Abstract
The thyrotropin receptor [thyroid-stimulating hormone receptor (TSHR)], a G-protein-coupled receptor (GPCR), is endogenously activated by thyrotropin, which binds to the extracellular region of the receptor. We previously identified a low-molecular-weight (LMW) agonist of the TSHR and predicted its allosteric binding pocket within the receptor’s transmembrane domain. Because binding of the LMW agonist probably disrupts interactions or leads to formation of new interactions among amino acid residues surrounding the pocket, we tested whether mutation of residues at these positions would lead to constitutive signaling activity. Guided by molecular modeling, we performed site-directed mutagenesis of 24 amino acids in this spatial region, followed by functional characterization of the mutant receptors in terms of expression and signaling, measured as cAMP accumulation. We found that mutations V421I, Y466A, T501A, L587V, M637C, M637W, S641A, Y643F, L645V, and Y667A located in several helices exhibit constitutive activity. Of note is mutation M637W at position 6.48 in transmembrane helix 6, which has a significant effect on the interaction of the receptor with the LMW agonist. In summary, we found that a high proportion of residues in several helices surrounding the allosteric binding site of LMW ligands in the TSHR when mutated lead to constitutively active receptors. Our findings of signaling-sensitive residues in this region of the transmembrane bundle may be of general importance as this domain appears to be evolutionarily retained among GPCRs.—Kleinau, G., Haas, A.-K., Neumann, S., Worth, C. L., Hoyer, I., Furkert, J., Rutz, Gershengorn, M. C., Schülein, R., Krause, G. Signaling-sensitive amino acids surround the allosteric ligand binding site of the thyrotropin receptor.
Keywords: allosteric small molecules, glycoprotein hormone receptors, constitutively activating mutations, activation mechanisms
The thyrotropin receptor [thyroid-stimulating hormone receptor (TSHR)] together with the lutropin/choriogonadotropin receptor (LHCGR) and the follitropin receptor (FSHR) are glycoprotein hormone receptors (GPHRs) that constitute a subclass of the rhodopsin-like (family A) G protein-coupled receptors (GPCRs). They are pivotal proteins with respect to a variety of physiological functions (reviewed in refs. 1,2,3,4,5,6,7). In accordance with other family A GPCRs, the GPHRs are characterized by the general structural topology of an N-terminal region, 7 transmembrane helices (TMHs) connected by 3 intracellular loops and 3 extracellular loops (ECLs) and a C-terminal tail. However, a unique structural feature of the GPHRs compared with other family A GPCRs is the large (300–400 aa) N-terminal region, which can be subdivided into 2 parts. The first part is the leucine-rich repeat domain (LRRD) comprising ∼260 aa, and the second part is the cysteine-rich so-called hinge region that links the LRRD with TMH1. Both extracellular regions together are responsible for hormone (endogenous heterodimeric ligand) binding and hormone selectivity (reviewed in ref. 8).
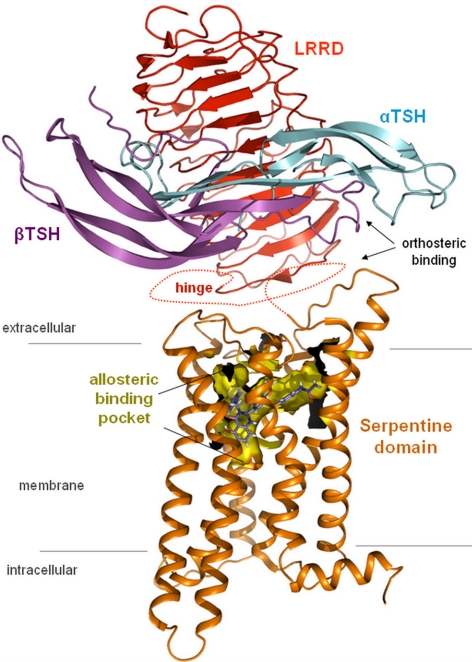
In contrast to this extracellular orthosteric hormone binding site, we have presented evidence that small, drug-like TSHR ligands bind allosterically in the transmembrane region of the TSHR (9,10,11) (Fig. 1). Interestingly, the transmembrane binding pocket is located in a region where endogenous ligands of other GPCRs (such as rhodopsin and β-adrenergic receptors) are bound (12). Thus, although the orthosteric hormone-binding pocket in the TSHR and other GPHRs is located extracellularly, it appears that the binding pocket in the transmembrane region is retained also in these receptors (11).
Figure 1.
Orthosteric and allosteric binding sites of the TSHR. TSHR is characterized by a unique structural feature compared with other family A GPCRs: a large extracellular region (∼400 aa), which can be subdivided into two parts: the LRRD (light orange) and the cysteine-rich hinge region (indicated by dotted line because no structure or homology model is available). Together, both extracellular parts are responsible for hormone binding and hormone selectivity. The hormone TSH consists of an α-subunit (cyan) and a β-subunit (lilac). In contrast, the small synthetic ligand (LMW-Ag, blue) is shown to bind allosterically in a pocket (yellow surface) located in the heptahelical transmembrane region (orange backbone) of the TSHR. The pocket is characterized by specific biophysical properties complementary to that of the ligand. Simultaneous binding of both ligands to the same receptor has not been shown to occur.
Little is known regarding the activation mechanism induced by low-molecular-weight (LMW) agonists of GPHRs (11, 13, 14). Because binding of LMW agonists probably disrupts interactions or leads to formation of new interactions among amino acid residues surrounding the allosteric binding pocket (15), we tested whether mutation of residues at these positions in the TSHR would lead to constitutive signaling activity. We found that mutation of a high proportion of residues surrounding the binding pocket leads to constitutive signaling and suggest that amino acids at these positions in other GPCRs may also be signaling sensitive.
MATERIALS AND METHODS
Construction of vectors and site-directed mutagenesis of TSHR
The restriction site KpnI was introduced into hTSHR-pSVL via a QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). The plasmid was digested with KpnI and BamHI (New England Biolabs, Ipswich, MA, USA), and the fragment encoding the wild-type (wt) TSHR sequence was inserted into the pCDNA3 vector (Invitrogen, Carlsbad, CA, USA). Mutations were introduced into wt TSHR in vector pCDNA3 via a QuikChange Site-Directed Mutagenesis Kit (Stratagene). All constructs were verified by nucleotide sequencing.
Cell culture and transfection
HEK 293 cells (DSMZ) were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (Biochrom, Berlin, Germany) at 37°C in a humidified 5% CO2 incubator. For determination of intracellular cAMP accumulation, cells were seeded in 24-well plates (7.5×104 cells/well) and transfected with 0.6 μg of DNA/well. For determination of cell surface expression, cells were seeded in 6-well plates (3×105 cells/well) and transfected with 2.4 μg of DNA/well. Cells were transiently transfected with Lipofectamine 2000 reagent (Invitrogen) 24 h after seeding according to the manufacturer’s protocol.
Determination of cell surface expression by FACS
The thyroid-stimulating hormone (TSH) receptor cell surface expression level was quantified on a FACS flow cytometer (FACSCalibur; BD, Franklin Lakes, NJ, USA). All steps were performed at 4°C or on ice. Approximately 48 h after transfection, cells were detached from the dishes with 1 mM EDTA and 1 mM EGTA in PBS. After detachment, they were spun at 300 g for 3 min, and the supernatant was discarded. Cells were incubated for 15 min in FACS buffer (PBS containing 0.5% BSA) and centrifuged again. Then they were incubated for 30 min with a 1:200 dilution of a mouse anti-extracellular human TSHR antibody (2C11; Serotec, Oxford, UK) in FACS buffer. Cells were washed twice in FACS buffer and incubated for 30 min with a 1:400 dilution of a DyLight488-conjugated goat anti-mouse IgG antibody (Serotec). Before FACS analysis, cells were washed twice and resuspended in FACS buffer. 7-Aminoactinomycin D (BD) was used for detection of damaged cells that were excluded from the analysis. The fluorescence of ≥104 cells/tube was assayed. Receptor expression was determined by the mean fluorescence intensity by comparison with wt TSHR.
Determination of intracellular cAMP accumulation by radioimmunoassay
Forty-eight hours after transfection, cells were washed with 1 ml of stimulation buffer [DMEM supplemented with 10 mM HEPES, 0.5% BSA, and 0.25 mM 3-isobutyl-1-methylxanthine (IBMX)] and incubated for 1 h at 37°C with stimulation buffer alone or stimulation buffer containing increasing concentrations of bovine TSH (1 μIU/ml to 0.1 IU/ml; Sigma Aldrich, St. Louis, MO, USA). The stimulation was terminated by aspiration of the medium and lysis of the cells with 750 μl of 0.1% trifluoroacetic acid and 0.005% Triton X-100 for 30 min at 4°C. The lysates were heated at 95°C for 10 min, dried in a rotation vacuum concentrator (Alpha-RCV; Martin Christ GmbH, Osterode am Harz, Germany) and finally stored at −20°C until use. After reconstitution and acetylation of the samples (16), the cAMP content was determined using 125I-cAMP-tyrosyl methyl ester (104 cpm; BioTrend, Köln, Germany) and polyclonal rabbit anti-cAMP-antibody (final dilution 1:160,000). After an overnight incubation at 4°C, the antibody-bound fraction was precipitated using 50 μl of Saccel (IBL, Hamburg, Germany). The radioactivity of the precipitate was determined in a gamma counter.
Determination of intracellular cAMP accumulation
Intracellular cAMP accumulation was determined by ELISA to test the effect of mutations at position M637 on activation induced by a LMW agonist (LMW-Ag) [N-(4-(5-(3-benzyl-5-hydroxy-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)-2-methoxybenzyloxy)-phenyl)acetamide; NIH Chemical Genomic Center number NCGC00161870-01; ref 11]. Transiently transfected HEK-EM293 cells were seeded into 96-well plates at a density of 7×104 cells/well in DMEM containing 10% FBS. Cells were cultured 24 h before incubation for 1 h in serum-free DMEM containing 1 mM IBMX (Sigma-Aldrich Corp., St. Louis, MO, USA) and TSH or a small-molecule ligand in a humidified 5% CO2 incubator at 37°C. After aspiration of the media, cells were lysed using lysis buffer of the cAMP-Screen Direct System (Applied Biosystems, Foster City, CA, USA). The cAMP content of the cell lysate was determined using the method described in the manufacturer’s protocol. The potencies (EC50) of the ligands were obtained from dose-response curves by data analysis with GraphPad Prism 4 for Windows (GraphPad, San Diego, CA, USA).
Structural bioinformatics and molecular modeling
Until recently, the available GPCR structures for generation of homology models were β2-adrenergic receptor, rhodopsin, and adenosine receptor (reviewed in refs. 17,18,19). The structures are silenced (keeping the receptors in a rigid conformation) in their signaling activity owing to the methods used to prepare these proteins for crystallization: 1) inverse agonists were used as ligands, 2) they were modified directly by mutations, or 3) they were fused with another protein, lysozyme (reviewed in refs. 17, 19, 20). The new X-ray structure of opsin (21) lacks the inverse agonist ligand, retinal, and has structural features of an active receptor conformation. For structural comparisons between the mutant-activated and wt TSHR, we generated homology models for both the basal TSHR based on rhodopsin and an activated TSHR using opsin as a template.
Several TSHR-specific corrections were made in the homology models. In opsin, the side chains of two consecutive threonines interact with the helical backbone of the preceding residues, causing a structural bulge in TMH2. In the TSHR, no consecutive threonines exist in TMH2, which suggests the presence of a regular α-helix. A minor change of orientation (a twist of 10–15°) of the N-terminal half of TMH5 was generated owing to the lack of a proline compared with opsin/rhodopsin. Gaps of missing residues in the loops of the template structure were closed by the Loop Search tool implemented in Sybyl 7.3.5 (Tripos Inc., St. Louis, MO, USA). Side chains and loops of homology models were subjected to conjugate gradient minimizations [until converging at a termination gradient of 0.05 kcal/(mol · Å)] and molecular dynamics simulation (2 ns) by fixing the backbone of the TMHs. Finally, the models were minimized without constraints. The quality and stability of the model were validated by checking the geometry using Procheck (22).
The amino acids for mutagenesis were chosen according to the following criteria: 1) they are in spatial proximity to the known constitutively activating mutation (CAM), I640V, in TMH6 (23, 24), 2) they form the potential binding pocket for LMW ligands in the TSHR and GPCRs (12), which is located in the TMH bundle close to the extracellular site. To facilitate comparison of different GPCRs, we used both the amino acid numbering scheme of the entire TSHR with its signal peptide and the Ballesteros-Weinstein nomenclature (25). All structure images were produced using PyMOL software (version 099; DeLano Scientific, San Carlos, CA, USA).
RESULTS
Guided by homology modeling, we performed site-directed mutagenesis of 24 amino acid residues that are in the transmembrane region of the TSHR in proximity to the predicted binding pocket for small ligands between TMHs 1, 2, 3, 5, 6, and 7 (9, 10). Fourteen mutations did not increase the basal activity of the TSHR, but we identified 10 new CAMs (V421I, Y466A, T501A, L587V, M637C, M637W, S641A, Y643F, L645V, and Y667A) at 9 TSHR positions. These mutations are characterized by increased ligand-independent (constitutive) basal cAMP accumulation (Table 1). To define constitutive activation, we used a criterion of >150% cAMP accumulation, given that for the TSHR and FSHR the lowest basal Gs signaling activity of activating pathogenic mutations was experimentally shown to be between ∼150 and 170% (supplemental data in ref. 26; see also database http://www.SSFA-GPHR.de).
TABLE 1.
Functional characterization of the constitutively activating TSHR mutations
| Location | Transfected construct | Ballesteros/Weinstein numbering | Cell surface expression: FACS (% TSHR wt) | cAMP accumulation |
|
|---|---|---|---|---|---|
| Basal (fold wt) | TSH stimulated (% TSHR) | ||||
| pcDNA3 | 0 | 0.3 ± 0.1 | ND | ||
| wt TSHR | 100 | 1 | 100 | ||
| TMH1 | V421I | 1.39 | 105.8 ± 8.1 | 2.1 ± 0.4 | 85.4 ± 3.1 |
| TMH2 | Y466A | 2.56 | 102.3 ± 8.0 | 2.8 ± 0.5 | 49.7 ± 7.7 |
| TMH3 | T501A | 3.32 | 142.2 ± 9.0 | 3.4 ± 0.6 | 128.7 ± 12.8 |
| TMH6 | L587V | 5.44 | 89.5 ± 11.0 | 1.7 ± 0.4 | 96.6 ± 2.4 |
| TMH6 | M637C | 6.48 | 96.1 ± 7.7 | 2.4 ± 0.6 | 112.5 ± 24.0 |
| M637W | 73.2 ± 6,0 | 4.8 ± 1.2 | 101.3 ± 13.1 | ||
| TMH6 | S641A | 6.52 | 74.4 ± 1.8 | 3.1 ± 0.2 | 96.8 ± 3.5 |
| TMH6 | Y643F | 6.54 | 117.1 ± 10.7 | 2.1 ± 0.3 | 88.9 ± 0.1 |
| TMH6 | L645V | 6.56 | 82.0 ± 7.2 | 2.1 ± 0.3 | 100.4 ± 11.8 |
| TMH7 | Y667A | 7.42 | 74.9 ± 3.3 | 1.7 ± 0.4 | 85.4 ± 6.9 |
HEK 293 cells were transfected with the wt TSHR or mutated TSHRs. TSHR cell surface expression level was quantified on a FACS flow cytometer. Receptor expression was determined by the mean fluorescence intensity by comparison to wt TSHR. Data are means ± se of 2 independent experiments, each carried out in duplicate. pcDNA3 vector was used as a control. ND, not determined.
The majority of the mutants showed levels of cell surface expression like that of wt TSHR. Only M637W, S641A, and Y667A are characterized by a 25% reduced expression compared with the wt. The maximum cAMP accumulation of the mutants after stimulation with TSH is similar to that of the wt, with the exception of Y466A, which has ∼50% activity compared with that of the wt. The basal (constitutive) activity of these mutants ranged between 1.7- and 4.8-fold over wt activity. Interestingly, the highest increase of basal activity (4.8-fold) was shown by the M637W mutant at position 6.48 (Table 1).
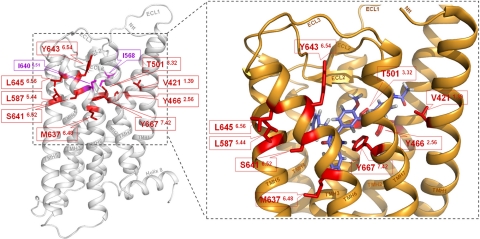
The homology model of the TSHR shows that these 9 new positions with 10 CAMs are located in the transmembrane helical bundle near the transition to the extracellular loops (Fig. 2, left panel). Moreover, these 9 residues are arranged in a cluster in spatial proximity to the potential allosteric transmembrane binding pocket for small-molecule agonists (Fig. 2, right panel). None of these residues have been characterized in the TSHR before or at corresponding positions of the homologous receptors LHCGR and FSHR.
Figure 2.
Newly identified CAMs in the thyrotropin receptor surround the allosteric binding site of a LMW agonist. Left panel: molecular homology model of the serpentine domain (aa C408–I698) without the large N-terminal tail of the human TSHR is based on the crystal structure of inactive bovine rhodopsin. Backbone (white) of the TSHR and the previously identified CAMs (magenta) I640V (TMH6) and I568V (ECL2) are displayed. Nine positions of the newly identified CAMs (red sticks) are localized in the upper part of the TMHs toward the extracellular loops. Right panel: closeup view of the activated TSHR conformation, which is based on the opsin crystal structure, occupied by LMW-Ag (11). Wild-type amino acids of the 9 CAM positions (red) are arranged in a cluster. They are spatially close or interact directly with each other and surround the allosteric ligand binding pocket of LMW-Ag (blue). Therefore, these amino acids may be involved in TSHR activation by small-molecule ligands.
V421 (V1.39) in TMH1 interacts with hydrophobic amino acids at TMH2 (L2.57 and A2.61) and TMH7 (L7.40). The CAM V421A involves a side-chain modification to a smaller and shorter residue and therefore disrupts the interplay in this hydrophobic patch which, most likely, leads to changes in the helix arrangement.
Y466 (Y2.56) in TMH2 is highly conserved in GPHRs and in the closely related leucine-rich repeat containing GPCRs 2, 4, 7, and 8. According to our homology model, this amino acid is oriented toward TMH3 (T3.32) and TMH7 (Y7.42). The CAM T501A (T3.32) in which alanine was substituted for threonine indicates that the hydroxy group with its H bond-forming ability is essential for maintaining the basal conformation. A potential interaction partner could be the side chain of Y466 (Y2.56) in TMH2.
Most of the CAMs are within TMH6. Wild-type amino acids S641 (S6.51) and Y643 (Y6.54) of the TSHR are oriented toward TMH5 or the transition of TMH7/ECL3. Y643 could establish a hydrogen bond with the backbone or amino acid side chains in ECL3, thereby stabilizing the basal TSHR conformation.
The residues L587 (L5.44) in TMH5 and L645 (L6.56) in TMH6 directly interact with each other by hydrophobic interactions. At both positions, mutations to a shorter valine interrupt this interaction and lead to changes in the interface between TMH5 and TMH6 that shift the receptor’s conformation toward the activated state. Such activation sensitivity at this interface is also described for other GPCRs such as the thyrotropin-releasing hormone receptor (27).
Based on this fact and the proximity of M6.37 to the potential small-molecule agonist binding pocket of the TSHR (Fig. 2, right panel), we decided to test the effect of the CAMs M637W and M637C on receptor activation by LMW-Ag. Both mutants are activated by TSH with potencies and efficacies similar to those of wt TSHR (wt TSHR EC50: 7.1±1.7 nM; M637W EC50: 11.6±1.0 nM; M637C EC50: 9.8±3.4 nM) (Fig. 3). In contrast, LMW-Ag activates the M637W receptor with a 14-fold decrease in potency (wt TSHR EC50: 0.2±0.02 μM; M637W EC50: 2.8±0.6 μM) (Fig. 3A). The M637C mutant exhibits similar receptor activation by LMW-Ag as wt TSHR (Fig. 3B).
Figure 3.
Mutant M637W decreases the TSHR activation potency of LMW-Ag. HEK-EM293 cells transiently transfected with wt TSHR or mutant receptors were exposed to increasing concentrations of TSH or the small-molecule ligand LMW-Ag (c2) in the presence of 1 mM IBMX as described in Materials and Methods. After 60 min, the cells were lysed, and cAMP levels were measured by ELISA. Data from 3 independent experiments with duplicate samples are shown. A) In contrast to TSH, LMW-Ag shows a 14-fold decrease in activation of the M637W mutant (wt TSHR EC50: 0.2±0.02 μM; M637W EC50: 2.8±0.6 μM). B) There is no difference between activation of TSHR and the M637C mutant by LMW-Ag (EC50 TSHR: 0.2±0.02 μM; EC50 M637C: 0.4 ±0.3 μM).
For the TSHR wt and mutants M637C and M637W, we designed molecular homology models based on the crystal structures of rhodopsin (wt conformation) and opsin (activated conformation). In the TSHR, M637 is located at the same position (6.48) as W265 in rhodopsin (Fig. 4A). The residues interacting with the bulky tryptophan (6.48) of rhodopsin are the small alanine (7.42) and serine (7.45) in TMH7, whereas those interacting with methionine (which is smaller than tryptophan) of the TSHR in the basal conformation are larger residues, tyrosine and asparagine, at positions 7.42 and 7.45 (Fig. 4A). The introduction of a tryptophan at position 6.48 in the TSHR basal state model causes a clash and thus repulsion with the large Y7.42 side chain in TMH7. This is not the case for the cysteine mutation. The bulkier tryptophan side chain clearly extends into the allosteric binding pocket and causes steric hindrance of LMW-Ag binding to the TSHR (docking studies for the agonist and orientation are described in ref. 11), whereas the smaller cysteine side chain does not influence the ligand binding site, similar to wt methionine (Fig. 4B).
Figure 4.
Unlike rhodopsin, a tryptophan at position 6.48 of TMH6 is not tolerated in the TSHR. A) Superimposition of the TSHR homology model (red residues) in the basal state with particular residues of the structural template (inactive bovine rhodopsin) are shown (pale beige residues). In the TSHR, M637 is located at the same position (6.48) as W265 in rhodopsin. Residues interacting with the bulky tryptophan (6.48) of rhodopsin are the small alanine and serine at positions 7.42 and 7.45 in TMH7, whereas those interacting with methionine (6.48) (which is smaller than tryptophan) of the TSHR in the basal conformation are larger residues, tyrosine and asparagine, at positions 7.42 and 7.45. Introduction of a tryptophan at position 6.48 in the TSHR basal state model causes a clash and thus repulsion with the large Y7.42 side chain in TMH7. B) TSHR model (orange) of the active state based on the structure of opsin is shown with the allosteric ligand binding pocket of wt TSHR. In addition to residue M637, superimpositions of the mutations M637W and M637C are shown at position 6.48. The bulkier tryptophan side chain (red) clearly extends into the allosteric binding pocket (yellow) and causes steric hindrance (red arrow) of the agonist c2 (blue)/TSHR interaction, whereas the smaller cysteine side chain (green) does not influence the ligand binding site similar to wt methionine (yellow).
DISCUSSION
We here consider intramolecular activating events of the TSHR by an allosteric LMW agonist, by endogenous hormones, and by mutations. Amino acid substitutions that modify receptor function are useful in elucidating structure-function relationships involved in signaling mechanisms of GPCRs (5, 28, 29). These modifications are helpful for gaining insights into the particular function of specific wt residues and their impact on protein properties such as conformation and stabilization or activation mechanisms. For the TSHR, numerous CAMs that are naturally occurring mutants have been observed or have been identified by random site-directed mutagenesis (reviewed in refs. 8, 30). Interestingly, in the region spatially adjacent to the transition between extracellular and TMHs, which is close to the predicted allosteric binding pocket region (11), only one CAM position (Ile640 at TMH6) was identified in the past (23). We hypothesized that other essential signaling-sensitive residues might exist in close spatial proximity to the position of this CAM in the area surrounding the allosteric transmembrane binding pocket region.
Indeed, we identified 9 new positions of CAMs and the wt residues are clustered in a region between TMHs 1, 2, 3, 5, 6, and 7 near the extracellular domain (Table 1 and Fig. 2). Two possible modes of action for CAMs are discussed in the literature: CAMs disrupt an interaction network important for stabilization of the basal conformation, which induces a shift toward the active conformation; or wt amino acids are not involved in the maintenance of the basal state but substituted side chains create new interactions and thereby shift the equilibrium between the inactive and active states toward the activated conformation. The molecular homology model of the TSHR indicates that several pairs of these residues are direct interaction partners [e.g., Y466 (TMH2) with T501 (TMH3)]. In accordance with the above-described mode 1 of CAM action, side chain modifications at these interacting amino acid positions cause ligand-independent shifts from the basal state toward the activated state.
Interestingly, in 303 of 372 family A GPCRs, a tryptophan located at position 6.48 in TMH6 (31) has been shown to function as an “aromatic side chain rotamer toggle switch” during receptor activation (reviewed in ref. 32). The tryptophan is located in the interior core of the transmembrane helical bundle surrounded by hydrophobic amino acids in TMH7 (Fig. 4A). Introduction of an aromatic and bulky tryptophan at position 6.48 instead of the methionine in the TSHR leads to receptor activation, caused by a reorientation of the tryptophan side chain toward TMH5 that probably causes movement of TMH6 around the pivot at Pro639 (P6.50). The M637C mutation leads to lower constitutive activity compared with M637W. Because cysteine is less bulky than methionine, this mutation indicates that M637 also stabilizes the nonactive state by constraining interactions. In the case of M637C, a loss of matching hydrophobic “knob-and-hole” interactions might cause constitutive receptor activation but does not lead to a strong steric repulsion as with the bulky tryptophan side chain. This conformational change does not affect the multiple mediated signal (33) induced by TSH, which binds to the extracellular region.
The two suggested mechanisms of constitutive activation induced by CAMs can also be assumed for activation caused by the allosterically bound small agonist (15, 34). Binding of the small-molecule agonist can either cause disruption of intramolecular interactions that stabilize the inactive state or stabilize the active state by generating new intramolecular interactions. The interaction of the TSHR with the small-molecule agonist LMW-Ag is characterized by an experimentally proven H bond between LMW-Ag and N590 (position N5.47 in TMH5) (11) and a predicted shift of the M637 side chain (position 6.48 in TMH6) toward TMH5 (Fig. 2). However, only the M637W mutant exhibits a significant effect on the interaction with LMW-Ag; mutation of M637 to cysteine has no effect on TSHR activation by LMW-Ag. Our model might provide an explanation for these observations because only the bulky side chain of tryptophan extends into the binding pocket and sterically hampers the interaction of the small agonist with the receptor, whereas the cysteine mutation with a smaller side chain variation does not influence the agonist binding pocket (Fig. 4B). Therefore, our data further support the conclusion that LMW-Ag binds in a pocket within the transmembrane helical bundle, which is surrounded by a cluster of signaling-sensitive amino acids (Fig. 2).
In summary, we found that a high proportion of residues in several helices surrounding the allosteric binding site of LMW ligands in the TSHR when mutated lead to constitutively active mutant receptors. A high density of signaling-sensitive residues in a discrete domain such as this has not been found previously in the TSHR (26). The wt amino acids of these mutations are likely to be key players in the stabilization of the basal receptor conformation, although M637 at position 6.48 in the TSHR is involved in both stabilizing the basal and supporting the active conformation. These positions are potential trigger points for activation that is induced by small agonistic molecules. In addition, steps potentially involved in the activation of the human TSHR by small allosteric ligands were explored. We show that LMW-Ag may mediate conformational changes toward the active receptor state directly at the key position M637. These findings regarding differentiation between intramolecular stabilization and determinants of activation may be helpful for rational design of small ligands, particularly for high-affinity LMW TSHR-antagonists that are unavailable at present. Our results may also be relevant for other GPCRs because the region of the allosteric transmembrane binding pocket of the TSHR is occupied by endogenous ligands in many other GPCRs. In addition, CAMs in this spatial region are known for several GPCRs [e.g., ghrelin receptor and G protein-coupled α-factor receptor (365 36)]. The allosteric binding region embedded in a signaling-sensitive part of the receptor might be evolutionarily retained in other receptors where the endogenous ligands bind elsewhere. This might also be relevant for family B and C GPCRs.
Acknowledgments
The authors thank Elena Eliseeva for her excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (KR1273/4-1) and by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
References
- Vassart G., Pardo L., Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci. 2004;29:119–126. doi: 10.1016/j.tibs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Themmen A. P. N., Huhtaniemi I. T. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- Szkudlinski M. W., Fremont V., Ronin C., Weintraub B. D. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol Rev. 2002;82:473–502. doi: 10.1152/physrev.00031.2001. [DOI] [PubMed] [Google Scholar]
- Simoni M., Gromoll J., Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- Schoneberg T., Schulz A., Biebermann H., Hermsdorf T., Rompler H., Sangkuhl K. Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol Ther. 2004;104:173–206. doi: 10.1016/j.pharmthera.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Kosugi S., Sugawa H., Mori T. TSH receptor and LH receptor, 1996. Endocr J. 1996;43:595–604. doi: 10.1507/endocrj.43.595. [DOI] [PubMed] [Google Scholar]
- Ascoli M., Fanelli F., Segaloff D. L. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- Kleinau G., Krause G. Thyrotropin and homologous glycoprotein hormone receptors: structural and functional aspects of extracellular signaling mechanisms. Endocr Rev. 2009;30:133–151. doi: 10.1210/er.2008-0044. [DOI] [PubMed] [Google Scholar]
- Jaschke H., Neumann S., Moore S., Thomas C. J., Colson A. O., Costanzi S., Kleinau G., Jiang J. K., Paschke R., Raaka B. M., Krause G., Gershengorn M. C. A low molecular weight agonist signals by binding to the transmembrane domain of thyroid-stimulating hormone receptor (TSHR) and luteinizing hormone/chorionic gonadotropin receptor (LHCGR) J Biol Chem. 2006;281:9841–9844. doi: 10.1074/jbc.C600014200. [DOI] [PubMed] [Google Scholar]
- Neumann S., Kleinau G., Costanzi S., Moore S., Jiang J. K., Raaka B. M., Thomas C. J., Krause G., Gershengorn M. C. A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology. 2008;149:5945–5950. doi: 10.1210/en.2008-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S., Huang W., Titus S., Krause G., Kleinau G., Alberobello A. T., Zheng W., Southall N. T., Inglese J., Austin C. P., Celi F. S., Gavrilova O., Thomas C. J., Raaka B. M., Gershengorn M. C. Small-molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc Natl Acad Sci U S A. 2009;106:12471–12476. doi: 10.1073/pnas.0904506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgand J. S., Rodrigo J., Kellenberger E., Rognan D. A chemogenomic analysis of the transmembrane binding cavity of human G-protein-coupled receptors. Proteins. 2006;62:509–538. doi: 10.1002/prot.20768. [DOI] [PubMed] [Google Scholar]
- Heitman L. H., Ijzerman A. P. G protein-coupled receptors of the hypothalamic-pituitary-gonadal axis: a case for Gnrh, LH, FSH, and GPR54 receptor ligands. Med Res Rev. 2008;28:975–1011. doi: 10.1002/med.20129. [DOI] [PubMed] [Google Scholar]
- Moore S., Jaeschke H., Kleinau G., Neumann S., Costanzi S., Jiang J. K., Childress J., Raaka B. M., Colson A., Paschke R., Krause G., Thomas C. J., Gershengorn M. C. Evaluation of small-molecule modulators of the luteinizing hormone/choriogonadotropin and thyroid stimulating hormone receptors: structure-activity relationships and selective binding patterns. J Med Chem. 2006;49:3888–3896. doi: 10.1021/jm060247s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnot C., Miserey-Lenkei S., Bardin S., Corvol P., Clauser E. Lessons from constitutively active mutants of G protein-coupled receptors. Trends Endocrinol Metab. 2002;13:336–343. doi: 10.1016/s1043-2760(02)00628-8. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Wales M. R., Perry M. J. Assays of cyclic nucleotides A review of current techniques. Appl Biochem Biotechnol. 1993;41:189–218. doi: 10.1007/BF02916422. [DOI] [PubMed] [Google Scholar]
- Hanson M. A., Stevens R. C. Discovery of new GPCR biology: one receptor structure at a time. Structure. 2009;17:8–14. doi: 10.1016/j.str.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum D. M., Rasmussen S. G., Kobilka B. K. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka B., Schertler G. F. New G-protein-coupled receptor crystal structures: insights and limitations. Trends Pharmacol Sci. 2008;29:79–83. doi: 10.1016/j.tips.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Lodowski D. T., Angel T. E., Palczewski K. Comparative analysis of GPCR crystal structures. Photochem Photobiol. 2009;85:425–430. doi: 10.1111/j.1751-1097.2008.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H., Scheerer P., Hofmann K. P., Choe H. W., Ernst O. P. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- Laskowski R. A., Moss D. S., Thornton J. M. Main-chain bond lengths and bond angles in protein structures. J Mol Biol. 1993;231:1049–1067. doi: 10.1006/jmbi.1993.1351. [DOI] [PubMed] [Google Scholar]
- Kleinau G., Claus M., Jaeschke H., Mueller S., Neumann S., Paschke R., Krause G. Contacts between extracellular loop two and transmembrane helix six determine basal activity of the thyroid-stimulating hormone receptor. J Biol Chem. 2007;282:518–525. doi: 10.1074/jbc.M606176200. [DOI] [PubMed] [Google Scholar]
- Gozu H. I., Bircan R., Krohn K., Muller S., Vural S., Gezen C., Sargin H., Yavuzer D., Sargin M., Cirakoglu B., Paschke R. Similar prevalence of somatic TSH receptor and Gsα mutations in toxic thyroid nodules in geographical regions with different iodine supply in Turkey. Eur J Endocrinol. 2006;155:535–545. doi: 10.1530/eje.1.02253. [DOI] [PubMed] [Google Scholar]
- Ballesteros J. A., Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relationships in G-protein coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- Kleinau G., Brehm M., Wiedemann U., Labudde D., Leser U., Krause G. Implications for molecular mechanisms of glycoprotein hormone receptors using a new sequence-structure-function analysis resource. Mol Endocrinol. 2007;21:574–580. doi: 10.1210/me.2006-0309. [DOI] [PubMed] [Google Scholar]
- Deflorian F., Engel S., Colson A. O., Raaka B. M., Gershengorn M. C., Costanzi S. Understanding the structural and functional differences between mouse thyrotropin-releasing hormone receptors 1 and 2. Proteins. 2008;71:783–794. doi: 10.1002/prot.21763. [DOI] [PubMed] [Google Scholar]
- Kleinau G., Jaeschke H., Mueller S., Worth C. L., Paschke R., Krause G. Molecular and structural effects of inverse agonistic mutations on signaling of the thyrotropin receptor—a basally active GPCR. Cell Mol Life Sci. 2008;65:3664–3676. doi: 10.1007/s00018-008-8450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R., Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- Rodien P., Ho S. C., Vlaeminck V., Vassart G., Costagliola S. Activating mutations of TSH receptor. Ann Endocrinol (Paris) 2003;64:12–16. [PubMed] [Google Scholar]
- Sum C. S., Tikhonova I. G., Costanzi S., Gershengorn M. C. Two arginine-glutamate ionic locks near the extracellular surface of FFAR1 gate receptor activation. J Biol Chem. 2009;284:3529–3536. doi: 10.1074/jbc.M806987200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz T. W., Frimurer T. M., Holst B., Rosenkilde M. M., Elling C. E. Molecular mechanism of 7TM receptor activation—a global toggle switch model. Annu Rev Pharmacol Toxicol. 2006;46:481–519. doi: 10.1146/annurev.pharmtox.46.120604.141218. [DOI] [PubMed] [Google Scholar]
- Kleinau G., Jaeschke H., Mueller S., Raaka B. M., Neumann S., Paschke R., Krause G. Evidence for cooperative signal triggering at the extracellular loops of the TSH receptor. FASEB J. 2008;22:2798–2808. doi: 10.1096/fj.07-104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka B. K., Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Holst B., Holliday N. D., Bach A., Elling C. E., Cox H. M., Schwartz T. W. Common structural basis for constitutive activity of the ghrelin receptor family. J Biol Chem. 2004;279:53806–53817. doi: 10.1074/jbc.M407676200. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Margarit S. M., Dube P. Mutation of Pro-258 in transmembrane domain 6 constitutively activates the G protein-coupled α-factor receptor. Proc Natl Acad Sci U S A. 1996;93:6764–6769. doi: 10.1073/pnas.93.13.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]