Abstract
Chimeras are organisms composed of at least two genetically distinct cell lineages originating from different zygotes. In the laboratory, mouse chimeras can be produced experimentally; various techniques allow combining different early stage mouse embryos with each other or with pluripotent stem cells. Identification of the progeny of the different lineages in chimeras permits to follow cell fate and function, enabling correlation of genotype with phenotype. Mouse chimeras have become a tool to investigate critical developmental processes, including cell specification, differentiation, patterning and the function of specific genes. In addition, chimeras can also be generated to address biological processes in the adult, including mechanisms underlying diseases or tissue repair and regeneration. This review summarizes the different types of chimeras and how they have been generated and provides examples of how mouse chimeras offer a unique and powerful system to investigate questions pertaining to cell and tissue function in the developing and adult organism.
Key words: chimera, developmental chimera, aggregation, blastocyst injection, embryonic stem cells, induced pluripotent stem cells, complementation, regeneration
Introduction
Chimeras are organisms composed of two or more genetically distinct cell lineages. In mammals, the natural formation of chimeras is unusual, and in many cases erroneous. Directed production of laboratory mouse chimeras, however, has had an enormous impact on biological research. Beyond serving as a means to propagate mutations through germ line contribution of mutated pluripotent stem cells, mouse chimeras have become a valuable tool to study cellular processes and gene function. Questions such as how cells become allocated to lineages, whether or not a gene is important for the formation of a specific lineage or tissue, or how genetic or epigenetic changes can influence the differentiation ability of cells within the organism, have been effectively addressed in chimeras.1,2 Key elements to using chimeras to answer such questions are the directed combination of genetically different mouse embryos or embryos with pluripotent stem cells such as embryonic stem (ES) cells, the use of markers to identify their progeny in the mixed organism, and the subsequent correlation of cell genotype with phenotype.
The chimera approach is not new—many techniques for mouse chimera production were developed decades ago—but remains uniquely useful, as illustrated by several recent studies on organ regeneration from pluripotent stem cells. These studies used mouse chimeras generated with pre-implantation stage embryos, in which different lineages co-develop from early stages onwards, to investigate the ability of induced pluripotent stem (iPS) cells to regenerate the liver in a mouse model of a fatal human liver disease,3 or to form an entire pancreas.4 In the following we introduce the different chimera types and their method of generation and discuss how mouse developmental chimeras can be used to study cell and tissue function, disease mechanisms and organ regeneration.
Definition and Types of Chimeras
Both chimeras and mosaics contain more than one genetically distinct cell lineage within the same organism. In chimeras, these lineages stem from different zygotes or at least two different fertilization events, while mosaics result from a mitotic event in the same zygotic lineage.5 Thus, successful aggregation of two pre-implantation stage embryos can produce an animal chimeric for all lineages. In contrast, transgenic founder mice resulting from pronuclear injection of DNA fragments are often mosaics due to delayed integration of the injected trans-gene into the genome of some but not all blastomeres during cleavage stages.
Developmental (or primary) chimeras are formed at early embryonic stages; their different lineages co-develop and can potentially mix throughout all tissues. Transplantation (or secondary) chimerism results from spontaneous or directed tissue transfer at stages post organogenesis, and is therefore mostly limited to the transplanted tissue, for example hematopoietic chimerism as a consequence of intraplacental blood exchange between twins or after bone marrow transplantation. While all known naturally formed chimeras occur within the same species (intraspecific chimeras), experimentally induced chimerism can also include the mixing of tissues of different species (interspecific chimeras), both as developmental and transplantation chimeras. The production of interspecific transplantation chimeras relies on using immune-deficient animals as tissue recipients. Engraftment and expansion of human hematopoietic stem cells or hepatocytes in immune-deficient mice with bone marrow or liver failure, respectively, yields “humanized mice” in which most immune cells6 or hepatocytes7 are derived from the human cell transplant. The efficiency of this organ-specific transplantation approach is high and thus in stark contrast to the extremely limited contribution of human cells to animals derived from mouse blastocysts injected or aggregated with human pluripotent stem cells.8 The generation, application and potential of human-animal transplantation chimeras have been extensively reviewed in references 9–11.
Spontaneous Chimerismin Mammals
In most mammalian species, naturally occurring developmental chimeras are considered unusual,5 rendering the recent discovery of extensive natural chimerism in marmosets (callitrichid primates) very intriguing.12 Marmoset mothers typically give birth to fraternal twins, and most twins exhibit hematopoietic chimerism due to intraplacental blood exchange with their sibling. In addition, microsatellite analysis of various other tissues has revealed extensive somatic and germ line chimerism of marmoset twin pairs—more than half of the males studied were functionally chimeric in the germ line.12 Marmosets frequently exhibit placental fusion as well as delayed development at the time of chorionic fusion, which presumably permits cell exchange between twins at very early stages of development leading to chimerism.
The rare cases of spontaneous developmental chimerism documented in humans are typically diagnosed based on a discrepancy between cytogenetic and apparent gender, ambiguous genitalia, hermaphroditism or hematopoietic chimerism. Same-sex chimerism with no apparent clinical phenotype is rarely identified, and if so, inadvertently. In one such case, the results of histocompatibility antigen blood testing of a family suggested that two of three sons could not be the biological offspring of the mother.13 However, microsatellite analysis of various tissues from the mother revealed that she was chimeric for two different 46,XX/46,XX cell lineages, with only one of these forming the blood.
About 40 cases of human whole body 46,XX/46XY chimerism have been documented. In about one fifth of these cases, chimerism identified by analysis of polymorphic genetic markers could be attributed to the spontaneous fusion of two separate zygotes to form a single embryo.14,15 Other potential mechanisms underlying XX/XY chimerism include the participation of parthenogenetic, that is oocyte-only, derived lineages. The spontaneous parthenogenetic activation of an oocyte followed by fertilization and endoreplication of pronuclei could explain the mixing of entirely maternally derived XX and XY cell lineages in a rare human parthenogenetic chimera.16 Another hypothesis suggests parthenogenetic oocyte activation and subsequent fertilization by two different sperm to produce two separate lineages with the same maternal but two paternal contributions.15,17
Human chimerism or mosaicism involving cells with only sperm-derived (androgenetic) genomes is predominantly found in the placenta. Androgenetic cell contribution can result in complete hydatidiform mole with no fetal tissue or cause substantial placental defects, with varying effects on the fetus—from completely normal development to fetal death.14,18 Because sperm are haploid, androgenetic mosaicism and chimerism are not always unequivocally distinguishable.18 For example, a growth retarded twin fetus that had been spontaneously aborted at 15 weeks of gestation, with a grossly enlarged and cystic placenta, consisted of both androgenetic and biparental cells in placental and fetal cell lineages. As the andro-genetic cells had genetic contribution from different sperm, this chimera may have formed through fertilization of an anucleate egg with two sperm, and subsequent aggregation with a second zygote.19 However, such a scenario could also result from uneven distribution of pronuclei during cleavage of a single oocyte fertilized by multiple sperm, a condition that is not strictly chimerism or mosaicism.
Twin blood chimerism, arising from the exchange of fetal hematopoietic stem cells between siblings through blood vessel anastomoses in the placenta, is common in some mammalian species, particularly marmoset and cattle. The incidence in humans is unknown. Studies of human multiple pregnancies using a sensitive method that can identify low levels of hematopoietic chimerism indicate that the frequency of blood chimerism in twin pairs (8%) and triplets (21%) may be quite substantial.20 Because both mono-zygotic and dizygotic human twins can share a placenta (monochorionic diamniotic placenta), hematopoietic chimerism may be more prevalent than assumed, as suggested by case reports of hematopoietic chimerism of dizygotic multiple births after assisted reproduction.21,22
Approaches to Producing Mouse Developmental Chimeras
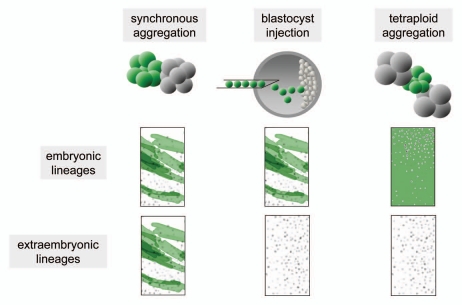
The production of mouse developmental chimeras requires combination of the different lineages at pre-implantation stages to facilitate joint post-implantation development. Commonly used experimental approaches to achieve this include aggregation of cleavage stage embryos, combination of pluripotent stem cells with pre-implantation embryos, frequently by injection into blastocysts or morulae, and the combination of cleavage-stage diploid embryos or pluripotent stem cells with tetraploid embryos (Fig. 1).
Figure 1.
The method of chimera generation determines the origin of embryonic and extraembryonic lineages.
The different genetic lineages can be distinguished using various markers of cellular origin established in the mouse, including eye pigment and coat color,5 mouse-strain-specific variants of glucose phosphate isomerase 1 (GPI1),23 repeat markers,24 retroviral insertion marking,25 or transgenic markers such as ubiquitously expressed lacZ (ROSA2626) or green fluorescent protein (GFP27), as well as cell-type specific transgenic markers. GPI1 and retroviral insertion permit quantification of overall tissue contribution levels but do not allow resolution at the cellular level that can be obtained with markers compatible with microscopy, such as lacZ or fluorescent proteins.
Aggregation chimeras.
Aggregation chimeras result from combining cleavage stage embryos either at equal (synchronous) or unequal (asynchronous) stages. Because cleavage stage blastomeres are still totipotent, aggregation chimeras are potentially highly chimeric throughout all derivatives of the epiblast, primitive endoderm (PE) and trophectoderm (TE). Therefore, in contrast to chimeras generated by injection of ES cells into blastocysts, aggregation chimeras can be useful for the analysis of extraembryonic tissues, but require that secondary effects of fetal-placental interactions be considered. The distribution of derivatives of mutant embryos in aggregation chimeras with normal embryos can reveal preferential or restricted differentiation as a consequence of the mutation, and can elucidate phenotypes masked by compensatory mechanisms or early demise in whole null mutant animals. For example, embryos with a deletion of the platelet-derived growth factor receptor β (PDGFRβ), an embryonic lethal mutation, exhibit substantially reduced ability to contribute to blood vessel walls and most other muscle lineages in aggregation chimeras with wild-type embryos, exposing a previously masked role of PDGFRβ in muscle development.28
Chimeras generated by aggregation of embryos distinguishable by ubiquitously expressed markers considered developmentally neutral, such as ROSA26 or GFP, have been used extensively to investigate cell growth patterns, origin of cell populations, clonality, cell mixing, migration of precursor cells and overall tissue formation during organogenesis, for example of the eye,29 kidney,30 pancreas,31 intestine32 or heart.33
Although nowadays less used due to the development of ES cell technology and ease of production of chimeras by blastocyst injection, aggregation chimeras retain their utility. Aggregating primary embryos largely circumvents the potential problem that epigenetic changes in ES cells affect their properties independently of the mutation in question. For example, parthenogenetic (PG) chimeras (between normal cells and those with only maternally derived genomes) produced by aggregation exhibit a number of phenotypes, including growth restriction, which are absent in chimeras produced by injection of PG ES cells into blastocysts.34,35 Because the contribution of PG cells to TE derivatives is very limited in aggregation chimeras,36,37 these phenotypic differences are likely not a consequence of the different chimera approach, but are instead caused by divergent properties of PG embryos and PG ES cells associated with epigenetic changes in the latter.
Blastocyst injection chimeras.
When pluripotent cells such as ES cells are injected into the blastocoel cavity of a blastocyst, they can combine at peri-implantation stages with the inner cell mass to form the epiblast, but will not contribute to PE or TE derivatives. When working with genetically modified mouse models, two variants are possible. First, injection of mutant ES or other pluripotent stem cells into normal blastocysts produces chimeras with normal extraembryonic tissues such that consequences of the mutation or type of injected cells will only be detectable in epiblast-derived tissues. Such chimeras can be the founders of mutant mouse lines, through the contribution of the injected cells to the germ line, but can also be utilized to investigate phenotypes beyond the stages to which the mutant embryos would develop, or to test the differentiation ability of presumptive pluripotent cells.38–42 Second, normal ES or other pluripotent stem cells can be injected into mutant blastocysts. In this scenario, all PE and TE derivatives are mutant, and the epiblast a presumptive mixture of normal and mutant cells. In particular cases, the deficiency of the host blastocyst provides an “empty” developmental niche that can be “filled” by the progeny of the injected normal ES or other pluripotent stem cells. Approaches taking advantage of this so-called “blastocyst complementation” will be discussed in more detail below.
Tetraploid complementation.
Electrofusion of blastomeres of a two-cell stage embryo can be used to produce an embryo with a tetraploid (4n) genome. Tetraploid mouse embryos can form blastocysts and implant, but do not develop much thereafter. In chimeras with diploid embryos or cells, tetraploid cells are excluded from the epiblast, but can form PE and TE derivatives. Thus, injection of ES or other pluripotent stem cells into tetraploid blastocysts or aggregation of diploid with tetraploid embryos yields chimeras in which the embryo proper is derived exclusively from the diploid cells, while the tetraploid cells form or contribute to the extraembryonic tissues.43 This approach has been instrumental in the analysis of phenotypic consequences of a mutation in epiblast-derived lineages.1
Tetraploid complementation also facilitates rapid generation of mutant mice derived only from pluripotent stem cells, avoiding the extensive breeding required to obtain mutant mice from germ line chimeras. However, only a minority of pluripotent stem cell lines is capable of producing entirely pluripotent stem cell-derived mice by tetraploid complementation. Even in those lines proven effective, the frequency of viable fetuses is low, rendering this benchmark a definitive but time and resource-consuming proof of pluripotency of ES44,45 or induced pluripotent stem (iPS) cell lines.46–48
Tetraploid complementation has also been used to improve the efficiency of somatic cell nuclear transfer (cloning) in the mouse; tetraploid complementation of ES cells derived from blastocyststage clones can generate viable animals at higher numbers than direct embryo transfer of clone blastocysts.45
Questions that can be Effectively Addressed in Developmental Chimeras
Looking at compensation in chimeras.
Analysis of cell fate in the mixed tissues of developmental chimeras can permit distinction of cell-autonomous from noncell-autonomous effects. Embryos deficient for the transcription factor forkhead box D3 (FOXD3), which would normally die early after implantation due to lack of epiblast maintenance, can be rescued to reach later developmental stages by injection of normal ES cells at the blastocyst stage, suggesting that the requirement for FOXD3 for epiblast maintenance is non-cell autonomous and can be compensated by wild-type cells.49 This approach has been used in other null mutants, such as mice lacking at least two of the three inhibitor of DNA binding (Id) genes, which exhibit severe cardiac defects and midgestation lethality, but can survive to term when rendered chimeric by blastocyst injection of wild-type ES cells.50 Although term ID chimeras are substantially smaller and almost half die within the first 4 weeks of life, surviving chimeras catch up in growth and exhibit normal adult heart morphology and size. Gene expression analyses of fetal-stage chimeras, as well as co-culture experiments, suggest that the normal ES cells afford this phenotypic rescue by altering gene expression in the mutant cells in a non-cell-autonomous manner through secreted factors, including long-ranging IGF1 and locally, WNT5a.
Conversely, the injection of mutant pluripotent stem cells into normal blastocysts can reveal properties of mutant cells, often at stages beyond which the mutant would develop. In the central nervous system of fetal and adult blastocyst injection chimeras, derivatives of retinoblastoma (Rb) gene null mutant ES cells continued to exhibit abnormal entry into the cell cycle, identifying this property as a cell-autonomous defect. Yet, the normal cells provided non-cell-autonomous rescue by suppressing apoptosis in RB deficient cells which survived and differentiated into neurons.51
Similarly, chimeras between normal embryos and ES cells deficient for the Ena/VASP proteins, a family of actin regulatory proteins, were used to investigate the role of these proteins in the nervous system; both normal and mutant cells contained an axon-specific fluorescent transgenic marker, and the absence of the mutant cell marker in axons of chimeric mice demonstrated a cell-autonomous requirement for Ena/Vasp proteins for axon fiber tract formation during cortical development.52
Addition of normal cells by blastocyst injection has also been used in an attempt to rescue more complex developmental defects, such as those of embryos produced by somatic cell nuclear transfer. Clone blastocysts injected with normal ES or inner cell mass cells formed more early stage fetuses than non-injected clones, suggesting that peri-implantation epiblast defects in clones are non-cell autonomous and can be complemented by normal cells. However, midgestation and term chimeras exhibited abnormal morphology and low viability similar to clones, without detectable rescue, presumably related to deficiencies of the trophoblast lineage.53
How many different genomes can contribute to developmental chimeras?
Both chimeras and mosaics have been used to trace lineage formation during mammalian development and to determine the number of founder cells of cell lineages.5 Aggregation chimera experiments suggested that at least three cells constitute the founder cells of the epiblast from which all somatic lineages are derived,54 while analysis of mice rendered mosaic through retroviral marking, an approach that obviated the need to disrupt the embryo, indicated that eight cells form all somatic lineages of the mouse.55 To answer the question of how many ES cells contribute to chimeras produced by blastocyst injection, in which the injected ES cells (usually 10–15) mix with the existing inner cell mass to form a combined epiblast, Wang et al. performed tetraploid and normal blastocyst injection experiments with varying numbers of cells from an ES cell mixture in which the individual cells and their derivatives could be distinguished by different retroviral marker insertion sites. The outcomes were indicative of a surprisingly low number of ES cells that contribute to the resulting entirely ES cell-derived mice or chimeras: in most cases, only one or two ES cells contributed, and three at a maximum.25 Thus, although mouse development is sufficiently flexible to allow the generation of chimeric animals through various approaches, it appears to limit the number of different genomes that can be combined. In the case of blastocyst injections, this could be a constraint imposed by the competition that occurs between the cells of the host blastocyst and the injected cells which compete for the developmental niche of the few cells that will form the embryo.25,54
Using Developmental Chimerasto Investigate Adult Cell and Tissue Function
Developmental chimeras are typically being used to address questions about processes that occur during embryonic and fetal stages. However, they are also effective systems for investigating processes in the adult, in particular, when combining pluripotent stem cells with blastocysts with a mutation known to cause a specific defect in a postnatal tissue, for example injury of hepatocytes lacking the enzyme fumarylacetoacetate hydrolase (FAH).3 In some cases, a mutation can even leave an empty developmental niche that can be filled entirely by the pluripotent stem cell progeny, for example T and B lymphocytes,56 the lens57 or pancreas.58 Such complementation models provide unique tools to test the ability of mutant or presumptive pluripotent stem cells to form the cell type or organ in question.
Adult tissue complementation using blastocyst injection chimeras.
A prominent example for this approach is the complementation of Rag2 (recombinationactivating gene 2)-deficient host embryos. Mice lacking RAG2 are devoid of mature T and B lymphocytes due to inability for VDJ recombination, such that the injection of RAG2-deficient blastocysts with ES cells produces mice with entirely ES cell-derived T and B cells. Termed RAG complementation, this model has been instrumental for assaying the function of various genes in lymphocytes, by complementing RAG2-deficient blastocysts with ES cells carrying varying mutations.56,59–62
Similarly, mice with a hematopoietic stem cell deficit due to homozygous mutation of the c-kit receptor (W41/W41) have been used in a combined complementation and transplantation approach to generate mice in which the entire hematopoietic system is derived from ES cells.63 In chimeras of W41/W41 blastocysts injected with normal ES cells, fetal liver hematopoiesis is dominated by ES cell derivatives. Transplantation of fetal liver cells from these chimeras into irradiated adult wild-type mice provides a rapid method for producing large numbers of animals with entirely ES cell-derived hematopoiesis.
Beyond the hematopoietic system, complementation has been achieved in homozygous aphakia (ak) mice, which fail to develop an ocular lens.57,64 Complementation of ak/ak blastocysts with normal ES cells results in phenotypic rescue and mice with entirely ES cell-derived lenses. Although primarily designed to investigate lens differentiation during development, this chimera model can also be used for postnatal studies such as adult lens regeneration.
Interspecific chimeras to produce xenogenic organs.
A recent study used developmental chimeras to study pancreas regeneration from iPS cells and to provide proof-of-principle for organ production in interspecific chimeras by producing an entirely rat-derived pancreas in mouse.4 Mice deficient for the transcription factor pancreatic and duodenal homeobox 1 (Pdx1) gene are incapable of pancreatogenesis and die soon after birth due to pancreatic insufficiency. However, complementation by injection of normal mouse ES or iPS cells into Pdx1-/- blastocysts permitted survival of chimeric mice to adulthood. In these mice, all pancreatic cell types except cell lineages forming blood vessels, nerves and connective tissue were pluripotent stem cell-derived, and transplantation of isolated pluripotent stem cell-derived islets into diabetic mice afforded protection from hypoglycemia, thus providing proof-of-principle for the generation of a functional organ from ES and iPS cells using chimera complementation. Furthermore, the authors succeeded in producing pancreatic tissue derived entirely form rat pluripotent stem cells in Pdx1-/- mice, thereby generating rodent interspecific chimeras. The authors injected mouse pluripotent stem cells into normal rat blastocysts and vice versa, and transferred these into recipients of the blastocyst species. In contrast to previous unsuccessful attempts at producing mouse-rat chimeras by injection of inner cell masses into blastocysts,65,66 some of the injected blastocysts developed into fetal and postnatal chimeras. However, these interspecific chimeras exhibited high embryonic lethality and poor postnatal survival. In general, a high contribution of cells of the other rodent species was associated with morphological abnormalities and lethality. Surviving postnatal chimeras exhibited contribution to various organs, including composite structures such as pancreatic islands of mixed mouse and rat origin, but never to the germ line. One key observation made in these interspecific chimeras was that the instructive ability of the blastocyst host not only determines overall animal size but also organ morphology. Mice, but not rats, have a gallbladder and this organ was only formed when mouse blastocysts were used as hosts. This result may explain why the new approach of producing chimeras by injecting ES or iPS cells into blastocysts prevailed in contrast to previous attempts based on injecting inner cell masses.67 Remarkably, the authors also succeeded in demonstrating interspecific chimera complementation; rat iPS cells injected into Pdx1-/- mouse blastocysts produced Pdx1-/ neonates with pancreatic epithelia derived entirely from rat iPS cells. Survival of the animals into adulthood was rare, but two viable chimeras were obtained. Thus, this landmark study established the feasibility and highlighted the clinical potential of generating an entire organ from xenogenic pluripotent stem cells in chimeras.
Organ regeneration in the adult.
Developmental chimeras can be used to explore the potential of new pluripotent stem cell types such as iPS cells for cell replacement strategies. The liver is a particularly promising target for cell transplantation because of its natural ability to support and integrate large numbers of newly generated hepatocytes. Because the adult human liver contains billions of hepatocytes, the efficacy of liver cell therapy would be greatly enhanced if pluripotent stem cell-derived hepatocytes were as capable of post-transplant expansion as primary hepatocytes.68 The FAH-deficient mouse provides a rigorous system to test the proliferative capabilities of hepatocytes derived from pluripotent stem cells; the liver injury inflicted by FAH deficiency confers a selective growth advantage on wild-type hepatocytes.69 Thus, postnatal expansion of iPS cell-derived hepatocytes in chimeric mice derived from injection of wild-type iPS cells into FAH-deficient blastocysts showed that these cells replicate the ability of primary hepatocytes to proliferate extensively.3 Because only differentiated hepatocytes, but not liver progenitor cells, are efficiently expanded in the FAH-deficient liver, liver repopulation with iPS cell-derived hepatocytes also suggests that these cells are fully differentiated. Along these lines, livers of chimeric mice in which virtually all FAH-deficient, blastocyst-derived hepatocytes were replaced by iPS cell-derived hepatocytes provided normal liver function. In the absence of compensation by blastocystderived hepatocytes and biases potentially introduced by imperfect in vitro differentiation protocols, these results provided evidence for the principal efficacy of iPS cells for liver cell therapy.
Tissue homeostasis and function in the adult.
Investigations of adult chimeric tissues composed of normal and GFP transgenic or otherwise mutant cells can elucidate mechanisms of tissue homeostasis. Imaging analysis of size and distribution of chimeric patches in the lung epithelium of aggregation chimeras between normal and GFP-tagged cells in tissue homeostasis or after slight injury suggested the involvement of many widely distributed progenitors, rather than airway stem cells, as patches were small and widely distributed. In contrast, severe injury resulted in the formation of large patches near presumptive stem cell niches, suggesting they may be of stem cell origin.70
Due to the monoclonal origin of crypts in the small intestine, chimeras are also excellent tools to investigate consequences of mutations in the intestinal epithelium, by comparison of adjacent mutant and non-mutant populations. Analysis of various mutants of the GTPase RAC1 using blastocyst injection chimeras suggested a crucial role of RAC1 in the regulation of normal intestinal stem cell differentiation.71
In a different approach, aggregation of two different mouse mutants—T and B cell-deficient (RAG or SCID) with thymus-deficient mice—followed by analysis of T cell repertoire selection in adult chimeras provided evidence that thymus epithelial cells were not required for major histocompatibility complex selection.72
Chimera analysis of cells mutant for zonula occludens (ZO) proteins illustrates how this approach can identify non-redundant functions of proteins in adult tissues that are masked by early embryonic lethality as well as compensatory mechanisms. Embryos deficient for either the ZO-1 or ZO-2 protein die shortly after implantation, but injection of ZO-2 null mutant ES cells into normal blastocysts resulted in viable term chimeras with extensive ES cell contribution, suggesting that ZO-2 function is more critical for extraembryonic tissues than the epiblast.73 In adult chimeras, reduced levels of ZO-2 in tissues were associated with abnormal kidneys, and male chimeras exhibited small testes and reduced fertility due to a deficient blood-testis barrier, demonstrating a non-redundant role of the ZO-2 protein in specific adult tissues.
Chimera analysis has also contributed substantially to the understanding of disease mechanisms in mouse mutants with a CNS phenotype, such as mutations of ion channels and neurotransmitter receptors. For instance, aggregation chimeras between normal and weaver mutant mice that are ataxic due to a near complete lack of granule cells in the cerebellum showed that this phenotype was caused by a cell-intrinsic defect within the granule cells leading to premature death.74 Similar experiments with other ataxic mice such as lurcher and scrambler have provided the basis for subsequent identification of the genes and mechanisms involved.75,76
Wound repair.
Adult chimeras can be used to investigate the function of mutant versus normal cells in response to a stimulus, such as an injury. For example, analysis of PDGFRβ-deficient cells in chimeras with normal cells has revealed roles of PDGF signaling in adult tissues. Although PDGFRβ-deficient cells form fibroblasts and endothelial lineages in chimeras during development, they are excluded from granulation tissue formation during wound repair in adult chimeras, indicating that PDGF signaling is required for connective tissue repair in the adult, but not for connective tissue formation in the embryo.77 In response to arterial injury, PDGF signaling appears to function largely in a chemotactic manner to attract smooth muscle cells to the newly forming neointima, based on the migration patterns of PDGFRβ-positive and PDGFRβ-negative smooth muscle cells in chimeric animals.78
How many normal cells are required for phenotypic rescue?
The stochastic distribution of cells within chimeras allows for a variation in contribution, such that phenotypes can vary. This variation can be used to investigate the proportion of cells required for phenotype induction or rescue, thus providing an estimate for the level of therapeutic engraftment that would be needed to restore normal tissue function.
Two studies have used injection of normal ES cells into blastocysts from shiverer homozygous mice (shi/shi) to correlate phenotypic rescue with the degree of normal cell distribution.79,80 Shiverer mice do not produce myelin basic protein (MBP) and represent a mouse model for CNS specific demyelination similar to that in multiple sclerosis, resulting in a postnatal phenotype characterized by early onset of severe tremor, subsequent hindlimb ataxia and short lifespan. The shiverer defect is cell-intrinsic to the myelinating oligodendrocytes, and a study using aggregation chimeras between normal and shiverer embryos showed that axons could contain both normal and deficient fibers.81 When MBP immunoreactivity was used to identify the degree of chimerism, the results of one study80 strongly suggested that rescue from severe defects such as tremor and ataxia requires extensive and widespread distribution of ES cell-derived MBP, with no or little benefit from patchy MBP expression. Varying degrees of rescue were also seen in a different study,79 in which the authors measured motor function in a graded assay that suggested that also lower levels of chimerism could lead to subtle improvements in motor function. However, as the regional distribution of ES cell derivatives or MBP was not analyzed, a correlation between the degree of chimerism and the level of rescue remains uncertain.
Developmental chimeras have also been used to investigate mechanisms, gene function and potential rescue by normal cells in mouse models of muscular dystrophy, particularly because many null mutants of genes involved in basement membrane and extracellular matrix formation and signaling cause demise very early in development. Adult mice chimeric for cells carrying the early developmental lethal null mutation of alpha 5 integrin develop muscular dystrophy.82 Similarly, deficiency for dystroglycans results in embryonic lethality shortly after implantation; however, null mutant ES cells could contribute substantially to term chimeras produced by blastocyst injection; chimeras with more than 50% mutant cell contribution demonstrated a progressive phenotype of muscle degeneration,83 in which the normal composition of basement membranes suggested that dystroglycans play more than a structural role, which was later confirmed to be signaling.84 Complementation of dystrophin mutant (mdx) mice by blastocyst injection of normal ES cells yielded phenotype correction with 10 to 30% contribution of normal cells to muscle syncytia, but upregulation of dystrophin expression to normal levels.85
In several mouse models with cell non-autonomous defects, smaller proportions of normal cells can be sufficient for phenotypic rescue. In aggregation chimeras, the contribution of 20% normal cells was sufficient to rescue mice carrying the lethal spotted mutation from death from intestinal obstruction as a consequence of lack of ganglion cells in the colon.86 The study revealed that the lack of colonization in the mutant is not the consequence of a cell-autonomous defect of the migrating neuroblasts, but rather, is caused by a deficiency in the mesenchyme. This defect could be rescued by a subset of normal cells permitting colonization of the hind-gut by mutant cells.
Similarly, surviving ID mutant mice rescued to term by complementation with normal ES cells injected into the blastocyst exhibited an average of 20% ES cell contribution to the heart, allowing apparently normal adult heart morphology and size.50 These models, however, require fetal codevelopment of normal and mutant cells to rescue an adult phenotype.
Concluding Remarks
Chimeras have contributed much to our knowledge of mammalian development and remain effective systems to address questions related to new areas of interest in biology such as pluripotent stem cells and organ regeneration. While technologies that use site-specific recombinases to introduce mutations in a tissue-specific or time-specific manner have become the leading tools to investigate gene function and lineage formation,87 chimera approaches provide both complementary and distinct features useful for many areas of investigation. Chimera analysis can pinpoint tissues of interest without the need to generate and analyze several tissue-specific null mutants. Chimera analysis allows determining the role of a gene in tissues in which specific induction of mutations is not yet possible and avoids excluding tissues in which this gene is not expected to play a role. For many naturally occurring or induced lethal mutations not derived by ES cell targeting, production and analysis of aggregation chimeras can be a much easier and faster approach than derivation and/or conditional targeting of ES cells, or the only approach if the mutation is unknown. Thus, chimera approaches support both classic and novel applications for exploring various aspects of mammalian biology.
References
- 1.Tam PP, Rossant J. Mouse embryonic chimeras: Tools for studying mammalian development. Development. 2003;130:6155–6163. doi: 10.1242/dev.00893. [DOI] [PubMed] [Google Scholar]
- 2.Gardner RL. Contributions of blastocyst micromanipulation to the study of mammalian development. Bioessays. 1998;20:168–180. doi: 10.1002/(SICI)1521-1878(199802)20:2<168::AID-BIES9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 3.Espejel S, Roll GR, McLaughlin KJ, Lee AY, Zhang JY, Laird DJ, et al. Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. J Clin Invest. 2010;120:3120–3126. doi: 10.1172/JCI43267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142:787–799. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 5.McLaren A. Mammalian chimaeras. Cambridge: Cambridge University Press; 1976. [Google Scholar]
- 6.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 7.Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, et al. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James D, Noggle SA, Swigut T, Brivanlou AH. Contribution of human embryonic stem cells to mouse blastocysts. Dev Bio. 2006;295:90–102. doi: 10.1016/j.ydbio.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Behringer RR. Human-animal chimeras in biomedical research. Cell Stem Cell. 2007;1:259–262. doi: 10.1016/j.stem.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Manz MG, Di Santo JP. Renaissance for mouse models of human hematopoiesis and immunobiology. Nat Immunol. 2009;10:1039–1042. doi: 10.1038/ni1009-1039. [DOI] [PubMed] [Google Scholar]
- 11.Strom SC, Davila J, Grompe M. Chimeric mice with humanized liver: Tools for the study of drug metabolism, excretion and toxicity. Methods Mol Biol. 2010;640:491–509. doi: 10.1007/978-1-60761-688-7_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross CN, French JA, Orti G. Germ-line chimerism and paternal care in marmosets (Callithrix kuhlii) Proc Natl Acad Sci USA. 2007;104:6278–6282. doi: 10.1073/pnas.0607426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu N, Kruskall MS, Yunis JJ, Knoll JH, Uhl L, Alosco S, et al. Disputed maternity leading to identification of tetragametic chimerism. N Engl J Med. 2002;346:1545–1552. doi: 10.1056/NEJMoa013452. [DOI] [PubMed] [Google Scholar]
- 14.Malan V, Vekemans M, Turleau C. Chimera and other fertilization errors. Clin Genet. 2006;70:363–373. doi: 10.1111/j.1399-0004.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- 15.Winberg J, Gustavsson P, Lagerstedt-Robinson K, Blennow E, Lundin J, Iwarsson E, et al. Chimerism resulting from parthenogenetic activation and dispermic fertilization. Am J Med Genet A. 2010;152:2277–2286. doi: 10.1002/ajmg.a.33594. [DOI] [PubMed] [Google Scholar]
- 16.Strain L, Warner JP, Johnston T, Bonthron DT. A human parthenogenetic chimaera. Nat Genet. 1995;11:164–169. doi: 10.1038/ng1095-164. [DOI] [PubMed] [Google Scholar]
- 17.Giltay JC, Brunt T, Beemer FA, Wit JM, van Amstel HK, Pearson PL, et al. Polymorphic detection of a parthenogenetic maternal and double paternal contribution to a 46,XX/46,XY hermaphrodite. Am J Hum Genet. 1998;62:937–940. doi: 10.1086/301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson WP, Lauzon JL, Innes AM, Lim K, Arsovska S, McFadden DE. Origin and outcome of pregnancies affected by androgenetic/biparental chimerism. Hum Reprod. 2007;22:1114–1122. doi: 10.1093/humrep/del462. [DOI] [PubMed] [Google Scholar]
- 19.Surti U, Hoffner L, Kolthoff M, Dunn J, Hunt J, Sniezek L, et al. Persistent gestational trophoblastic disease after an androgenetic/biparental fetal chimera: A case report and review. Int J Gynecol Pathol. 2006;25:366–372. doi: 10.1097/01.pgp.0000215295.45738.ed. [DOI] [PubMed] [Google Scholar]
- 20.van Dijk BA, Boomsma DI, de Man AJ. Blood group chimerism in human multiple births is not rare. Am J Med Genet. 1996;61:264–268. doi: 10.1002/(SICI)1096-8628(19960122)61:3<264::AID-AJMG11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Kuhl-Burmeister R, Simeoni E, Weber-Matthiesen K, Milde A, Herwartz C, Neppert J, et al. Equal distribution of congenital blood cell chimerism in dizygotic triplets after in-vitro fertilization. Hum Reprod. 2000;15:1200–1204. doi: 10.1093/humrep/15.5.1200. [DOI] [PubMed] [Google Scholar]
- 22.Bogdanova N, Siebers U, Kelsch R, Markoff A, Ropke A, Exeler R, et al. Blood chimerism in a girl with Down syndrome and possible freemartin effect leading to aplasia of the Mullerian derivatives. Hum Reprod. 2010;25:1339–1343. doi: 10.1093/humrep/deq048. [DOI] [PubMed] [Google Scholar]
- 23.Chapman VM, Ansell JD, McLaren A. Trophoblast giant cell differentiation in the mouse: expression of glucose phosphate isomerase (GPI-1) electrophoretic variants in transferred and chimeric embryos. Dev Biol. 1972;29:48–54. doi: 10.1016/0012-1606(72)90042-5. [DOI] [PubMed] [Google Scholar]
- 24.Lo CW, Coulling M, Kirby C. Tracking of mouse cell lineage using microinjected DNA sequences: analyses using genomic Southern blotting and tissue-section in situ hybridizations. Differentiation. 1987;35:37–44. doi: 10.1111/j.1432-0436.1987.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Jaenisch R. At most three ES cells contribute to the somatic lineages of chimeric mice and of mice produced by ES-tetraploid complementation. Dev Biol. 2004;275:192–201. doi: 10.1016/j.ydbio.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich G, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- 27.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 28.Crosby JR, Seifert RA, Soriano P, Bowen-Pope DF. Chimaeric analysis reveals role of Pdgf receptors in all muscle lineages. Nat Genet. 1998;18:385–388. doi: 10.1038/ng0498-385. [DOI] [PubMed] [Google Scholar]
- 29.Collinson JM, Hill RE, West JD. Analysis of mouse eye development with chimeras and mosaics. Int J Dev Biol. 2004;48:793–804. doi: 10.1387/ijdb.041885jc. [DOI] [PubMed] [Google Scholar]
- 30.Gattone VH, 2nd, Goldowitz D. The renal glomerulus and vasculature in ‘aggregation’ chimeric mice. Nephron. 2002;90:267–272. doi: 10.1159/000049062. [DOI] [PubMed] [Google Scholar]
- 31.Eberhard D, Jockusch H. Clonal and territorial development of the pancreas as revealed by eGFPlabelled mouse chimeras. Cell Tissue Res. 2010;342:31–38. doi: 10.1007/s00441-010-1028-y. [DOI] [PubMed] [Google Scholar]
- 32.Ponder BA, Schmidt GH, Wilkinson MM, Wood MJ, Monk M, Reid A. Derivation of mouse intestinal crypts from single progenitor cells. Nature. 1985;313:689–691. doi: 10.1038/313689a0. [DOI] [PubMed] [Google Scholar]
- 33.Eberhard D, Jockusch H. Patterns of myocardial histogenesis as revealed by mouse chimeras. Dev Biol. 2005;278:336–346. doi: 10.1016/j.ydbio.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez L, Kozlov S, Piras G, Stewart CL. Paternal and maternal genomes confer opposite effects on proliferation, cell-cycle length, senescence and tumor formation. Proc Natl Acad Sci USA. 2003;100:13344–13349. doi: 10.1073/pnas.2234026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sturm KS, Berger CN, Zhou SX, Dunwoodie SL, Tan S, Tam PP. Unrestricted lineage differentiation of parthenogenetic ES cells. Dev Genes Evol. 1997;206:377–388. doi: 10.1007/s004270050067. [DOI] [PubMed] [Google Scholar]
- 36.Thomson JA, Solter D. The developmental fate of androgenetic, parthenogenetic and gynogenetic cells in chimeric gastrulating mouse embryos. Genes Dev. 1988;2:1344–1351. doi: 10.1101/gad.2.10.1344. [DOI] [PubMed] [Google Scholar]
- 37.Clarke HJ, Varmuza S, Prideaux VR, Rossant J. The development potential of parthenogenetically derived cells in chimeric mouse embryos: Implications for action of imprinted genes. Development. 1988;104:175–182. doi: 10.1242/dev.104.1.175. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Mann JR, Gadi I, Harbison ML, Abbondanzo SJ, Stewart CL. Androgenetic mouse embryonic stem cells are pluripotent and cause skeletal defects in chimeras: Implications for genetic imprinting. Cell. 1990;62:251–260. doi: 10.1016/0092-8674(90)90363-j. [DOI] [PubMed] [Google Scholar]
- 40.Barton SC, Ferguson-Smith AC, Fundele R, Surani MA. Influence of paternally imprinted genes on development. Development. 1991;113:679–687. doi: 10.1242/dev.113.2.679. [DOI] [PubMed] [Google Scholar]
- 41.Maandag EC, van der Valk M, Vlaar M, Feltkamp C, O'Brien J, van Roon M, et al. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 1994;13:4260–4268. doi: 10.1002/j.1460-2075.1994.tb06746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiner A, Del Mar N, Meade CA, Yang H, Dragatsis I, Zeitlin S, et al. Neurons lacking huntingtin differentially colonize brain and survive in chimeric mice. J Neurosci. 2001;21:7608–7619. doi: 10.1523/JNEUROSCI.21-19-07608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarkowski AK, Witkowska A, Opas J. Development of cytochalasin in B-induced tetraploid and diploid/tetraploid mosaic mouse embryos. J Embryol Exp Morphol. 1977;41:47–64. [PubMed] [Google Scholar]
- 44.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eggan K, Akutsu H, Loring J, Jackson-Grusby L, Klemm M, Rideout WM, 3rd, et al. Hybrid vigor, fetal overgrowth and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc Natl Acad Sci USA. 2001;1:1. doi: 10.1073/pnas.101118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boland MJ, Hazen JL, Nazor KL, Rodriguez AR, Gifford W, Martin G, et al. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461:91–94. doi: 10.1038/nature08310. [DOI] [PubMed] [Google Scholar]
- 47.Zhao XY, Li W, Lv Z, Liu L, Tong M, Hai T, et al. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461:86–90. doi: 10.1038/nature08267. [DOI] [PubMed] [Google Scholar]
- 48.Kang L, Wang J, Zhang Y, Kou Z, Gao S. iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell. 2009;5:135–138. doi: 10.1016/j.stem.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Hanna LA, Foreman RK, Tarasenko IA, Kessler DS, Labosky PA. Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Genes Dev. 2002;16:2650–2661. doi: 10.1101/gad.1020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fraidenraich D, Stillwell E, Romero E, Wilkes D, Manova K, Basson CT, et al. Rescue of cardiac defects in id knockout embryos by injection of embryonic stem cells. Science. 2004;306:247–252. doi: 10.1126/science.1102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipinski MM, Macleod KF, Williams BO, Mullaney TL, Crowley D, Jacks T. Cell-autonomous and noncell-autonomous functions of the Rb tumor suppressor in developing central nervous system. EMBO J. 2001;20:3402–3413. doi: 10.1093/emboj/20.13.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwiatkowski AV, Garner CC, Nelson WJ, Gertler FB. Cell autonomous defects in cortical development revealed by two-color chimera analysis. Mol Cell Neurosci. 2009;41:44–50. doi: 10.1016/j.mcn.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jouneau A, Zhou Q, Camus A, Brochard V, Maulny L, Collignon J, et al. Developmental abnormalities of NT mouse embryos appear early after implantation. Development. 2006;133:1597–1607. doi: 10.1242/dev.02317. [DOI] [PubMed] [Google Scholar]
- 54.Markert CL, Petters RM. Manufactured hexaparental mice show that adults are derived from three embyronic cells. Science. 1978;202:56–58. doi: 10.1126/science.694518. [DOI] [PubMed] [Google Scholar]
- 55.Soriano P, Jaenisch R. Retroviruses as probes for mammalian development: allocation of cells to the somatic and germ cell lineages. Cell. 1986;46:19–29. doi: 10.1016/0092-8674(86)90856-1. [DOI] [PubMed] [Google Scholar]
- 56.Chen J, Lansford R, Stewart V, Young F, Alt FW. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci USA. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liegeois NJ, Horner JW, DePinho RA. Lens complementation system for the genetic analysis of growth, differentiation and apoptosis in vivo. Proc Natl Acad Sci USA. 1996;93:1303–1307. doi: 10.1073/pnas.93.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulinpromoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 59.Chen J. Analysis of gene function in lymphocytes by RAG-2-deficient blastocyst complementation. Adv Immunol. 1996;62:31–59. doi: 10.1016/s0065-2776(08)60427-7. [DOI] [PubMed] [Google Scholar]
- 60.Young FM, Pinkert CA, Bottaro A. Analysis of lymphocyte development and function using the RAG-deficient blastocyst complementation system. Methods Mol Biol. 2004;271:77–90. doi: 10.1385/1-59259-796-3:077. [DOI] [PubMed] [Google Scholar]
- 61.Kim JM, White JM, Shaw AS, Sleckman BP. MAPK p38 alpha is dispensable for lymphocyte development and proliferation. J Immunol. 2005;174:1239–1244. doi: 10.4049/jimmunol.174.3.1239. [DOI] [PubMed] [Google Scholar]
- 62.Cotta-de-Almeida V, Westerberg L, Maillard MH, Onaldi D, Wachtel H, Meelu P, et al. Wiskott Aldrich syndrome protein (WASP) and N-WASP are critical for T cell development. Proc Natl Acad Sci USA. 2007;104:15424–15429. doi: 10.1073/pnas.0706881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jansson L, Larsson J. W41/W41 blastocyst complementation: A system for genetic modeling of hematopoiesis. Blood. 2010;115:47–50. doi: 10.1182/blood-2009-07-235622. [DOI] [PubMed] [Google Scholar]
- 64.Zhao H, Yang Y, Partanen J, Ciruna BG, Rossant J, Robinson ML. Fibroblast growth factor receptor 1 (Fgfr1) is not essential for lens fiber differentiation in mice. Mol Vis. 2006;12:15–25. [PMC free article] [PubMed] [Google Scholar]
- 65.Gardner RL, Johnson MH. Investigation of cellular interaction and deployment in the early mammalian embryo using interspecific chimaeras between the rat and mouse. Ciba Found Symp. 1975;0:183–200. doi: 10.1002/9780470720110.ch9. [DOI] [PubMed] [Google Scholar]
- 66.Gardner RL, Johnson MH. Investigation of early mammalian development using interspecific chimaeras between rat and mouse. Nat New Biol. 1973;246:86–89. doi: 10.1038/newbio246086a0. [DOI] [PubMed] [Google Scholar]
- 67.Solter D. Viable rat-mouse chimeras: where do we go from here? Cell. 2010;142:676–678. doi: 10.1016/j.cell.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 68.Yamanouchi K, Zhou H, Roy-Chowdhury N, Macaluso F, Liu L, Yamamoto T, et al. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology. 2009;49:258–267. doi: 10.1002/hep.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willenbring H, Bailey AS, Foster M, Akkari Y, Dorrell C, Olson S, et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med. 2004;10:744–748. doi: 10.1038/nm1062. [DOI] [PubMed] [Google Scholar]
- 70.Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci USA. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stappenbeck TS, Gordon JI. Rac1 mutations produce aberrant epithelial differentiation in the developing and adult mouse small intestine. Development. 2000;127:2629–2642. doi: 10.1242/dev.127.12.2629. [DOI] [PubMed] [Google Scholar]
- 72.Martinic MM, Rulicke T, Althage A, Odermatt B, Hochli M, Lamarre A, et al. Efficient T cell repertoire selection in tetraparental chimeric mice independent of thymic epithelial MHC. Proc Natl Acad Sci USA. 2003;100:1861–1866. doi: 10.1073/pnas.252641399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu J, Anuar F, Ali SM, Ng MY, Phua DC, Hunziker W. Zona occludens-2 is critical for blood-testis barrier integrity and male fertility. Mol Biol Cell. 2009;20:4268–4277. doi: 10.1091/mbc.E08-12-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldowitz D. The weaver granuloprival phenotype is due to intrinsic action of the mutant locus in granule cells: Evidence from homozygous weaver chimeras. Neuron. 1989;2:1565–1575. doi: 10.1016/0896-6273(89)90045-7. [DOI] [PubMed] [Google Scholar]
- 75.Yang H, Jensen P, Goldowitz D. The community effect and Purkinje cell migration in the cerebellar cortex: Analysis of scrambler chimeric mice. J Neurosci. 2002;22:464–470. doi: 10.1523/JNEUROSCI.22-02-00464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mullen RJ, Hamre KM, Goldowitz D. Cerebellar mutant mice and chimeras revisited. Perspect Dev Neurobiol. 1997;5:43–55. [PubMed] [Google Scholar]
- 77.Crosby JR, Tappan KA, Seifert RA, Bowen-Pope DF. Chimera analysis reveals that fibroblasts and endothelial cells require platelet-derived growth factor receptorbeta expression for participation in reactive connective tissue formation in adults but not during development. Am J Pathol. 1999;154:1315–1321. doi: 10.1016/s0002-9440(10)65384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buetow BS, Tappan KA, Crosby JR, Seifert RA, Bowen-Pope DF. Chimera analysis supports a predominant role of PDGFRbeta in promoting smooth-muscle cell chemotaxis after arterial injury. Am J Pathol. 2003;163:979–984. doi: 10.1016/s0002-9440(10)63457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kiel ME, Chen CP, Sadowski D, McKinnon RD. Stem cell-derived therapeutic myelin repair requires 7% cell replacement. Stem Cells. 2008;26:2229–2236. doi: 10.1634/stemcells.2008-0218. [DOI] [PubMed] [Google Scholar]
- 80.Low HP, Greco B, Tanahashi Y, Gallant J, Jones SN, Billings-Gagliardi S, et al. Embryonic stem cell rescue of tremor and ataxia in myelin-deficient shiverer mice. J Neurol Sci. 2009;276:133–137. doi: 10.1016/j.jns.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mikoshiba K, Yokoyama M, Inoue Y, Takamatsu K, Tsukada Y, Nomura T. Oligodendrocyte abnormalities in shiverer mouse mutant are determined in primary chimaeras. Nature. 1982;299:357–359. doi: 10.1038/299357a0. [DOI] [PubMed] [Google Scholar]
- 82.Taverna D, Disatnik MH, Rayburn H, Bronson RT, Yang J, Rando TA, et al. Dystrophic muscle in mice chimeric for expression of alpha5 integrin. J Cell Biol. 1998;143:849–859. doi: 10.1083/jcb.143.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cote PD, Moukhles H, Lindenbaum M, Carbonetto S. Chimaeric mice deficient in dystroglycans develop muscular dystrophy and have disrupted myoneural synapses. Nat Genet. 1999;23:338–342. doi: 10.1038/15519. [DOI] [PubMed] [Google Scholar]
- 84.Bozzi M, Morlacchi S, Bigotti MG, Sciandra F, Brancaccio A. Functional diversity of dystroglycan. Matrix Biol. 2009;28:179–187. doi: 10.1016/j.matbio.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Stillwell E, Vitale J, Zhao Q, Beck A, Schneider J, Khadim F, et al. Blastocyst injection of wild type embryonic stem cells induces global corrections in mdx mice. PLoS One. 2009;4:4759. doi: 10.1371/journal.pone.0004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kapur RP, Yost C, Palmiter RD. Aggregation chimeras demonstrate that the primary defect responsible for aganglionic megacolon in lethal spotted mice is not neuroblast autonomous. Development. 1993;117:993–999. doi: 10.1242/dev.117.3.993. [DOI] [PubMed] [Google Scholar]
- 87.Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cel. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]