Abstract
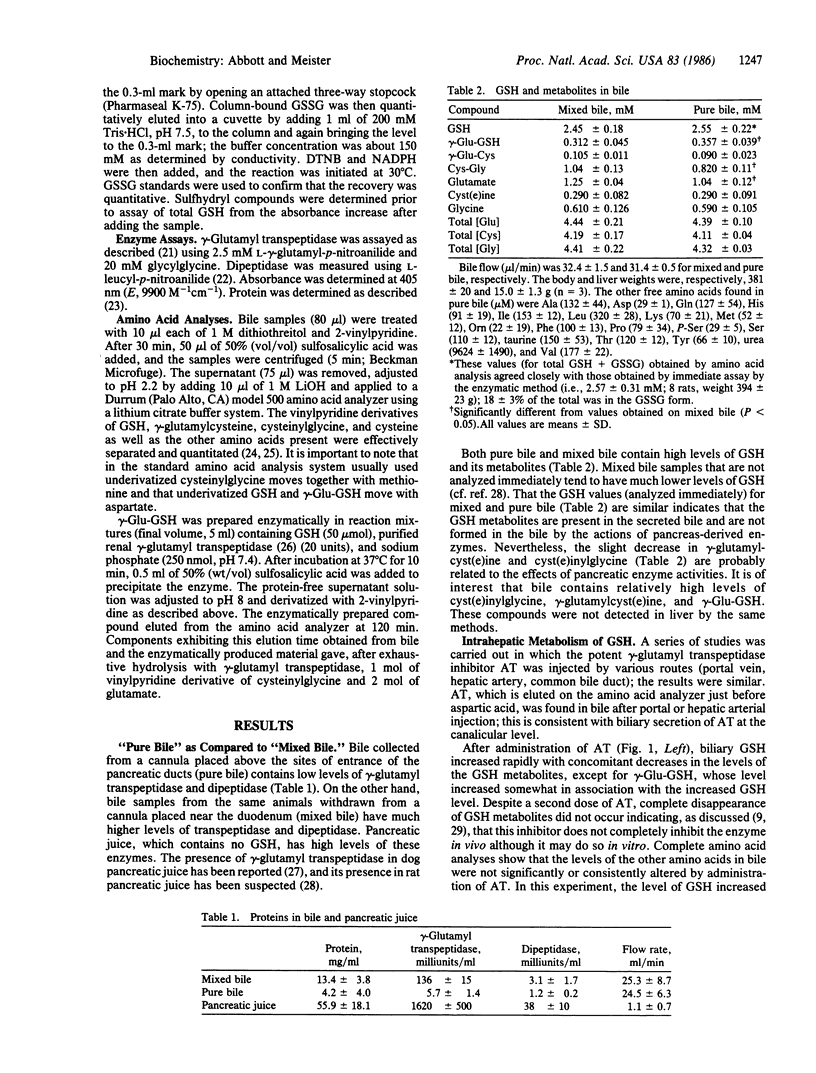
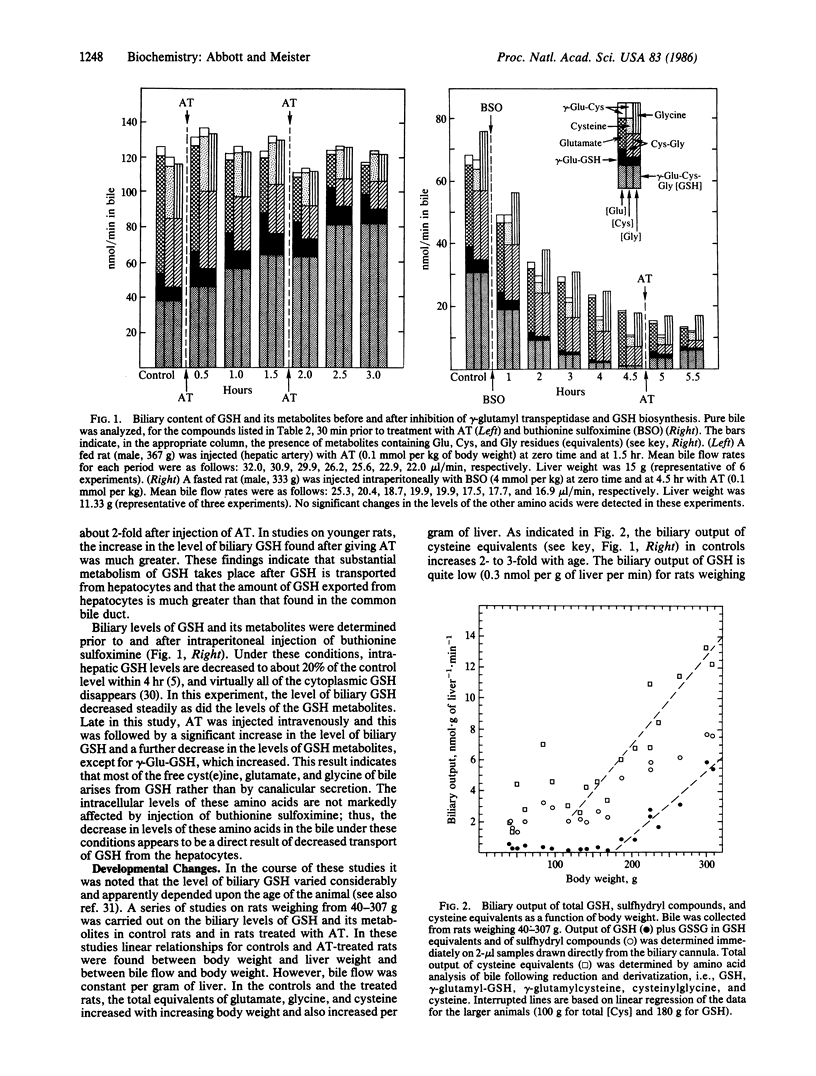
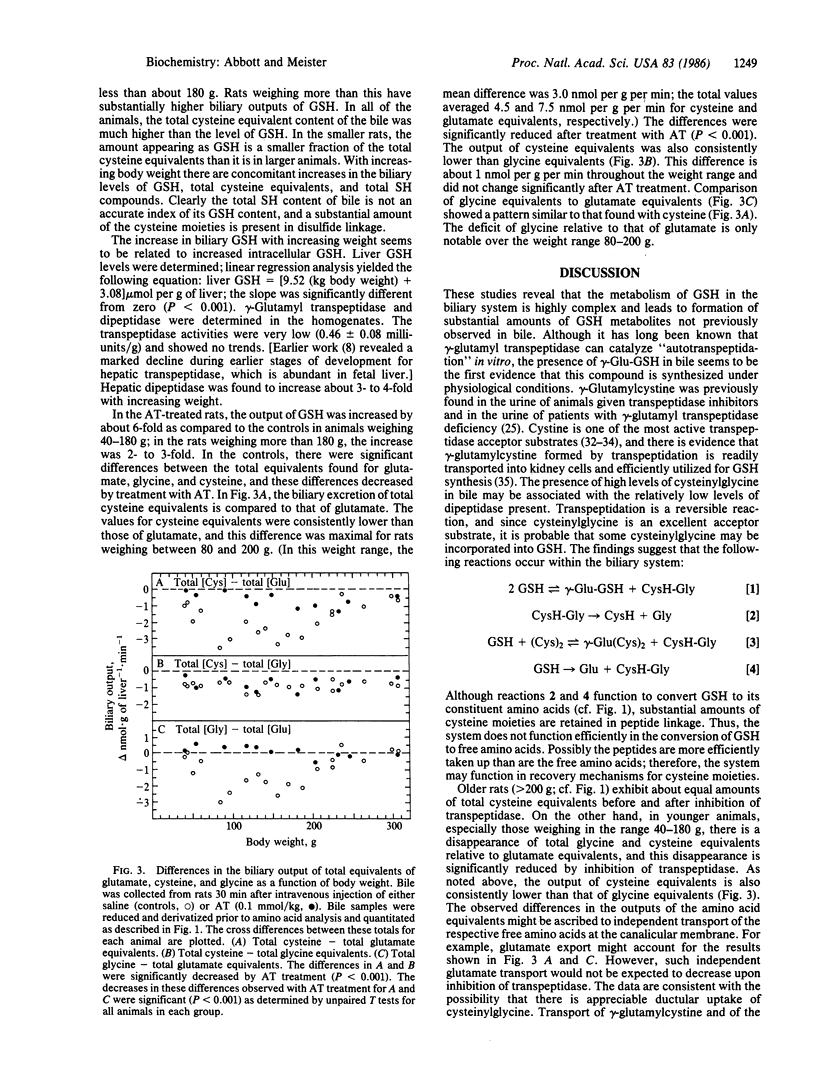
Glutathione transported by hepatocytes into the bile canaliculi is metabolized by the actions of gamma-glutamyl transpeptidase and dipeptidase located on the biliary ductular epithelium. This pathway is revealed by the finding of high levels of cyst(e)inylglycine, gamma-glutamylglutathione, gamma-glutamylcyst(e)ine, glutamate, glycine, and cyst(e)ine in bile, by studies in which intrahepatic metabolism of glutathione was inhibited by administration of a potent inhibitor of gamma-glutamyl transpeptidase and by experiments in which glutathione synthesis was inhibited. Canalicular transport of glutathione, as estimated from totals of metabolites found, is much greater than the glutathione found in bile. Glutathione and glutathione metabolites found in bile increase with age, in association with an increase in hepatic glutathione. In younger rats there is apparent uptake of cysteine and glycine moieties that may reflect uptake of cysteinylglycine at the ductular level. This intrahepatic pathway of glutathione transport and metabolism, which resembles that which occurs in the kidney, seems to function as a cellular protective mechanism in the processing of glutathione conjugates and as a recovery system for cysteine moieties.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott W. A., Bridges R. J., Meister A. Extracellular metabolism of glutathione accounts for its disappearance from the basolateral circulation of the kidney. J Biol Chem. 1984 Dec 25;259(24):15393–15400. [PubMed] [Google Scholar]
- Abbott W. A., Meister A. Modulation of gamma-glutamyl transpeptidase activity by bile acids. J Biol Chem. 1983 May 25;258(10):6193–6197. [PubMed] [Google Scholar]
- Akerboom T. P., Bilzer M., Sies H. The relationship of biliary glutathione disulfide efflux and intracellular glutathione disulfide content in perfused rat liver. J Biol Chem. 1982 Apr 25;257(8):4248–4252. [PubMed] [Google Scholar]
- Anderson M. E., Bridges R. J., Meister A. Direct evidence for inter-organ transport of glutathione and that the non-filtration renal mechanism for glutathione utilization involves gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1980 Sep 30;96(2):848–853. doi: 10.1016/0006-291x(80)91433-3. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):707–711. doi: 10.1073/pnas.80.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatori N., Clarkson T. W. Developmental changes in the biliary excretion of methylmercury and glutathione. Science. 1982 Apr 2;216(4541):61–63. doi: 10.1126/science.7063871. [DOI] [PubMed] [Google Scholar]
- Capraro M. A., Hughey R. P. Use of acivicin in the determination of rate constants for turnover of rat renal gamma-glutamyltranspeptidase. J Biol Chem. 1985 Mar 25;260(6):3408–3412. [PubMed] [Google Scholar]
- Eberle D., Clarke R., Kaplowitz N. Rapid oxidation in vitro of endogenous and exogenous glutathione in bile of rats. J Biol Chem. 1981 Mar 10;256(5):2115–2117. [PubMed] [Google Scholar]
- Griffith O. W., Bridges R. J., Meister A. Transport of gamma-glutamyl amino acids: role of glutathione and gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6319–6322. doi: 10.1073/pnas.76.12.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982 Nov 25;257(22):13704–13712. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Excretion of cysteine and gamma-glutamylcysteine moieties in human and experimental animal gamma-glutamyl transpeptidase deficiency. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3384–3387. doi: 10.1073/pnas.77.6.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):268–272. doi: 10.1073/pnas.76.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata E., Takahashi H. Degradation of methyl mercury glutathione by the pancreatic enzymes in bile. Toxicol Appl Pharmacol. 1981 May;58(3):483–491. doi: 10.1016/0041-008x(81)90101-0. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. The mechanism of biliary secretion of reduced glutathione. Analysis of transport process in isolated rat-liver canalicular membrane vesicles. Eur J Biochem. 1983 Aug 15;134(3):467–471. doi: 10.1111/j.1432-1033.1983.tb07590.x. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N., Eberle D. E., Petrini J., Touloukian J., Corvasce M. C., Kuhlenkamp J. Factors influencing the efflux of hepatic glutathione into bile in rats. J Pharmacol Exp Ther. 1983 Jan;224(1):141–147. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lauterburg B. H., Smith C. V., Hughes H., Mitchell J. R. Biliary excretion of glutathione and glutathione disulfide in the rat. Regulation and response to oxidative stress. J Clin Invest. 1984 Jan;73(1):124–133. doi: 10.1172/JCI111182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A., Tate S. S., Griffith O. W. Gamma-glutamyl transpeptidase. Methods Enzymol. 1981;77:237–253. doi: 10.1016/s0076-6879(81)77032-0. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Toda Y., Hayakawa T., Suzuki T., Noda A. Secretory characteristics of pancreatic gamma-glutamyl transpeptidase. Pflugers Arch. 1973;345(4):271–279. doi: 10.1007/BF00585846. [DOI] [PubMed] [Google Scholar]
- ORLOWSKI M., MEISTER A. GAMMA-GLUTAMYL-P-NITROANILIDE: A NEW CONVENIENT SUBSTRATE FOR DETERMINATION AND STUDY OF L- AND D-GAMMA-GLUTAMYLTRANSPEPTIDASE ACTIVITIES. Biochim Biophys Acta. 1963 Aug 6;73:679–681. doi: 10.1016/0006-3002(63)90348-2. [DOI] [PubMed] [Google Scholar]
- OWENS C. W., BELCHER R. V. A COLORIMETRIC MICRO-METHOD FOR THE DETERMINATION OF GLUTATHIONE. Biochem J. 1965 Mar;94:705–711. doi: 10.1042/bj0940705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin B. B., McIntyre T. M., Curthoys N. P. Brush border membrane hydrolysis of S-benzyl-cysteine-p-nitroanilide, and activity of aminopeptidase M. Biochem Biophys Res Commun. 1980 Oct 16;96(3):991–996. doi: 10.1016/0006-291x(80)90050-9. [DOI] [PubMed] [Google Scholar]
- Refsvik T., Norseth T. Methyl mercuric compounds in rat bile. Acta Pharmacol Toxicol (Copenh) 1975 Jan;36(1):67–78. doi: 10.1111/j.1600-0773.1975.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Roberts R. J., Klaassen C. D., Plaa G. L. Maximum biliary excretion of bilirubin and sulfobromophthalein during anesthesia-induced alteration of rectal temperature. Proc Soc Exp Biol Med. 1967 May;125(1):313–316. doi: 10.3181/00379727-125-32080. [DOI] [PubMed] [Google Scholar]
- Sies H., Koch O. R., Martino E., Boveris A. Increased biliary glutathione disulfide release in chronically ethanol-treated rats. FEBS Lett. 1979 Jul 15;103(2):287–290. doi: 10.1016/0014-5793(79)81346-0. [DOI] [PubMed] [Google Scholar]
- Thompson G. A., Meister A. Hydrolysis and transfer reactions catalyzed by gamma-glutamyl transpeptidase; evidence for separate substrate sites and for high affinity of L-cystine. Biochem Biophys Res Commun. 1976 Jul 12;71(1):32–36. doi: 10.1016/0006-291x(76)90245-x. [DOI] [PubMed] [Google Scholar]
- Thompson G. A., Meister A. Interrelationships between the binding sites for amino acids, dipeptides, and gamma-glutamyl donors in gamma-glutamyl transpeptidase. J Biol Chem. 1977 Oct 10;252(19):6792–6798. [PubMed] [Google Scholar]
- Thompson G. A., Meister A. Utilization of L-cystine by the gamma-glutamyl transpeptidase-gamma-glutamyl cyclotransferase pathway. Proc Natl Acad Sci U S A. 1975 Jun;72(6):1985–1988. doi: 10.1073/pnas.72.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]



