Abstract
The mother–infant bond provides the foundation for the infant's future mental health and adaptation and depends on the provision of species-typical maternal behaviors that are supported by neuroendocrine and motivation-affective neural systems. Animal research has demonstrated that natural variations in patterns of maternal care chart discrete profiles of maternal brain–behavior relationships that uniquely shape the infant's lifetime capacities for stress regulation and social affiliation. Such patterns of maternal care are mediated by the neuropeptide Oxytocin and by stress- and reward-related neural systems. Human studies have similarly shown that maternal synchrony—the coordination of maternal behavior with infant signals—and intrusiveness—the excessive expression of maternal behavior—describe distinct and stable maternal styles that bear long-term consequences for infant well-being. To integrate brain, hormones, and behavior in the study of maternal–infant bonding, we examined the fMRI responses of synchronous vs intrusive mothers to dynamic, ecologically valid infant videos and their correlations with plasma Oxytocin. In all, 23 mothers were videotaped at home interacting with their infants and plasma OT assayed. Sessions were micro-coded for synchrony and intrusiveness. Mothers were scanned while observing several own and standard infant-related vignettes. Synchronous mothers showed greater activations in the left nucleus accumbens (NAcc) and intrusive mothers exhibited higher activations in the right amygdala. Functional connectivity analysis revealed that among synchronous mothers, left NAcc and right amygdala were functionally correlated with emotion modulation, theory-of-mind, and empathy networks. Among intrusive mothers, left NAcc and right amygdala were functionally correlated with pro-action areas. Sorting points into neighborhood (SPIN) analysis demonstrated that in the synchronous group, left NAcc and right amygdala activations showed clearer organization across time, whereas among intrusive mothers, activations of these nuclei exhibited greater cross-time disorganization. Correlations between Oxytocin with left NAcc and right amygdala activations were found only in the synchronous group. Well-adapted parenting appears to be underlay by reward-related motivational mechanisms, temporal organization, and affiliation hormones, whereas anxious parenting is likely mediated by stress-related mechanisms and greater neural disorganization. Assessing the integration of motivation and social networks into unified neural activity that reflects variations in patterns of parental care may prove useful for the study of optimal vs high-risk parenting.
Keywords: maternal behavior, attachment, synchrony, imaging, motivation, emotion regulation
INTRODUCTION
The mother's attachment to her infant is a complex process involving biological, behavioral, and psychological components that aim to ensure reproductive success and provide the foundation for the infant's well-being and adaptation (Bowlby, 1958; Carter et al, 2005). Across mammalian species, infant growth and survival depend on the provision of a unique set of maternal behaviors that appear immediately after birth and function to organize the infant's physiology and behavior (Leckman and Herman, 2002; Meaney, 2001). In humans, the maternal behavioral repertoire includes gaze at infant face, ‘motherese' high-pitched vocalizations, expression of positive affect, affectionate touch, and the synchronous adaptation of these behaviors to moments of infant responsiveness (Barrett and Fleming, 2011; Feldman and Eidelman, 2007). Such human maternal behaviors parallel the licking-and-grooming and arched-back nursing of other mammals, which have been linked with the affiliation-related neuropeptide Oxytocin and the functioning of motivational and affective neural systems (Champagne et al, 2004; Lee et al, 2000; Oxley and Fleming, 2000; Shahrokh et al, 2010; Strathearn et al, 2009; Toscano et al, 2009). However, to date, little research examined the integration of brain circuits, affiliative hormones, and maternal behavior in human mothers.
The formation of the mother–infant bond draws on the timely provision of well-adapted maternal behaviors during the early post-partum period (Bowlby, 1969; Feldman et al, 2009; Tronick, 1989). Research in rodents indicates that naturally occurring variations in maternal behavior chart distinct patterns of mothering each associated with a specific bio-behavioral profile in mother and child. These patterns of maternal care initiate a cascade of epigenetic processes that uniquely shape gene expression, organize the Oxytocinergic system that supports bond formation in mammals, and determine the infant's lifetime capacity to handle stress (Champagne, 2008; Weaver et al, 2004). Studies in humans have similarly shown that optimal mothering involves the synchronous coordination between maternal behavior and the infant's social readiness (Feldman, 2007), and that the degree of interactive synchrony is associated with peripheral measures of Oxytocin in both parent and child (Feldman et al, 2010b, 2011). On the other hand, intrusive mothering describes a pattern of care that correlates with maternal anxiety and is expressed in excessive maternal behavior that disregards the child's communications and provides stimulation when the infant signals a need for rest (Kaitz and Maytal, 2005). The human synchronous and intrusive maternal styles, like patterns of maternal care observed in other mammals, are naturally expressed variations in the general population and represent distinct bio-behavioral profiles of parenting. Longitudinal studies have shown that the synchronous and intrusive maternal constellations are individually stable from birth to adolescence, are associated with distinct patterns of maternal and infant HPA and parasympathetic response, and are uniquely predictive of children's social–emotional outcomes across childhood and up to adult life (Feldman, 2010; Feldman et al, 2010c; Isabella and Belsky, 1991; Sroufe, 2005).
Maternal–infant bonding is based on the co-activation of motivational mechanisms that index stress, such as heightened vigilance and threat detection, and those associated with reward, which address the high incentive value placed on the infant (Barrett and Fleming, 2011). Such stress- and reward-related mechanisms must integrate to form the parent–infant bond in ways that balance the activity of these two components (Leckman et al, 2004). As human attachment develops in the context of the parent–infant relationship during the first months of life (Bowlby, 1969; Sroufe, 2005), describing the mechanisms that link brain and behavior in the formation of affiliative bonds and may reflect underlying neural variations in motivation, vigilance, and reward systems among typical mothers may be of theoretical and clinical importance for the study of healthy and at-risk parenting.
The stress and reward components of maternal attachment are supported by distinct motivational and threat-related networks. The nucleus accumbens (NAcc) and amygdala are limbic structures involved in the ‘labeling' of a stimulus with an emotional valance and have shown to play a role in maternal behavior and bond formation in mammals (Cardinal et al, 2002). The NAcc, part of the mesolimbic dopaminergic reward circuit, imbues the infant with intuitive reinforcement and provides motivational drive to maternal behavior (Aron et al, 2005; Cardinal et al, 2002; Leckman and Herman, 2002; Lee et al, 2000). Lesions (Champagne et al, 2004) and DA manipulation studies (Li and Fleming, 2003) have demonstrated that the NAcc is critical for maternal behavior, particularly for maternal motivation to nest and retrieve pups (Champagne et al, 2004; Hansen, 1994; Hansen et al, 1991; Keer and Stern, 1999; Li and Fleming, 2003; Vernotica et al, 1999). The amygdala, a central node of the limbic affective system, has similarly been implicated in social affiliation and maternal attachment (Fleming and Korsmit, 1996; Lee et al, 2000; Oxley and Fleming, 2000). Lesions to the amygdala reduce maternal behavior (Toscano et al, 2009) and significant amygdala c-fos changes are observed following mother–pup interaction (Fleming and Korsmit, 1996). However, whereas the NAcc is likely to play a role in the reward-related aspects of mothering, the amygdala, which mediates fear processing and vigilance (Cardinal et al, 2002), may be important for the stress-related components. Both the NAcc and amygdala work in concert with several cortical areas, including the medial preoptic area, which integrates infant sensory cues and foster adequate parenting (Insel and Young, 2001; Lee et al, 2000; Oxley and Fleming, 2000), the anterior cingulate (ACC), and the dorso-medial prefrontal cortex (dmPFC) (MacLean et al, 1990; MacLean and Newman, 1988; Murphy et al, 1981; Slotnick, 1967; Stamm, 1955). Some of these cortical areas, particularly the ACC and dmPFC, have been implicated in empathy and ‘theory of mind' skills (Assaf et al, 2009; Gallagher and Frith, 2003; Vollm et al, 2006), and may increase the mother's capacity to read and respond to her infant's signals. As such, the neural basis of mothering is supported by sub-cortical motivational limbic areas as well as by higher-level emotion modulation networks.
Consistent with research in animals, functional imaging studies have underscored the importance of the NAcc and amygdala for human parenting (Barrett and Fleming, 2011; Strathearn et al, 2009; Swain et al, 2007). Similarly, fMRI studies have demonstrated the involvement of reward dopaminergic circuits, salience areas containing Oxytocin projections (Strathearn et al, 2009), the hippocampus (Swain et al, 2007), ACC, and insula in human parenting (Bartels and Zeki, 2004; Lorberbaum et al, 2002; Noriuchi et al, 2008; Swain et al, 2007). Yet, to date, most fMRI studies of parenting reported activations in discrete brain areas in response to infant pictures or cries and did not explore the parents' systemic brain response to dynamic infant stimuli or examined the relations between brain activations and naturally occurring variations in maternal behavior.
In light of the above, this study sought to extend knowledge on the neurobiological basis of human parenting. We hypothesized that similar to the findings observed in other mammals (Meaney, 2001), patterns of maternal care in human mothers, specifically synchronous and intrusive maternal behavior, would chart distinct bio-behavioral profiles and would be expressed in unique patterns of brain–behavior correlations. We videotaped mother–infant interaction and sessions were micro-coded for maternal synchrony and intrusiveness. We then assessed the brain responses in these two groups of mothers to video vignettes of their own infant to provide an individually-tailored ecological trigger to the neural processes that underlie mothering in these two groups. Plasma Oxytocin levels were sampled to examine the integration of maternal behaviors, parenting networks, and affiliation-related neuropeptides. We hypothesized that among synchronous mothers, infant cues would activate networks related to reward circuitry and those would be associated with maternal Oxytocin. Among mothers presenting intrusive behaviors, we expected that the vigilance component of mothering would be more pronounced, resulting in greater amygdala-associated activations.
MATERIALS AND METHODS
Participants
In all, 28 mothers (ages 22–37, 12–21 years of education) were recruited when their infant was 4–6 months old. Mothers were screened for high depression and anxiety symptoms using the Beck Depression Inventory (BDI) (Beck, 1978) and the State-Trait Anxiety Inventory (Spielberger and Lushene, 1970). Five mothers were excluded from the final analysis: two mothers owing to high levels of depressive symptoms (BDI scores of 12 and 14) and three owing to an incomplete imaging procedure. The study was approved by the Institutional Review Board and all participants signed an informed consent.
Procedure
The study included two sessions. In the first, families were visited at home and several sessions of mother–infant interaction and infant solitary play were videotaped. Films were used for both behavioral analysis and as fMRI stimuli. In the second session, mothers participated in brain scanning at the Tel-Aviv Sourasky Medical Center.
fMRI Paradigm
Mothers were presented with a series of infant-related video vignettes while lying in the scanner. Stimuli included a 2-min movie of their own infant during solitary play and a 2-min movie of mother–own–infant interactions (Figure 1, red frame). Control conditions included unfamiliar infants and unfamiliar mother–infant interactions, which were standard across subjects (Figure 1, green frame). Stimuli were counterbalanced and were randomly presented in five different order patterns.
Figure 1.
Header: (a) Experimental procedure. Footer: Mothers and infants were recruited 4–6 months post-partum and videotaped during a home visit. Video vignettes of mother–infant interaction were micro-coded for maternal synchrony and intrusiveness. Vignettes of interaction and of infant solitary play were used as functional magnetic resonance imaging (fMRI) stimuli. Header: (b) Experimental paradigm. Footer: Mothers were presented with infant-related vignettes of individually tailored stimuli of own infant and own mother–infant interaction (shown in red). Control stimuli of unfamiliar infants and interactions were fixed for all subjects and included stranger infants, typical mother–infant interaction, and pathological mother–infant interactions (shown in green). Each vignette was presented once and lasted 2 min. Clips were previewed by rest with fixation period of 1 min. Rest with fixation periods of 15–18 s were presented in between the films.
fMRI Acquisition
Imaging was performed on a GE 3T Sigma Horizon echo speed scanner with a resonant gradient echoplanar imaging system. Functional images were acquired using a single-shot echo-planar T2*-weighted sequence. The following parameters were used: 128 × 128 matrix; field of view of 20 × 20 cm; 39 slices with 3 mm thickness and no gap; TR/TE 3000/35; and flip angle 90°, acquisition orientation was of the fourth ventricle plane. In addition, each functional scan was accompanied by a three-dimensional anatomical scan using T1-SPGR sequence (1 × 1 × 1 mm3).
fMRI Analysis
BrainVoyager QX version 2.1 (Brain Innovation, Maastricht, the Netherlands) was used to analyze the recorded fMRI data (Goebel et al, 2006). The first six functional volumes, before signal stabilization, were excluded from the analysis. Preprocessing included the following operations: 3D motion correction using trilinear interpolation, linear trend removal and high-pass filtering. A 4-mm full-width at half-maximum gaussian smoothing was used to overcome differences in inter-subject localization. Functional 2D data were manually aligned and co-registered with 3D anatomical data, which were normalized into Talairach space. To account for a hemodynamic response, predictors were convolved with 6-s hemodynamic response filter for all participants.
Whole Brain Analysis
Statistical maps were prepared for each subject using a general linear model (GLM), in which the various activation blocks were defined as district predictors. Following, multi-subject analysis was computed (fixed and corrected effect).
Region-of-Interest Analysis
Region-of-interest (ROI) analysis was conducted on bilateral NAcc and amygdala. ROI selection was a priori and theory-based. ROI's were defined functionally and anatomically as a box-shaped volume of 7 voxels diameter around the peak of activations in all subjects. This was obtained by a whole brain contrast of all conditions vs rest (fixed and corrected effect, q(FDR)<0.05). Signal values were extracted from each ROI for all conditions and submitted to a descriptive statistical analysis using the ‘Statistica' software. Signal change was calculated for each condition as % of baseline. Baseline was considered as the average signal collected at all rest periods throughout the paradigm. A secondary index of ‘own infant–stranger infant' was calculated by subtracting the ‘stranger infant' signal change from the ‘own infant' signal change to assess the ROI activity that is specific to own infant and does not address a general reaction to infants.
Functional Correlation Maps Analysis
The correlation analysis was based on each ROI's time courses (Friston et al, 1993; Greicius et al, 2003), which were functionally defined for each subject as stated above. Average ‘seed' time courses were obtained for each subject by averaging the time series of all voxels in the specific ROI (left NAcc and right amygdala, which differentiated the groups in the ROI analysis). These average time courses were used as a GLM predictor to compute a voxel-by-voxel fit (analogous to linear correlation, fixed and corrected effect) along the entire paradigm (including all standard as well as individually tailored stimuli, see Figure 1). A second-level t-test was applied to determine the brain areas that showed significant functional connectivity between groups (fixed and uncorrected effect).
Behavioral Coding
To define the mother's synchronous vs intrusive style, interactions were micro-coded by trained coders on a computerized system (Noldus, Wageningen, the Netherlands) in 0.01 s frames, consistent with previous research on parent–infant interaction (Feldman and Eidelman, 2004). Four non-verbal categories of parenting behavior were coded and each category included a set of mutually exclusive codes (an ‘uncodable' code was added to each category to address moments when codes could not be determined). Categories and codes were as follows: parent gaze—to infant, to object or environment, gaze aversion; parent affect—positive, neutral, negative; parent vocalizations—motherese (high-pitched, sing-song vocalization), adult speech to infant, adult speech to other adult, none; and parent touch—affectionate touch (eg, hugging, kissing, stroking), touch of infant extremities, functional touch, proprioceptive touch (ie, changing infant position in space), object presentation, stimulatory touch, and no touch. Infant behavior was coded in a separate viewing for similar categories, including infant gaze—to parent, to object or environment, gaze aversion; infant affect—negative, neutral, positive; infant vocalizations—cry, fuss, neutral vocalizations, positive vocalizations (giggling or laughing), no vocalizations; and infant exploration—reaching to a toy, playing with one hand, playing with two hands, mouthing a toy, and no play.
Inter-rater reliability was conducted for 10% of the interactions and averaged 98% (κ=0.84). For each behavior, we computed the proportions of time out of the entire interaction this behavior occurred. Maternal synchrony and intrusiveness were defined as conditional probabilities that address the simultaneous behaviors of mother and infant, consistent with previous research (Feldman et al, 2011, 2010c). Maternal synchrony was defined as mother coordinating moments of social gaze and affectionate touch with episodes of infant-positive affect, vocalizations, or social gaze. Maternal intrusiveness was defined as mothers providing stimulatory or proprioceptive touch or presenting objects when infants showed gaze aversion and a need for rest. On the basis of our previous research, maternal intrusiveness was defined as the overall proportion of intrusive behavior observed across the interaction, a measure that has shown to correlate with maternal and infant HPA and parasympathetic responses (Feldman et al, 2010c). Synchrony was defined as the mean durations of synchronous episodes—moments in which mother and infant coordinate their positive social engagement—which we found to be associated with plasma and salivary OT (Feldman et al, 2011; Weisman et al, 2011). The behavioral coding resulted in two groups: synchronous mothers (N=13) and intrusive mothers (N=10).
Sorting Points into Neighborhood
Sorting points into neighborhood (SPIN) is an unsupervised method for sorting and visualizing multidimensional data (Tsafrir et al, 2005). The algorithm iteratively creates an optimal ordering of the objects/signal being studied, such that the distances are small along the diagonal of the pair-wise distance matrix and increase as they move away from it. This ordering enables to sort distinct groups of subjects that display similar patterns over several samples (Tsafrir et al, 2005).
Oxytocin
Oxytocin data were available for 13 synchronous mothers and six intrusive mothers. Mothers were visited at home during the evening hours (1600–2000 hours) and blood was drawn from the antecubital vein into 9 ml chilled vacutainer tubes containing lithium heparin that were supplemented with 400 KIU of Trasylol (Bayer, Leverkusen, Germany) per 1 ml blood. Oxytocin samples were kept ice-chilled for up to 2 h before being centrifuged at 4 °C at 1000 g for 15 min. Supernatants were collected and stored at −80 °C until assayed. Mothers were asked to refrain from food intake 30 min before blood draw. Maternal blood was drawn at least 30 min after nursing and 30 min before nursing. Determination of OT was performed using a commercial OT enzyme-linked immunosorbent assay kit (Assay Design, Ann Arbor, Michigan) as described in earlier studies (Carter, 2007; Feldman et al, 2007b; Gordon et al, 2008; Levine et al, 2007). Measurements were performed in duplicate and the concentrations of samples were calculated by using MATLAB-7 (The MathWorks, Natick, Massachusetts) according to relevant standard curves. The intra- and inter-assay coefficients were less than 7% and 15.8%, respectively.
RESULTS
Behavioral Analysis
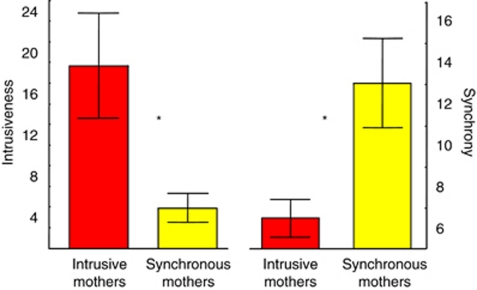
To validate our classification of the two groups of mothers, we examined the degree of synchrony and intrusiveness in each group and the findings are presented in Figure 2. As seen, among synchronous mothers, the degree of synchrony was significantly higher than that among the intrusive mothers (F=4.89, p=0.038). In parallel, among intrusive group, the amount of intrusive behavior was significantly higher than that in the synchronous group (F=8.98, p=0.0069).
Figure 2.
Header: Mean behavioral scores of synchrony and intrusiveness in synchronous and intrusive mothers. Footer: Among the synchronous mothers, the degree of synchrony was significantly higher than among the intrusive mothers (F=4.89, p=0.038). Among the intrusive mothers, the degree of intrusiveness was significantly higher than that in the synchronous group (F=8.98, p=0.0069). *p<0.05.
Whole Brain GLM
To explore the functional neural networks that are activated when mothers observe their own infants, a whole-brain contrast was performed between own infant vignette and a strange infant vignette. This analysis revealed several areas that are activated while mothers observed their own infant as compared to a strange infant (p<0.03, random and uncorrected effect, N=23, see Figure 3 and Table 1).
Cortical attention areas oriented to perception–action, including (a) visual association areas and (b) mirror neuron system areas, including superior temporal sulcus, ventral premotor, left inferior parietal cortex, and bilateral inferior frontal gyrus (IFG).
Limbic/paralimbic vigilance areas, including bilateral amygdala, right NAcc, right cingulate cortex, and left insula.
Figure 3.
Header: Brain response to own infant video. Footer: Blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) statistical maps for 23 mothers presented with the contrast own infant (shown in orange)>stranger infant (shown in blue), showing activation in limbic areas such as the right nucleus accumbens and amygdala, and cortical activations such as the frontal, parietal temporal, and occipital gyri and insula.
Table 1. Brain Regions Showing Significant Activations in the Contrast: Own Infant>Unfamiliar Infant.
| x | y | z | BA | t score | p score | |
|---|---|---|---|---|---|---|
| Confidence threshold min=3.23 | ||||||
| Right inferior parietal lobule | 59 | −38 | 27 | 40 | 3.97555 | 0.00064 |
| Right inferior temporal gyrus | 41 | −68 | −3 | 5.07622 | 0.000044 | |
| Right precentral gyrus | 38 | −11 | 60 | 6 | 4.47142 | 0.000191 |
| Right claustrum | 29 | 19 | 9 | 5.15995 | 0.000036 | |
| Right precuneus | 23 | −56 | 54 | 7 | 4.62824 | 0.00013 |
| Right precuneus | 5 | −77 | 51 | 7 | 5.65198 | 0.000011 |
| Right caudate head | 14 | 16 | 3 | 4.39934 | 0.000227 | |
| Right nucleus accumbens | 14 | −2 | −9 | 4.40801 | 0.000223 | |
| Right medial frontal gyrus | 2 | −20 | 48 | 6 | 4.92285 | 0.000064 |
| Right superior frontal gyrus | 5 | 10 | 57 | 6 | 3.85059 | 0.000868 |
| Right cingulate gyrus | 5 | 19 | 30 | 32 | 4.53295 | 0.000164 |
| Left medial frontal gyrus | −4 | 40 | 33 | 9 | 4.26043 | 0.000319 |
| Left postcentral gyrus | −1 | −53 | 69 | 7 | 4.1617 | 0.000407 |
| Left caudate body | −10 | 4 | 15 | 3.95063 | 0.00068 | |
| Left inferior occipital gyrus | −40 | −71 | −3 | 19 | 5.03215 | 0.000049 |
| Left ventro-lateral thalamus | −19 | −14 | 18 | 4.36408 | 0.000248 | |
| Left insula | −37 | 13 | 3 | 4.21708 | 0.000355 | |
| Left supramarginal gyrus | −52 | −50 | 27 | 40 | 4.53688 | 0.000163 |
| Left inferior parietal lobule | −64 | −38 | 27 | 40 | 5.41971 | 0.000019 |
| Confidence threshold min=2.23 | ||||||
| Left amygdala | −10 | −5 | −12 | 34 | 3.04573 | 0.00593 |
| Right amygdala | 29 | 1 | −18 | 34 | 3.49067 | 0.00207 |
x, y and z represent talairach coordinates. N=23, random effect, uncorrected, cluster size >6, confidence threshold min=3.2.
ROI analysis
Signal ANOVA analysis
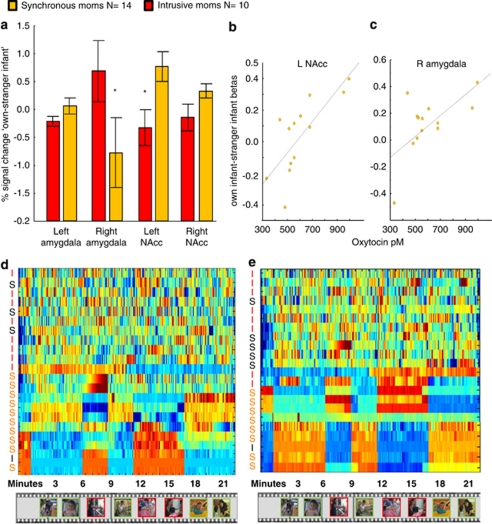
A contrast index of own–other baby signal was calculated for each mother and each region (see Materials and Methods). A repeated measure 2 × 4 ANOVA was conducted to bilateral amygdala and NAcc according to the type of maternal care—synchronous (N=13) and intrusive (N=10) mothering. Analysis revealed a significant two-way interaction effect between ROI and maternal caregiving group (F(3, 57)=3.8048, p=0.01480). Post hoc LSD test (df=74.284) showed that higher activations in the left NAcc was observed in the synchronous as compared to the intrusive group (p=0.015), and significantly higher left NAcc activations than right amygdala activations were observed among the synchronous mothers (p=0.004939). The right amygdala also differentiated between the groups and showed a significantly higher signal change in the intrusive mothers group (p=0.008) (Figure 4a).
Figure 4.
Header: Region-of-Interest (ROI) signal analysis from nucleus accumbens (NAcc) and amygdala in synchronous and intrusive mothers. Footer: (a) Signal change was calculated as % from baseline, and then subtractions of stranger baby from own baby was preformed. A repeated measure 2 × 4 general linear model (GLM) revealed significant interaction effect (F(3, 57)=3.8048, p=0.01480). Post hoc least significant difference (LSD) test mark the signal of left NAcc in synchronous moms as significantly higher than intrusive moms (p=0.015275), and also higher than the right amygdala of the same group (p=0.004939). Right amygdala's signal of intrusive moms were significantly higher than right amygdala's signal of synchronous moms (p=0.008181), which are higher than the left NA in the same group (trend, p=0.078460). (b) Correlation between Oxytocin plasma levels and left NAcc β weights in the contrast own infant>stranger infant are evident only in synchronous moms (N=14, r=0.7673, p=0.0014) and intrusive moms (N=6, r=−0.3877, p=0.4475). (c) Correlation between Oxytocin plasma levels and right amygdala β weights in the contrast own infant>stranger infant are evident only in synchronous moms (r=0.6490, p=0.012) and intrusive moms (r=0.5863, p=0.2213), not shown. (d) Sorting points into neighborhood (SPIN) analysis. Synchrony clustering according to the activation time course of the left NAcc. (e) SPIN analysis. Synchrony clustering according to the activation time course of the right amygdala. Mothers were presented with infant-related vignettes of individually tailored stimuli of own infant and own mother–infant interaction (shown in red). Control stimuli of unfamiliar infants and interactions were fixed for all subjects and included stranger infants, typical mother–infant interaction, and pathological mother–infant interactions (shown in green). An intrusive mother in the intrusive cluster is marked with red ‘I'. A synchronous mother in the synchrony cluster is marked with orange ‘S'. Mothers who appear outside of their cluster are marked with a black initial. *p<0.05.
Plasma oxytocin levels and correlations of oxytocin with left NAcc and right amygdala
No mean-level differences in Oxytocin levels were found between the synchronous and intrusive groups (F(Pearson,1, 18)=0.0381, p=0.8474). However, when considering the correlation between the activation of ROI and Oxytocin, a between-group categorized correlation was found. In the synchronous group, plasma Oxytocin levels were significantly correlated with the left NAcc β-values (r=0.7673, p=0.0014) (Figure 4b). In the intrusive group, on the other hand, there was a negative nonsignificant trend (r=−0.3877, p=0.4475). These results should be interpreted with caution owing to the small number of intrusive mothers with Oxytocin data. In addition, among the synchronous group, plasma Oxytocin levels showed a significant positive correlation with right amygdala β-values (r=0.6490, p=0.012) (Figure 4c). Among the intrusive mothers, there was a positive nonsignificant trend of correlation (r=0.5863, p=0.2213), and this may again relate to the low number of intrusive mothers with Oxytocin data. However, no significant correlations were found between the ROI activation and Oxytocin levels when the entire sample was considered (left NAcc: r=−0.0160, p=0.9331; right amygdala: r=0.2785, p=0.1361) and correlations emerged only when the synchronous group was examined separately, suggesting that the origin of the correlations lies in the synchronous group.
Correlations between plasma oxytocin levels and maternal behavior
Finally, for a full model of the inter-relationship between brain, hormones, and behavior, we examined the correlations between Oxytocin and maternal behavior. Oxytocin correlated with mother–infant synchrony, r=0.6107, p=0.0204, indicating that higher levels of synchrony are associated with higher maternal baseline Oxytocin.
SPIN
This expression matrix is sorted according to correlation in the activity along the corresponding conditions between subjects, with blue denoting low activity and red indicating high activity. This type of data presentation visualizes the activation pattern of the brain regions of interest throughout the time course of the entire paradigm, thus providing additional information beyond the simple analysis of activation/deactivation to a certain condition. As can be seen in the matrix, some mothers show an organized activations pattern, whereas others display disorganized activations. Interestingly, we found that most of the synchronous mothers (10 out of 13) were grouped together and displayed organized left NAcc and right amygdala activity patterns. On the other hand, the majority of intrusive mothers (eight out of nine) displayed a non-organized pattern of activations of the same limbic nuclei. It is of interest to note that the brain's pattern of organization is more predictive of the mother's caregiving behavior than the direction of activations to a certain condition. It is possible that intrinsic brain organization may lead to the development of synchronous maternal behavior, whereas disorganized limbic activation may result in intrusive mothering (Figure 4d and e).
Whole brain contrasts of functional connectivity maps
Results of the functional connectivity analysis described two distinct brain networks according to patterns of maternal care—synchrony and intrusiveness. Seed regions were chosen on the basis of the previously described ROI analysis, which demonstrated that the left NAcc and the right amygdala differentiated between the two groups. Following, we computed whole brain functional connectivity maps using these two seed regions to statistically assess the brain areas that correlate with the activity of the left NAcc and right amygdala across the paradigm.
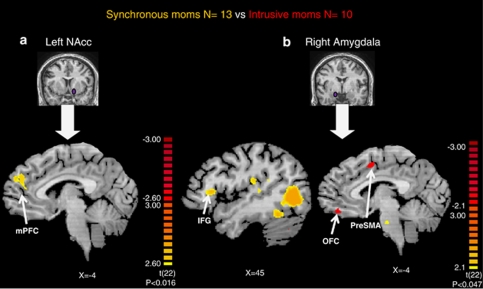
Seed region left NAcc: contrast map of synchronous mothers>intrusive mothers
When comparing the two groups (random effect, t-test, p<0.016, uncorrected), it was found that the intrusive mothers group displayed higher correlations in occipital, lateral–frontal, temporal and cerebellar cortices, whereas the synchronous mothers group showed higher cortical correlation in mPFC: BA10 (Figure 5a and Table 2).
Figure 5.
Header: Inter-regional functional connectivity analysis from left nucleus accumbens (NAcc) and right amygdala in synchronous (N=13) and intrusive mothers (N=10), random effect. Footer: (a) A whole brain t-test between groups with left NAcc as seed region show significantly higher functional correlation with paracingulate medial prefrontal cortex (PFC) among synchronous moms than among intrusive moms. (b) A whole brain t-test between groups with right amygdala as seed region showing significantly higher functional correlation in orbito-frontal cortex (OFC) and pre-supplementary motor area (pre-SMA) among intrusive moms than synchronous moms. Synchronous moms display amygdala correlation to inferior frontal gyrus (IFG), superior temporal gyrus (STG), and insula.
Table 2. Brain Regions Showing Significant Correlations to Left NAcc as Seed Region.
| x | y | z | BA | t score | p score | |
|---|---|---|---|---|---|---|
| Intrusive>synchronous | ||||||
| Left inferior occipital gyrus | −31 | −98 | −12 | 18 | −4.342 | 0.00029 |
| Right middle occipital gyrus | 50 | −81 | 6 | 19 | −4.3216 | 0.0003 |
| Right fusiform | 21 | −53 | −8 | 19 | −4.0954 | 0.00052 |
| Left cerebellum | −25 | −80 | −36 | −3.8422 | 0.00095 | |
| Left uncus | −25 | −5 | −39 | 20 | −3.707 | 0.00131 |
| Right cerebellum | 11 | −80 | −39 | −3.6861 | 0.00137 | |
| Left cerebellum | −4 | −74 | −36 | −3.614 | 0.00163 | |
| Right middle frontal gyrus | 14 | 25 | 39 | 8 | −3.5255 | 0.00201 |
| Right middle frontal gyrus | 26 | 55 | −6 | 10 | −3.4311 | 0.00251 |
| Left middle occipital gyrus | −28 | −95 | 3 | 18 | −3.0772 | 0.00572 |
| Synchronous>intrusive | ||||||
| Right insula | 38 | 1 | 9 | 13 | 2.98469 | 0.00707 |
| Left caudate head | −10 | 16 | −6 | 3.00433 | 0.00676 | |
| Left uncus | −16 | 4 | −24 | 28 | 3.41192 | 0.00262 |
| Left temporal pole | −40 | 23 | −30 | 38 | 3.49369 | 0.00216 |
| Left inferior frontal gyrus | −49 | 31 | 9 | 46 | 3.51655 | 0.00205 |
| Left medial frontal gyrus | −1 | 46 | 27 | 9 | 4.1208 | 0.00049 |
| Right subcallosal gyrus | 8 | 22 | −12 | 25 | 4.38748 | 0.00026 |
x, y and z represent talairach coordinates. N=23, random effect, uncorrected, cluster size >6, confidence threshold min=2.2.
Seed region right amygdala: contrast map of synchronous group>intrusive group
Functional connectivity maps with right amygdala as seed region revealed a different pattern. Intrusive mothers, significantly more than synchronous mothers (random effect, t-test, p<0.047, uncorrected), displayed higher cortical correlation in mPFC (BA11), cingulate gyrus, superior temporal gyrus (STG), and cerebellum (Figure 5b and Table 3). On the other hand, the synchronous group showed higher amygdala correlations in temporal, occipital, insular, lateral–frontal, and cerebellar cortices.
Table 3. Brain Regions Showing Significant Correlations to Right Amygdala as Seed Region.
| x | y | z | BA | t score | p score | |
|---|---|---|---|---|---|---|
| Intrusive>synchronous | ||||||
| Left medial frontal gyrus | −4 | 43 | −15 | 11 | −4.1068 | 0.0005 |
| Left OFC | −13 | 46 | −18 | 12 | −3.943 | 0.0007 |
| Left supramarginal gyrus | −58 | −56 | 36 | 40 | −3.2628 | 0.0037 |
| Left superior temporal gyrus | −19 | 20 | −33 | 38 | −3.142 | 0.0049 |
| Right cerebellum | 50 | −62 | −36 | −3.0831 | 0.0056 | |
| Left cyngulate gyrus | −4 | 4 | 45 | 24 | −2.8673 | 0.0092 |
| Right middle frontal gyrus | 26 | 28 | 33 | 9 | −2.8646 | 0.0093 |
| Synchronous>intrusive | ||||||
| Right insula | 41 | −41 | 21 | 28 | 2.75317 | 0.0119 |
| Right cuneus V2 | 23 | −86 | 27 | 18 | 3.03171 | 0.0063 |
| Right inferior frontal gyrus | 47 | 22 | 9 | 45 | 3.05545 | 0.006 |
| Right cerebellum | 44 | −50 | −18 | 3.07224 | 0.0058 | |
| Left superior temporal gyrus | −64 | −50 | 15 | 22 | 3.11481 | 0.0052 |
| Left uncus | −16 | −17 | −31 | 28 | 3.22338 | 0.0041 |
| Right lingual gyrus | 11 | −95 | −3 | 17 | 3.35723 | 0.003 |
| Right postcentral gyrus | 50 | −26 | 21 | 40 | 3.42512 | 0.0025 |
| Left uncus | −16 | −8 | −30 | 36 | 3.51478 | 0.0021 |
| Left superior temporal gyrus | −34 | 10 | −39 | 38 | 3.58812 | 0.0017 |
| Right uncus | 17 | 1 | −27 | 28 | 3.65478 | 0.0015 |
| Left fusiform gyrus | −43 | −23 | −15 | 20 | 4.80621 | 1E-04 |
| Right middle occipital gyrus | 47 | −71 | 3 | 37 | 5.77649 | 1E-05 |
x, y and z represent talairach coordinates. N=23, random effect, uncorrected, cluster size >6, confidence threshold min=2.2.
Focusing on the limbic–mPFC connectivity axis of emotional regulation, it appears that the medial–prefrontal areas are activated in concordance with the left NAcc only among the synchronous group. On the other hand, in the intrusive group, the limbic–mPFC connectivity analysis shows a more extensive functional association with the right amygdala. In addition, several lateral cortex areas were found as differentially associated with the right amygdala. In the synchronous group, we found activation of mirror neuron system (MNS) areas, including insula and STG, which did not appear in the intrusive group (Figure 5b and Table 3).
Finally, we examined whether the synchronous and intrusive patterns of maternal care indeed represent discrete profiles of maternal brain–behavior correlations or whether synchrony and intrusiveness are two poles of the same continuum. For this end, we computed correlations between the degree of synchrony and the degree of intrusiveness in the entire group with left NAcc and right amygdale activations. No significant correlations were found for either structure. Examination of the pattern of correlations showed that the nonsignificant effects were due to opposite patterns of correlations in each group, which led to a nonsignificant overall effect.
DISCUSSION
Results of this study are the first, to our knowledge, to chart the coordinated functioning of neural networks, affiliation hormones, and micro-level maternal behavior in an attempt to further specify the neurobiological basis of mothering. Consistent with methodology developed in animal research and research showing that patterns of maternal care in mammals chart distinct bio-behavioral profiles in mothers, we examined two naturally occurring patterns of human maternal care—synchronous and intrusive mothering—and assessed their brain and neurohormonal correlates. The study is also among the first to assess maternal brain response to ongoing, dynamic, and ecologically valid infant stimuli and describe the neural networks associated with the synchronous and intrusive maternal styles on the basis of functional connectivity analysis. Similar to the analysis of the mother's brain response to an ongoing infant stimulus, our behavioral measures were defined on a temporal continuum and addressed the ongoing coordination between maternal behavior and infant social signals. The findings, therefore, may provide new insights into the interface between the maternal brain response and the moment-by-moment expression of maternal behavior in the formation of maternal–infant bonding in humans.
Overall, the results chart three functional neural networks that are activated in response to infant stimuli and may, in combination, describe the brain basis of human mothering. The first refers to a limbic motivational network that includes the NAcc and amygdala (Assaf et al, 2009). The second is an attention network (Pessoa et al, 2002) oriented at perception–action integration (Dayan et al, 2007), which includes the IFG, medial frontal gyrus, associative visual areas, insula, pre-supplementary motor area and parietal cortices that are thought to compose the mirror–neuron network (Iacoboni and Dapretto, 2006; Keysers and Fadiga, 2008). The third network includes the mPFC emotional modulation areas (Bryant et al, 2008; Gilboa et al, 2004; Liddell et al, 2005; Zaretsky et al, 2010), which we found to be functionally correlated with the limbic activation of the left NAcc in the synchronous mothers and the right amygdala in the intrusive group. The discrete activations of these structures have been previously associated with maternal response to own infant stimuli (Bartels and Zeki, 2004; Leckman et al, 2004; Lorberbaum et al, 2002; Swain et al, 2007). Here, we describe the coordinated functioning of these three neural networks into a unified time-locked brain activity and suggest that this integration is related to the mother's social behavior. The finding that a unique integrative profile of the three neural systems emerged for mothers showing synchronous vs intrusive styles of maternal care may suggest that addressing the coordination between various emotional and social brain systems can prove useful in the study of normative vs high-risk parenting.
The synchronous maternal style was characterized by four features. At the motivational level, the left NAcc in this group showed greater activations as compared to the right amygdala during the observation of own infant cues. This may suggest that the motivational mechanisms underlying synchronous maternal care are based on the reward components of parenting to a greater extent than on the stress elements. Second, at the connectivity level, the left NAcc in the synchronous group was functionally correlated with the left dmPFC and the right subcallosal ACC. In the context of rodent maternal behavior, these structures were found to control the motivational element of maternal behavior rather than the behavior per se (Lorberbaum et al, 2002; Slotnick, 1967; Stamm, 1955), whereas human research has implicated these structures in cognitive empathy and ‘Theory of Mind' skills (Assaf et al, 2009; Gallagher and Frith, 2003; Vollm et al, 2006). In addition, in the synchronous maternal care group, functional connectivity was found between the right amygdala and regions included in the so-called MNS, including the STG, insula, and IFG. Such mirror system activations may reflect the mother's ability to better understand the intentions and desires of her infant and to respond with a synchronized conduct. Thirdly, the hormonal analysis revealed that among the synchronous group, plasma Oxytocin levels were correlated with the activation of the left NAcc and the right amygdala. Oxytocin is known to mediate social affiliation in mammals (Insel et al, 1997), and thus the connection between Oxytocin and activations of these parenting-related motivational limbic areas may suggest that parenting for these mothers is more closely linked with subcortical motivational mechanisms. Finally, the SPIN analysis indicated that among the synchronous group, the time course of the left NAcc and right amygdala activations showed a relatively organized pattern across the paradigm, whereas the intrusive group displayed less organized activity of these nuclei. This study is the first to apply SPIN analysis in fMRI research of human attachment, and may thus open new directions for future research. Such analysis focuses on the organization of ROI activity across time, rather than on the direction of effects and underscores the importance of signal organization in the description of maternal care profiles.
In contrast to the synchronous group, the intrusive group was characterized by a different set of features. Among these mothers, right amygdala activations were significantly greater than left NAcc activations, suggesting that the motivational components of fear and anxiety may be more pronounced than those associated with reward and affiliation. In addition, the organizing activity of the dmPFC was missing in this group and wide activations of pro-action areas were observed, including the right premotor cortex and the left OFC. This pattern may point to insufficient behavioral inhibition, which may lead to the excessive, non-modulated maternal behavior typical of the intrusive style. Another feature that reflected intrusive mothering was the lower organization of the left NAcc and right amygdala across time. SPIN analysis revealed that the activity of these motivational nuclei showed lower levels of intrinsic organization in this group and this corresponded with their lower behavioral synchronization. Taken together, the findings chart distinct profiles of limbic and cortical activity and their time-locked integration that differentiated mothers whose caregiving style was characterized by affect synchrony from those whose behaviors were marked by overstimulation, excessive parenting, and miscoordination.
The correlations between the left NAcc and right amygdala activity with plasma Oxytocin in the synchronous group are interesting in light of recent findings on the role of Oxytocin in social affiliation. Oxytocin has been consistently implicated in the onset of maternal behavior in mammals and with more adaptive maternal care in humans (Feldman et al, 2010a; Gordon et al, 2010a, 2010b; Levine et al, 2007; Pedersen et al, 1994). Oxytocin has also been linked with social approach behavior (Kosfeld et al, 2005) and empathy (Domes et al, 2007; Hurlemann et al, 2010). Recently, the infusion of Oxytocin directly into rat mothers' ventral tegmental area was found to enhance dopamine signals in the NAcc shell and to increase maternal behavior (Shahrokh et al, 2010). The involvement of NAcc dopamine in social reward, including maternal attachment, may be linked with Oxytocin projections. It is possible that social reward relies on different mechanisms than those implicated in other types of reward (eg, food) and that social reward depends to a greater extent on the functioning of the Oxytocinergic system (Strathearn et al, 2009). Although the precise nature of such Oxytocin–dopamine interactions in the support of maternal behavior is not fully understood, their combined activity may be central to the formation of affiliative bonds (Douglas, 2010; Strathearn et al, 2009). Future endocrine, imaging, and animal studies are required to explore the relations between the Oxytocin and NAcc dopaminergic systems within a model that addresses their combined contribution to human attachment.
Results of this study may provide further insights on the adaptive roles of both the reward and stress components of parenting. The fact that the infant is a powerful reinforcement to its mother (Barrett and Fleming, 2011) serves an important evolutionary role and may be mediated by the NAcc (Champagne et al, 2004; Hansen, 1994; Hansen et al, 1991; Keer and Stern, 1999; Li and Fleming, 2003; Vernotica et al, 1999). However, a certain amount of heightened arousal and vigilance is also required for infant care, which involves some anxious conduct (Barrett and Fleming, 2011; Leckman and Herman, 2002; Leckman and Mayes, 1999; Leckman et al, 1999). The amygdala, which processes negative emotions, may participate in directing attention toward the infant and charging the incentive value placed on the infant with acuteness and highly aroused energy. Such finely tuned co-activations of structures within the limbic system are central for the formation of the maternal–infant bond. However, this delicate balance is also highly sensitive to early disruptions and slight deviations from normative functioning may result in non-optimal patterns of maternal care that may bear negative consequences for the infant's future mental health. Indeed, recent studies have shown that up to 29% of mothers and infants suffer from some sort of disruptions to early bonding, due to marked increases in cases of maternal post-partum depression and anxiety, as well as in premature birth (ACOG, 2006; Andersson et al, 2006; Silverman et al, 2007; Vesga-Lopez et al, 2008). Such conditions pose a risk for the infant's adaptation and well-being and may lead to a variety of epigenetic, behavioral, and mental processes that would compromise the infant's future well-being, health, and adaptation.
The findings demonstrate that the motivational components of stress and affiliation are evident not only at the limbic level, but also at the cortical and mental levels. Synchronous mothers showed higher activations of mirror-neuron and empathy-related cortical systems, which may point to more adaptive theory-of-mind skills and more adequate representations of the infant's needs. Conversely, intrusive mothers consistently activated pro-action areas, which may explain the non-matched excessive maternal behavior and the lack of sufficient mentalizing of the infant's state. These results support the notion that in addition to conserved biological and behavioral aspects, human parenting includes higher-order mental components that involve the capacity to reflect on infant state, differentiate infant signals on the basis of past experience, and plan a course of caregiving actions. Such perception–action systems include primary and associative cortices of different modalities, premotor areas, and mirror neuron areas such as insula, STG, IFG, and parietal cortex that have been associated with empathy (Adolphs et al, 2000; Rizzolatti, 2005), theory-of-mind abilities (Gallagher and Frith, 2003), and maternal response to infant cries (Swain et al, 2007).
The findings that maternal synchrony and intrusiveness chart two unique profiles of brain–behavior correlations and that these associations did not emerge for the entire group is consistent with the findings for other mammals showing that distinct patterns of maternal care are underpinned by specific biological processes (Meaney, 2001). Future research may extend research from patterns of maternal care observed in the normative population to profiles of brain–behavior correlations in clinical populations. Similarly, future research may integrate other physiological systems with constellations of observed parenting, including the immune system and epigenetic processes.
Two reasons guided our choice to sample plasma Oxytocin at the home and not in the hospital context, where the fMRI data was collected. First, most current knowledge on maternal plasma Oxytocin is based on samples collected in the home and it was important to compare our Oxytocin levels with previously published data. Second, we wished to avoid the potential anxiety-provoking effects of a blood draw on the mother's brain response (if sampled before imaging) or the effects of exposure to infant cues on Oxytocin levels (if sampled after imaging). Plasma Oxytocin levels were found to be highly stable within individuals in several independent samples (Gordon et al, 2010a, 2010b; Levine et al, 2007) and to be unrelated to demographic conditions, such as parent gender, age, height, weight, body mass index, smoking, use of medications, and time since last meal. Similarly, when not sampled immediately before or after breastfeeding, maternal OT levels are unrelated to menstrual cycle phase, contraceptive intake, mode of delivery (vaginal vs c-section), feeding style (breastfeeding vs bottle-feeding), post-partum interval (weeks from birth to date of assessment), or the interval from prior breastfeeding (Feldman et al, 2010b). Thus, our plasma Oxytocin levels likely reflect baseline levels that are not affected by the situational distress of the fMRI procedure.
Limitations of the study include the small number of intrusive mothers with Oxytocin data and future research with larger samples are required to better describe the hormonal profile of this group and its brain correlates. The father's data would have been important to shed light on the paternal brain and its relation to patterns of paternal care. Attachment patterns in infants were also not tested and this is clearly a study limitation. Assessing infant attachment would have pointed to the relations between bio-behavioral profiles of maternal care and infant attachment security vs insecurity. In general, it is important to emphasize that our use of the term ‘attachment' was general and does not imply that infant attachment was studied. Such use is consistent with Bowlby's (1958, 1969) description of the mother's attachment to her infant and its biological and behavioral concomitants. Furthermore, as brain, hormonal, and behavioral data were collected concurrently, it is not possible to infer causality, only relationships between variables. Follow-up data would have been important to examine whether maternal brain activations in the post-partum can predict infants' later social–emotional development and assessment of maternal Oxytocin during pregnancy could have shown predictions from hormonal levels to brain activations. Much further research is required to integrate imaging, hormonal analysis, and careful assessments of social behavior with genetic and epigenetic markers to help describe integrative profiles that may later assist in the diagnosis of specific high-risk conditions.
The use of functional magnetic resonance imaging combined with micro-level behavioral analysis and bonding-related hormones may enhance our understanding of typical and high-risk parenting. Our results show that a priori categorization of patterns of maternal care that are expressed in the general population can reliably chart different neural networks. Future research is required to apply integrative assessments to various clinical populations that involve risk to the mother–infant bond due to post-partum depression, premature birth, or social adversity. Understanding the motivational basis of healthy and at-risk parenting may open new theoretical vistas and clinical opportunities and may lead to the construction of more specific interventions that can target disruptions to maternal–infant bonding at an earlier stage and in a more accurate manner.
Acknowledgments
We thank Dr Roi Avraham for his help in the SPIN analysis, and Dr Maya Bleich-Cohen, Daniella Perry, and Dr Michal Ziv from the functional brain center, Tel-Aviv Sourasky Medical Center, for their contribution to the fMRI analysis. We also thank Dr Orna Zagoory-Sharon, Hila Azulay, and Yana Gavriolov from the Early Developmental Laboratory at Bar-Ilan. Research at Prof. Feldman's lab was supported by the NARSAD independent investigator award, by the Israel Science Foundation (No. 1318/08), and by the Irving B. Harris Foundation.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- ACOG ACOG Committee Opinion No. 343: psychosocial risk factors: perinatal screening and intervention. Obstet Gynecol. 2006;108:469–477. doi: 10.1097/00006250-200608000-00046. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J Neurosci. 2000;20:2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M. Depression and anxiety during pregnancy and six months postpartum: a follow-up study. Acta Obstet Gynecol Scand. 2006;85:937–944. doi: 10.1080/00016340600697652. [DOI] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Assaf M, Kahn I, Pearlson GD, Johnson MR, Yeshurun Y, Calhoun VD, et al. Brain activity dissociates mentalization from motivation during an interpersonal competitive game. Brain Imag Behav. 2009;3:24–37. doi: 10.1007/s11682-008-9047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Fleming AS. Annual Research Review: all mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry. 2011;52:368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Beck AT.1978. Beck Depression Inventory. Harcourt Brace Jovanovich Psychological Corporation: San Antonio, TX [Google Scholar]
- Bowlby J. The nature of the child's tie to his mother. Int J Psychoanal. 1958;39:350–373. [PubMed] [Google Scholar]
- Bowlby J. Attachment and Loss. Basic Books: New York, NY; 1969. [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders. Behav Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Carter CS, Ahnert L, Grossmann KE, Hrdy SB, Lamb ME, Porges SW, et al. Attachment and Bonding: A New Synthesis. The MIT Press: Boston, MA; 2005. [Google Scholar]
- Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Casile A, Levit-Binnun N, Giese MA, Hendler T, Flash T. Neural representations of kinematic laws of motion: evidence for action–perception coupling. Proc Natl Acad Sci USA. 2007;104:20582–20587. doi: 10.1073/pnas.0710033104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves ‘mind-reading' in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Douglas AJ. Baby love? Oxytocin–dopamine interactions in mother–infant bonding. Endocrinology. 2010;151:1978–1980. doi: 10.1210/en.2010-0259. [DOI] [PubMed] [Google Scholar]
- Feldman R. Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J Child Psychol Psychiatry. 2007;48:329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Feldman R. The relational basis of adolescent adjustment: trajectories of mother–child interactive behaviors from infancy to adolescence shape adolescents' adaptation. Attach Hum Dev. 2010;12:173–192. doi: 10.1080/14616730903282472. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI. Parent–infant synchrony and the social–emotional development of triplets. Dev Psychol. 2004;40:1133–1147. doi: 10.1037/0012-1649.40.6.1133. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI. Maternal postpartum behavior and the emergence of infant–mother and infant–father synchrony in preterm and full-term infants: the role of neonatal vagal tone. Dev Psychobiol. 2007;49:290–302. doi: 10.1002/dev.20220. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology. 2010a;35:1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. The cross-generation transmission of oxytocin in humans. Horm Behav. 2010b;58:669–676. doi: 10.1016/j.yhbeh.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: considering stress and affiliation components of human bonding. Dev Sci. 2011;14:752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry. 2009;48:919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Feldman R, Singer M, Zagoory O. Touch attenuates infants' physiological reactivity to stress. Dev Sci. 2010c;13:271–278. doi: 10.1111/j.1467-7687.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother–infant bonding. Psychol Sci. 2007b;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M. Plasticity in the maternal circuit: effects of maternal experience on Fos–Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behav Neurosci. 1996;110:567–582. doi: 10.1037//0735-7044.110.3.567. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind'. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, et al. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry. 2004;55:263–272. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biol Psychiatry. 2010a;68:377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin, cortisol, and triadic family interactions. Physiol Behav. 2010b;101:679–684. doi: 10.1016/j.physbeh.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Schneiderman I, Leckman JF, Weller A, Feldman R. Oxytocin and cortisol in romantically unattached young adults: associations with bonding and psychological distress. Psychophysiology. 2008;45:349–352. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. Maternal behavior of female rats with 6-OHDA lesions in the ventral striatum: characterization of the pup retrieval deficit. Physiol Behav. 1994;55:615–620. doi: 10.1016/0031-9384(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Lofberg L, Svensson K. The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav. 1991;39:71–77. doi: 10.1016/0091-3057(91)90399-m. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young L, Wang Z. Central oxytocin and reproductive behaviours. Rev Reprod. 1997;2:28–37. doi: 10.1530/ror.0.0020028. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Isabella RA, Belsky J. Interactional synchrony and the origins of infant–mother attachment: a replication study. Child Dev. 1991;62:373–384. [PubMed] [Google Scholar]
- Kaitz M, Maytal H. Interactions between anxious mothers and their infants:an integration of theory and research findings. Infant Ment Health J. 2005;26:570–597. doi: 10.1002/imhj.20069. [DOI] [PubMed] [Google Scholar]
- Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67:659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Keysers C, Fadiga L. The mirror neuron system: new frontiers. Soc Neurosci. 2008;3:193–198. doi: 10.1080/17470910802408513. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Feldman R, Swain JE, Eicher V, Thompson N, Mayes LC. Primary parental preoccupation: circuits, genes, and the crucial role of the environment. J Neural Transm. 2004;111:753–771. doi: 10.1007/s00702-003-0067-x. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Herman AE. Maternal behavior and developmental psychopathology. Biol Psychiatry. 2002;51:27–43. doi: 10.1016/s0006-3223(01)01277-x. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Mayes LC. Preoccupations and behaviors associated with romantic and parental love. Perspectives on the origin of obsessive–compulsive disorder. Child Adolesc Psychiatr Clin N Am. 1999;8:635–665. [PubMed] [Google Scholar]
- Leckman JF, Mayes LC, Feldman R, Evans DW, King RA, Cohen DJ. Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessive–compulsive disorder. Acta Psychiatr Scand Suppl. 1999;396:1–26. doi: 10.1111/j.1600-0447.1999.tb10951.x. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 2000;108:215–231. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal–fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Li M, Fleming AS. Differential involvement of nucleus accumbens shell and core subregions in maternal memory in postpartum female rats. Behav Neurosci. 2003;117:426–445. doi: 10.1037/0735-7044.117.3.426. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, et al. A direct brainstem–amygdala–cortical ‘alarm' system for subliminal signals of fear. NeuroImage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, et al. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- MacLean MR, Raizada MK, Sumners C. The influence of angiotensin II on catecholamine synthesis in neuronal cultures from rat brain. Biochem Biophys Res Commun. 1990;167:492–497. doi: 10.1016/0006-291x(90)92050-a. [DOI] [PubMed] [Google Scholar]
- MacLean PD, Newman JD. Role of midline frontolimbic cortex in production of the isolation call of squirrel monkeys. Brain Res. 1988;450:111–123. doi: 10.1016/0006-8993(88)91550-8. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Murphy MR, MacLean PD, Hamilton SC. Species-typical behavior of hamsters deprived from birth of the neocortex. Science. 1981;213:459–461. doi: 10.1126/science.7244642. [DOI] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother's response to infant's attachment behaviors. Biol Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Oxley G, Fleming AS. The effects of medial preoptic area and amygdala lesions on maternal behavior in the juvenile rat. Dev Psychobiol. 2000;37:253–265. [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti GCL.2005Mirror neuron: a neurological approach to empathyIn: Changeux ARDJ-P, Singer W, Christen Y (eds).Neurobiology of Human Values Springer-Verlag: Berlin, Heidelberg; 107–123. [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin–dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman ME, Loudon H, Safier M, Protopopescu X, Leiter G, Liu X, et al. Neural dysfunction in postpartum depression: an FMRI pilot study. CNS Spectr. 2007;12:853–862. doi: 10.1017/s1092852900015595. [DOI] [PubMed] [Google Scholar]
- Slotnick BM. Disturbances of maternal behavior in the rat following lesions of the cingulate cortex. Behaviour. 1967;29:204–236. doi: 10.1163/156853967x00127. [DOI] [PubMed] [Google Scholar]
- Spielberger CDGR, Lushene SHRE. Manual for the State-Trait Anxiety Inventory. Palo Alto Consulting Psychologist Press: Palo Alto, CA; 1970. [Google Scholar]
- Sroufe LA. Attachment and development: a prospective, longitudinal study from birth to adulthood. Attach Hum Dev. 2005;7:349–367. doi: 10.1080/14616730500365928. [DOI] [PubMed] [Google Scholar]
- Stamm JS. The function of the median cerebral cortex in maternal behavior of rats. J Comp Physiol Psychol. 1955;48:347–356. doi: 10.1037/h0042977. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent–infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano JE, Bauman MD, Mason WA, Amaral DG. Interest in infants by female rhesus monkeys with neonatal lesions of the amygdala or hippocampus. Neuroscience. 2009;162:881–891. doi: 10.1016/j.neuroscience.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick EZ. Emotions and emotional communication in infants. Am Psychol. 1989;44:112–119. doi: 10.1037//0003-066x.44.2.112. [DOI] [PubMed] [Google Scholar]
- Tsafrir D, Tsafrir I, Ein-Dor L, Zuk O, Notterman DA, Domany E. Sorting points into neighborhoods (SPIN): data analysis and visualization by ordering distance matrices. Bioinformatics. 2005;21:2301–2308. doi: 10.1093/bioinformatics/bti329. [DOI] [PubMed] [Google Scholar]
- Vernotica EM, Rosenblatt JS, Morrell JI. Microinfusion of cocaine into the medial preoptic area or nucleus accumbens transiently impairs maternal behavior in the rat. Behav Neurosci. 1999;113:377–390. doi: 10.1037//0735-7044.113.2.377. [DOI] [PubMed] [Google Scholar]
- Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65:805–815. doi: 10.1001/archpsyc.65.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage. 2006;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Poster Presentation at the 10th World Congress of Biological Psychiatry. Prague, Czech Republic; 2011. Intranasal Oxytocin Improves the Autonomic, Hormonal and Social basis of Fatherhood. [Google Scholar]
- Zaretsky M, Mendelsohn A, Mintz M, Hendler T. In the eye of the beholder: internally driven uncertainty of danger recruits the amygdala and dorsomedial prefrontal cortex. J Cogn Neurosci. 2010;22:2263–2275. doi: 10.1162/jocn.2009.21402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.