initiation complexes: regulation by guanine nucleotides and by phosphorylation of initiation factor eIF-2. Biochemistry. 1983 Feb 15;22(4):726–733. doi: 10.1021/bi00273a003. [DOI] [PubMed] [Google Scholar]
- Panniers R., Henshaw E. C. A GDP/GTP exchange factor essential for eukaryotic initiation factor 2 cycling in Ehrlich ascites tumor cells and its regulation by eukaryotic initiation factor 2 phosphorylation. J Biol Chem. 1983 Jul 10;258(13):7928–7934. [PubMed] [Google Scholar]
- Peterson D. T., Merrick W. C., Safer B. Binding and release of radiolabeled eukaryotic initiation factors 2 and 3 during 80 S initiation complex formation. J Biol Chem. 1979 Apr 10;254(7):2509–2516. [PubMed] [Google Scholar]
- Ranu R. S. Regulation of protein synthesis in rabbit reticulocyte lysates by guanosine triphosphate. Biochem Biophys Res Commun. 1982 Dec 15;109(3):872–880. doi: 10.1016/0006-291x(82)92021-6. [DOI] [PubMed] [Google Scholar]
- Siekierka J., Mauser L., Ochoa S. Mechanism of polypeptide chain initiation in eukaryotes and its control by phosphorylation of the alpha subunit of initiation factor 2. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2537–2540. doi: 10.1073/pnas.79.8.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N. S., Matts R. L., Levin D. H., London I. M. The 60 S ribosomal subunit as a carrier of eukaryotic initiation factor 2 and the site of reversing factor activity during protein synthesis. J Biol Chem. 1985 Aug 15;260(17):9860–9866. [PubMed] [Google Scholar]
- Thomas N. S., Matts R. L., Petryshyn R., London I. M. Distribution of reversing factor in reticulocyte lysates during active protein synthesis and on inhibition by heme deprivation or double-stranded RNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6998–7002. doi: 10.1073/pnas.81.22.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and characterization of heme-reversible translational inhibitor. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3654–3658. doi: 10.1073/pnas.75.8.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Staehelin T. Binding and release of eukaryotic initiation factor eIF-2 and GTP during protein synthesis initiation. Proc Natl Acad Sci U S A. 1978 Jan;75(1):204–208. doi: 10.1073/pnas.75.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]