Abstract
Mammalian polo-like kinases (Plks) are characterized by the presence of an N-terminal protein kinase domain and a C-terminal polo-box domain (PBD) involved in substrate binding and regulation of kinase activity. Plk1-4 have traditionally been linked to cell cycle progression, genotoxic stress and, more recently, neuron biology. Recently, a fifth mammalian Plk family member, Plk5, has been characterized in murine and human cells. Plk5 is expressed mainly in differentiated tissues such as the cerebellum. Despite apparent loss of catalytic activity and a stop codon in the middle of the human gene, Plk5 proteins retain important functions in neuron biology. Notably, its expression is silenced by epigenetic alterations in brain tumors, such as glioblastomas, and its re-expression prevents cell proliferation of these tumor cells. In this review, we will focus on the non-cell cycle roles of Plks, the biology of the new member of the family and the possible kinase- and PBD-independent functions of polo-like kinases.
Key words: cell cycle, kinase evolution, neuron differentiation, polo-box domain, polo-like kinases, tumor suppression
General Overview of the Mammalian Polo-Like Kinase Family
During the 1980s, genetic screens in yeast and Drosophila led to the discovery of crucial mitotic regulators including polo kinase.1–4 Successive studies revealed that this kinase is well conserved through evolution, from budding yeast (Cdc5) to Drosophila (polo), Xenopus (Plxs) and mammals [polo-like kinases (Plks)] (Fig. 1). All polo kinases share a conserved N-terminal kinase catalytic domain and one or more C-terminal polo boxes (polobox domain or PBD; Fig. 2), which are involved in binding to substrates (reviewed in ref. 5).
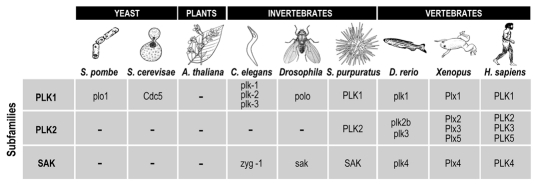
Figure 1.
Polo-like kinases in different taxons. Polo kinases are found in all eukaryotic lineages other than plants and apicomplexans. Yeast such Saccharomyces cerevisae and Schizosaccharomyces pombe only have one polo-like kinase. Invertebrates, like Caenorhabditis elegans or fruit flies (Drosophila), have Plk genes belonging to the PLK1 and SaK, but not PLK2, subfamilies. The PLK2 subfamily is present in vertebrates as well as in some other bilaterians, such as sea urchin (Strongylocentrotus purpuratus). Plk5 is present in mammals, although amphibians (Xenopus laevis) also contain a divergent but probably true ortholog of this protein (Plx5).
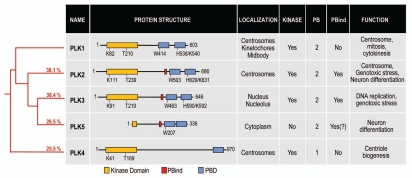
Figure 2.
The human Plk family. The red tree on the left represents the phylogeny of the five human Plk proteins. Numbers indicate the percentage of sequence indentity of each Plk compared to PLK1. The protein structure of each Plk is aligned using the kinase domain (yellow box). Polo boxes are shown in blue, and the PBind motif is represented as a red box. Key residues of the kinase domain (acceptor Lysine and T-loop Threonine) are indicated. Similarly, the key residues for substrate recognition in the PBD are shown.
The founding member of the family, Plk1, and its orthologs polo and Cdc5 are master regulators of cell division. By phosphorylating different substrates, Plk1 controls a number of processes throughout the cell cycle, including centrosome maturation,6 mitotic entry,7–10 chromosome segregation9,11,12 and cytokinesis13–19 (Fig. 3). Accordingly, Plk1 localizes to the cytoplasm and centrosomes in interphase and concentrates to the kinetochores and the cytokinetic bridge during cell division. Inhibition of Plk1 function by RNA interference or small-molecule inhibitors results in failure to establish a bipolar spindle and to properly attach kinetochores to microtubules.20,21 Plk1 is an essential gene. Cdc5 and Drosophila polo-null mutants are lethal,1,22 and Plk1-null mice die at the morula stage due to a massive mitotic arrest (Wachowicz P, de Cárcer G, Malumbres M, unpublished data).
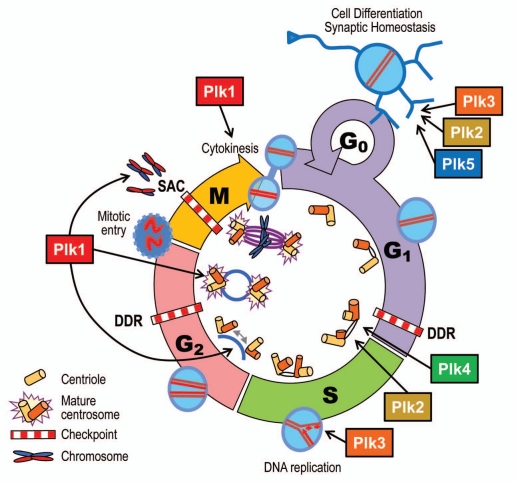
Figure 3.
Cellular functions of Plks. Plk1-4 are implicated in cell cycle processes, such as centriole duplication (Plk2 and Plk4); DNA replication (Plk3); centrosome separation and maturation (Plk1); mitotic entry (Plk1); spindle formation, chromosome segregation and cytokinesis (Plk1). On the other hand, Plk2 and Plk5 (and probably Plk3) are involved in non-proliferative functions, such as neuron differentiation (Plk2 and Plk5) and synaptic homeostasis (Plk2). DDR, DNA damage response; SAC, spindle assembly checkpoint.
The other mammalian Plks belong to two different subfamilies with distinctive functions and evolutionary histories. Plk4 (also known as SAK23) is found in all animals and scattered other eukaryotes, and it acts as a critical regulator of centriole duplication both in Drosophila and mammals.24–27> Alteration of Plk4 levels leads to aberrations in centriole biogenesis and subsequent problems in cell division due to severe mitotic disruption, eventually inducing aneuploidy. Plk4-null mice die at midgestation28 and heterozygous mice develop aneuploid tumors.29 ,30
Plk2, 3 and 5 belong to the PLK2 subfamily, which is found only in some bilaterian animals (Figs. 1 and 2). Plk2 (also known as SNK31,32) expression and activity peak as cells enter S phase.32,33 Plk2 is concentrated at the centrosomes, and inhibition of Plk2 results in an aberrant number of centrioles.34–36 However, Plk2 knockout mice are viable, suggesting that Plk2 is not essential and might be replaced by other Plks.33 Plk3 (also named FNK or PRK37,38) mRNA levels peak in G1 phase, although the protein levels are constant across the cell cycle.38,39 Plk3 is required at the G1-S phase transition, where it promotes the accumulation of cyclin E and activation of Cdc25A, favoring DNA replication.40,41 It has also been proposed that Plk3 might sense genotoxic stress, leading to cell cycle arrest and apoptosis by several mechanisms, including a p53-dependent pathway.39,42–44 Plk3-deficient mice are also viable, although prone to tumor development.45
A fifth member of the family has been recently described. Human Plk5 was first identified as a pseudogene containing a single stop codon within the kinase domain.46 The orthologous mouse locus was found to encode a full-length protein.47 Surprisingly, two independent groups have recently demonstrated that both the murine and human Plk5 genes encode expressed proteins, whereas the mouse protein contains a complete kinase domain and PBD, the human ortholog appears to re-initiate translation after the stop, expressing only a small portion of the kinase domain along with the PBD (Fig. 4).48,49 Plk5 is upregulated in serum-starved fibroblasts as they exit the cell cycle, and overexpression of mouse or human Plk5 arrests cells at a G0/G1-like stage, with increased expression of the cell cycle inhibitor p21Cip1. Plk5 does not need kinase activity to exert the cell cycle arrest, since this phenotype can be achieved by expressing the human protein, which lacks the kinase domain, or a kinase-dead form of the mouse protein.48
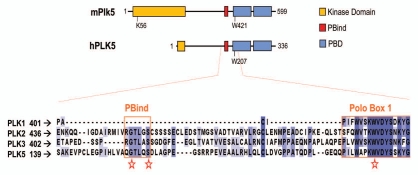
Figure 4.
Plk5 structure and the PBind motif. The murine Plk5 (as well as most other mammalian Plk5s) presents a full kinase domain although it lacks key T-loop activatory residues (See Fig. 2 for a comparison). The human PLK5 protein lacks almost the whole kinase domain due to the presence of a STOP codon. Both orthologs lack the main residues for phosphosubstrate recognition in the polo-box domain (PBD). Murine (not shown) and human PLK5 proteins display high sequence similarity in the PBind motif present in PLK2 and PLK3, but not in PLK1 (bottom alignment).
Evolution of the Polo-Like Kinase Family
Polo kinases are found in all eukaryotic lineages other than plants and apicomplexans. The PLK1 subfamily is universal within this group, highlighting its critical role in the cell cycle (Figs. 1 and 3). SAK/Plk4 is found in all animals, and a few other lineages (some fungi and ciliates). This protein emerged from a Plk1like ancestor and specialized in centriole biogenesis, uncoupling this activity from other more general functions in the cell cycle retained by Plk1.50 PLK2 is restricted to bilaterian animals and is absent from many of those, including the model systems, Drosophila and C. elegans. Most animals have a single PLK2 gene, but in vertebrates, this has duplicated to generate Plk2 and Plk3. A second duplication gave rise to Plk5, which is found in most mammals as well as in Xenopus, but has been secondarily lost in birds and is not found in fish (Fig. 1). The Plk5 sequence has evolved much faster than those of Plk2 or Plk3, including three separate mutations that at first appeared to be pseudogenization events. The human Plk5 transcript encodes a premature stop codon that disrupts the open reading frame near the end of the kinase domain and is followed by a frameshifting indel. Curiously, all human expressed sequences skip the constitutive exon following the indel and, so, restore the proper reading frame, though at the cost of losing part of the first polo box. This stop and frameshift are not seen in primate orthologs, but the chimp sequence has an independent stop, also within the kinase domain. Yet another disabling mutation is seen in the cow: a frameshift between the kinase domain and the first PBD, which should also lead to a truncated protein. Plk5 genes can be predicted from most other vertebrate draft genomes, though many of the assemblies are incomplete and lack parts of the gene. All Plk5 orthologs have lost the main activatory autophosphorylation site (T210 in human Plk1) and have changed the kinase motif DFG to DLG, which is not seen in any other Plks. These changes may have lead to the lack of observable kinase activity in the mouse Plk5.48 In addition, Plk5 has lost the conserved key residues in the second PBD that are involved in phospho-substrate binding (Figs. 2 and 4).
Tissue-Specific Expression of Mammalian Plks
Mammalian Plks have distinctive tissue-specific expression.51 Human Plk1 and Plk4 are highly expressed in all embryonic tissues, matching their high rate of proliferation, and, in adults, are predominantly found in proliferative tissues, such as the testis and bone marrow. Plk1 is not significantly detected in other tissues, such as liver, kidney, brain or lung, indicating that cell proliferation is the main driver of Plk1 expression.52
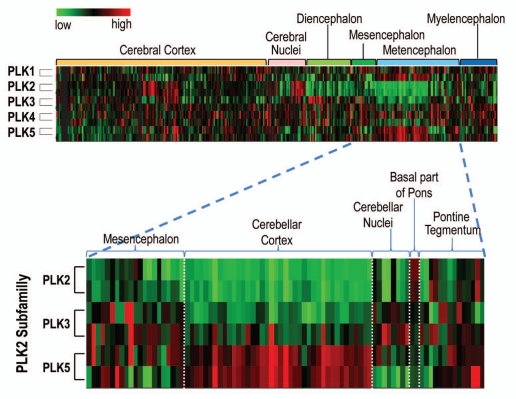
By contrast, Plk2 and Plk3 transcripts are more widely expressed throughout distinct proliferative and non-proliferative tissues. For instance, Plk2 is found in testis, mammary gland, spleen, uterus, trachea and cardiomyocytes. Plk2 is also highly expressed in the central nervous system and is involved in synaptic homeostasis and neuronal differentiation (see below). Plk2 is most highly expressed in the cerebral cortex, hippocampus, occipital, temporal and parietal lobes, putamen and trigeminal ganglion (Fig. 5), but surprisingly, there is almost no expression at the cerebellum. Plk3 is mainly found in the human respiratory organs, such as lungs, trachea and bronchus as well as in gastrointestinal mucosa.37 ,53
Figure 5.
Expression of human PLK1-5 in the central nervous system. PLK2 and PLK5 are highly expressed in certain anatomical areas of the brain. The bottom blown up part shows in detail how PLK5 complements precisely the low expression of PLK2 into the cerebellar cortex. The mRNA expression data was obtained from the allen institute for Brain Science (http://human.brain-map.org). High expression shown in red, low in green.
Plk5 is only expressed in a few non-proliferative tissues.48 Plk5 is highly expressed in the central nervous system, mainly in the brain cortical neurons and glia cells and in the granular layer of the cerebellum. According to available mRNA expression arrays, Plk5 is mostly transcribed in the cerebellum (Fig. 5). Interestingly, both Plk2, the other polo-like kinase with neuronal functions, and Plk3 are almost absent from the cerebellum. This suggests that that Plk5 may have a Plk2-like role in the cerebellum. In addition, using human tissue arrays, we have previously found milder Plk5 expression in highly differentiated tissues, such as cells of the serous acini in the parotid gland, distal and proximal tubules of the kidney and tubules of the seminal gland.48
Polo-Like Kinase Roles Beyond Cell Cycle Progression
As mentioned above, Plks are traditionally implicated in cell cycle progression. However, there is increasing evidence for Plk functions beyond cell proliferation, particularly for the PLK2 subfamily (reviewed in ref. 54). Although first described more than 10 years ago,55 it was only recently that the role of Plk2 (and possibly Plk3) in neurons has been explored. Plk2 is upregulated during neuronal activity, and its activation influences the modulation of synapsis in both proximal and distal dendrites. Plk2 phosphorylates SPAR (spine-associated protein), leading to SPAR degradation via a β-TrCP phosphodegron.56 Plk2 interacts with SPAR through its PBD, and this requires a priming phosphorylation of SPAR by Cdk5.57 SPAR promotes the growth of dendritic spines, the postsynaptic compartment of excitatory synapses. SPAR degradation causes the depletion of a core postsynaptic scaffolding molecule (PSD-95), leading to loss of mature dendritic spines and synapses.58 Plk2 overexpression causes depletion of mature spines and the overgrowth of thin filopodia-like spines, promoting synapse loss. In addition, Plk2 leads to modulation of distal dendrite synapses during chronic overexcitation in a kinase- and PBD-independent manner.59 Plk2 interacts directly with the N-ethylmaleimide-sensitive fusion protein (NSF), causing it to dissociate from GluA2-type glutamate receptors. This leads to intracellular sequestration of GluA2 in complex with adaptor proteins and the subsequent reduction in signal transmission through AMPA receptors in rat hippocampal neurons.59
Experimental evidence also implicates Plk2 in neuronal cell differentiation.60 Using different stimuli on neuroblasts, Plk2 was found to be required for neuronal differentiation driven by neuronal growth factor (NGF). Plk2 and Plk3 might also be important elements in certain neurological diseases, since Plk2, and possibly Plk3, are able to phosphorylate neuronal alpha-synuclein, a phospho-protein accumulated in neurodegenerative diseases, such as Parkinson disease and dementia with Lewy bodies.61 Plk5 also seems to modulate neurite formation in response to NGF, brain-derived growth factor (BDGF) or Ras pathway activation both in rat PC12 cells and in mouse primary hippocampus neuroblasts.48 Since Plk5 is expressed in specific regions of the central nervous system, where Plk2 or Plk3 are not significantly expressed (Fig. 5), it is tempting to speculate that Plk5 may perform in these cells at least some of the functions exerted by Plk2/3 in other areas of the brain.
Are the Kinase or Polo Domains Essential for Plk Function?
The lack of a functional kinase domain and the partial degeneration of the PBD in Plk5 highlight the question of whether other Plks have kinase- or PBD-independent roles. Specific mutations in critical residues of the PBD are sufficient to delocalize yeast or mammalian Plk1 and disrupt its function without affecting kinase activity.62,63 Later studies confirmed that the PBD is also required for proper localization and function of other Plks.64 In a phosphopeptide binding screen65 and a crystallographic study,66 M. Yaffe and colleagues described in 2003 how Plks recognize their substrates through the PBD domain. The PBD contains a phosphopeptide-binding motif, having a strong substrate affinity for either phospho-Serine or phospho-Threonine residues located in the consensus peptide Ser-pSer/pThr-Pro. Structurally, each polo box folds close to the other one creating a “pincer” that is able to grab the phosphopeptide. Phosphorylation of a substrate by other kinases (such as Cdk1 or Cdk5) provides a docking site, allowing Plks to additionally phosphorylate the substrate. This is probably the main cause of the specific and variable subcellular localization of Plks during the cell cycle (for instance the centrosomes and kinetochores during mitosis for Plk1).66
Other studies, however, have shown that the PBD is not completely required for Plk regulation and localization. Plk1 can bind unphosphorylated substrates, and mutants that cannot bind phosphorylated residues still localize to the centrosomes.67 Moreover, Drosophila polo localizes to interphase microtubules by interacting with a microtubule-associated protein, Map205, in a manner that requires both the PBD and the kinase domain but not the priming phosphorylation.68 In a similar fashion, the interaction between Plk1 and Bora might not require priming phosphorylation, and either the PBD or the kinase domain appear to be sufficient for the interaction.69 Finally, Plk4 kinases have an additional region called the “crypto-polo box.”70,71 This crytopolo-box domain displays weak homology with the consensus PBD, although it is also able to target Plk4 to the centrosome. Only when both the canonical PBD and the crypto-box are removed from Plk4 does the kinase fail to localize at the centrosome, and its function is suppressed.
It is therefore possible that Plks have additional scaffolding functions, since they are much longer than just the kinase or polo-box domains (which account for only half of the length for all mammalian Plks). A good illustration of this is the fact that Plk2 can bind to proteins using another region in the PBD not implicated in the classical substrate recognition model. Plk2 binds to NSF using a motif (PBind; Fig. 4) located at the boundary of the PB1 and the linker region. This region contains two residues that impair binding when mutated to alanine (Gly451 and Ser455). These residues are conserved throughout vertebrate Plk5 and Plk3 (which also binds to NSF) but are not found in Plk1. This might reflect a new functional mode for Plks in non-dividing tissues, where the classical upstream activators and priming kinases might not be present.
The dispensability of the kinase domain is less documented, although there is some support in the literature. First, Plk2 seems to control synaptic homeostasis independently of the kinase activity.59 The expression of the Plk2 kinase-dead mutant does not impair reduction of GluA2 levels in dendritic spines, exerting the very same phenotype as the wild-type Plk2. However, the most extreme example of dispensability of the kinase domain is represented by Plk5. The murine Plk5 lacks kinase activity,48 most probably due to the mutations in the activation of T-loop and the DFG motif. Even more dramatically, the premature stop codon results in deletion of most of the kinase domain in the human ortholog (Fig. 4). The recurrent disruption of the protein in human, chimp and cow can therefore be partially explained by the presence of a dominant function that is independent of the kinase domain.
Since Plk2 does not require either the kinase domain or the PBD for these functions, it is tempting to speculate that Plk5 has evolved to maintain these functions in the cerebellum, where Plk2 is not expressed, without the need to maintain a complete and active kinase domain. One possibility is that binding of these inactive kinases to putative substrates competes with their phosphorylation by other active Plks. However, the exclusive expression of PLK5 in tissues such as the cerebellum argues that it does not act just by sequestering interaction partners. Altogether, these findings suggest that Plks can also interact with some of their partners through a PBD-independent binding mechanism and may have kinase-independent functions.
Polo-Like Kinases in Tumor Development and Therapy
A number of mitotic targets have been proposed as cancer therapeutic targets.72–74 The requirement for PLK1 during mitotic entry led to the development of small-molecule inhibitors of this kinase, some of which are now in clinical trials.75–77 However, although PLK1 displays some oncogenic activity, all Plks may also function as tumor suppressors.75 Human PLK2 is methylated and silenced in hematopoietic,78–80 ovarian81 and liver82 neoplasias, and Plk1-(reviewed in ref. 83 and Wachowicz P, de Cárcer G and Malumbres M, unpublished data), Plk3-45 and Plk4,30-deficient mice display increased susceptibility to tumor development.
Similarly, the novel Plk5 seems to act as an antiproliferative signal both in humans and rodents. Plk5 might play a role in sensitizing genotoxic stress,49 and its expression leads to a p53 and p21Cip1-dependent cell cycle arrest.48 Moreover, silencing of the murine Plk5 in fibroblasts synergizes with oncogenic processes driven by the Ras oncogene.48 In humans, PLK5 is hypermethylated and silenced in brain tumors, and its re-expression leads to apoptotic death of glioblastoma cells in a kinase-independent manner.48
Nowadays there is an increasing list of small compounds intended to inhibit PLK1 for cancer therapy. These drugs are not only classical ATP competitors, but also are able to inhibit Plk1 allosterically by impairing PBD binding to substrates.73 The fact that some members of the Plk family seem to play a role as tumor suppressors requires reevaluating the possible negative secondary effects of these drugs. In addition, the new roles of some Plks in non-dividing tissues, such as brain tissues, raises similar concerns regarding the non-desired side effects of pan-Plk inhibitors.
Conclusions
The experimental evidence accumulated in recent years suggests that the biology of polo-like kinases is much more complex than previously anticipated from the Drosophila or yeast proteins. How these proteins have evolved to control divergent functions, such as centriole function or neuron biology, is an intriguing question. Besides the “classical” functions of Plks in the regulation of the cell cycle (Fig. 3), it is now obvious that these are important regulators of non-proliferative events, such as cell differentiation or synaptic homeostasis. The fact that some of these functions may be independent of the polo-box or even the kinase domain is even more surprising and adds an extra grade of molecular complexity to the biology of “polo-like kinases.” As frequently happens in the human genome, not everything is in the name.
Acknowledgments
This work was funded by grants from the Association for International Cancer Research (AICR #08-0188), Foundation Ramón Areces andthe Ministerio de Ciencia e Innovación (MICINN; SAF2009-07973) and NIH grant HG004164. The Cell Division and Cancer Group of the CNIO is supported by the OncoCycle Programme (S-BIO-0283-2006) from the Comunidad de Madrid, the OncoBIO Consolider-Ingenio 2010 Programme (CSD2007-00017) from the MICINN, Madrid andthe European Community's Seventh Framework Programme (MitoSys; HEALTH-F5-2010-241548).
References
- 1.Llamazares S, Moreira A, Tavares A, Girdham C, Spruce BA, Gonzalez C, et al. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 1991;5:2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- 2.Sunkel CE, Glover DM. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988;89:25–38. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- 3.Hartwell LH, Smith D. Altered fidelity of mitotic chromosome transmission in cell cycle mutants of S. cerevisiae. Genetics. 1985;110:381–395. doi: 10.1093/genetics/110.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood JS, Hartwell LH. A dependent pathway of gene functions leading to chromosome segregation in Saccharomyces cerevisiae. J Cell Biol. 1982;94:718–726. doi: 10.1083/jcb.94.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 6.Lane HA, Nigg EA. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrieu A, Brassac T, Galas S, Fisher D, Labbe J, Doree M. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J Cell Sci. 1998;111:1751–1757. doi: 10.1242/jcs.111.12.1751. [DOI] [PubMed] [Google Scholar]
- 8.van Vugt MATM, Brás A, Medema RH. Polo-like Kinase-1 Controls Recovery from a G2 DNA Damage-Induced Arrest in Mammalian Cells. Molecular Cell. 2004;15:799–811. doi: 10.1016/j.molcel.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Sumara I, Giménez-Abián JF, Gerlich D, Hirota T, Kraft C, de la Torre C, et al. Roles of Polo-like Kinase 1 in the Assembly of Functional Mitotic Spindles. Curr Biol. 2004;14:1712–1722. doi: 10.1016/j.cub.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 10.Arnaud L, Pines J, Nigg EA. GFP tagging reveals human Polo-like kinase 1 at the kinetochore/centromere region of mitotic chromosomes. Chromosoma. 1998;107:424–429. doi: 10.1007/s004120050326. [DOI] [PubMed] [Google Scholar]
- 11.Godinho S, Tavares AA. A role for Drosophila Polo protein in chromosome resolution and segregation during mitosis. Cell Cycle. 2008;7:2529–2534. doi: 10.4161/cc.7.16.6439. [DOI] [PubMed] [Google Scholar]
- 12.Kang YH, Park JE, Yu LR, Soung NK, Yun SM, Bang JK, et al. Self-Regulated Plk1 Recruitment to Kinetochores by the Plk1-PBIP1 Interaction Is Critical for Proper Chromosome Segregation. Molecular Cell. 2006;24:409–422. doi: 10.1016/j.molcel.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Seong YS, Kamijo K, Lee JS, Fernandez E, Kuriyama R, Miki T, et al. A spindle checkpoint arrest and a cytokinesis failure by the dominant-negative Polo-box domain of Plk1 in U-2 OS cells. J Biol Chem. 2002;277:32282–32293. doi: 10.1074/jbc.M202602200. [DOI] [PubMed] [Google Scholar]
- 14.Qian YW, Erikson E, Maller JL. Mitotic effects of a constitutively active mutant of the Xenopus Polo-like kinase Plx1. Mol Cell Biol. 1999;19:8625–8632. doi: 10.1128/mcb.19.12.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neef R, Preisinger C, Sutcliffe J, Kopajtich R, Nigg EA, Mayer TU, et al. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J Cell Biol. 2003;162:863–876. doi: 10.1083/jcb.200306009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkard ME, Randall CL, Larochelle Sp, Zhang C, Shokat KM, Fisher RP, et al. Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc Natl Acad Sci USA. 2007;104:4383–4388. doi: 10.1073/pnas.0701140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkard ME, Maciejowski J, Rodriguez-Bravo Vn, Repka M, Lowery DM, Clauser KR, et al. Plk1 Self-Organization and priming phosphorylation of HsCYK-4 at the spindle midzone regulate the onset of division in human cells. PLoS Biol. 2009;7:1000111. doi: 10.1371/journal.pbio.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfe BA, Takaki T, Petronczki M, Glotzer M. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol. 2009;7:1000110. doi: 10.1371/journal.pbio.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petronczki M, Glotzer M, Kraut N, Peters JM. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev Cell. 2007;12:713–725. doi: 10.1016/j.devcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Erikson RL. Activation of Cdc2/cyclin B and inhibition of centrosome amplification in cells depleted of Plk1 by siRNA. Proc Natl Acad Sci USA. 2002;99:8672–8676. doi: 10.1073/pnas.132269599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenart P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, et al. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 22.Kitada K, Johnson AL, Johnston LH, Sugino A. A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5. Mol Cell Biol. 1993;13:4445–4457. doi: 10.1128/mcb.13.7.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fode C, Motro B, Yousefi S, Heffernan M, Dennis JW. Sak, a murine protein-serine/threonine kinase that is related to the Drosophila polo kinase and involved in cell proliferation. Proc Natl Acad Sci USA. 1994;91:6388–6392. doi: 10.1073/pnas.91.14.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues-Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt-Dias M. Revisiting the Role of the Mother Centriole in Centriole Biogenesis. Science. 2007;316:1046–1050. doi: 10.1126/science.1142950. [DOI] [PubMed] [Google Scholar]
- 25.Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA. Plk4-induced centriole biogenesis in human cells. Dev Cell. 2007;13:190–202. doi: 10.1016/j.devcel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, et al. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol. 2005;15:2199–2207. doi: 10.1016/j.cub.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 27.Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. The Polo kinase Plk4 functions in centriole duplication. Nat Cell Biol. 2005;7:1140–1146. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- 28.Hudson JW, Kozarova A, Cheung P, Macmillan JC, Swallow CJ, Cross JC, et al. Late mitotic failure in mice lacking Sak, a polo-like kinase. Curr Biol. 2001;11:441–446. doi: 10.1016/s0960-9822(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 29.Rosario CO, Ko MA, Haffani YZ, Gladdy RA, Paderova J, Pollett A, et al. Plk4 is required for cytokinesis and maintenance of chromosomal stability. Proc Natl Acad Sci USA. 2010;107:6888–6893. doi: 10.1073/pnas.0910941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko MA, Rosario CO, Hudson JW, Kulkarni S, Pollett A, Dennis JW, et al. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nat Genet. 2005;37:883–888. doi: 10.1038/ng1605. [DOI] [PubMed] [Google Scholar]
- 31.Liby K, Wu H, Ouyang B, Wu S, Chen J, Dai W. Identification of the human homologue of the early-growth response gene Snk, encoding a serum-inducible kinase. DNA Seq. 2001;11:527–533. doi: 10.3109/10425170109041337. [DOI] [PubMed] [Google Scholar]
- 32.Simmons DL, Neel BG, Stevens R, Evett G, Erikson RL. Identification of an early-growth-response gene encoding a novel putative protein kinase. Mol Cell Biol. 1992;12:4164–4169. doi: 10.1128/mcb.12.9.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma S, Charron J, Erikson RL. Role of Plk2 (Snk) in mouse development and cell proliferation. Mol Cell Biol. 2003;23:6936–6943. doi: 10.1128/MCB.23.19.6936-6943.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warnke S, Kemmler S, Hames RS, Tsai HL, Hoffmann-Rohrer U, Fry AM, et al. Polo-like Kinase-2 Is Required for Centriole Duplication in Mammalian Cells. Curr Biol. 2004;14:1200–1207. doi: 10.1016/j.cub.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 35.Chang J, Cizmecioglu O, Hoffmann I, Rhee K. PLK2 phosphorylation is critical for CPAP function in procentriole formation during the centrosome cycle. EMBO J. 2010;29:2395–2406. doi: 10.1038/emboj.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cizmecioglu O, Warnke S, Arnold M, Duensing S, Hoffmann I. Plk2 regulated centriole duplication is dependent on its localization to the centrioles and a functional polo-box domain. Cell Cycle. 2008;7:3548–3555. doi: 10.4161/cc.7.22.7071. [DOI] [PubMed] [Google Scholar]
- 37.Li B, Ouyang B, Pan H, Reissmann PT, Slamon DJ, Arceci R, et al. Prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be downregulated in lung carcinomas. J Biol Chem. 1996;271:19402–19408. doi: 10.1074/jbc.271.32.19402. [DOI] [PubMed] [Google Scholar]
- 38.Donohue PJ, Alberts GF, Guo Y, Winkles JA. Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J Biol Chem. 1995;270:10351–10357. doi: 10.1074/jbc.270.17.10351. [DOI] [PubMed] [Google Scholar]
- 39.Bahassi el M, Conn CW, Myer DL, Hennigan RF, McGowan CH, Sanchez Y, et al. Mammalian Polo-like kinase 3 (Plk3) is a multifunctional protein involved in stress response pathways. Oncogene. 2002;21:6633–6640. doi: 10.1038/sj.onc.1205850. [DOI] [PubMed] [Google Scholar]
- 40.Zimmerman WC, Erikson RL. Finding Plk3. Cell Cycle. 2007;6:1314–1318. doi: 10.4161/cc.6.11.4275. [DOI] [PubMed] [Google Scholar]
- 41.Bahassi el M, Hennigan RF, Myer DL, Stambrook PJ. Cdc25C phosphorylation on serine 191 by Plk3 promotes its nuclear translocation. Oncogene. 2004;23:2658–2663. doi: 10.1038/sj.onc.1207425. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Gao J, Dai W, Lu L. Activation of Polo-like kinase 3 by hypoxic stresses. J Biol Chem. 2008;283:25928–25935. doi: 10.1074/jbc.M801326200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie S, Wu H, Wang Q, Kunicki J, Thomas RO, Hollingsworth RE, et al. Genotoxic stress-induced activation of Plk3 is partly mediated by Chk2. Cell Cycle. 2002;1:424–429. doi: 10.4161/cc.1.6.271. [DOI] [PubMed] [Google Scholar]
- 44.Xie S, Wu H, Wang Q, Cogswell JP, Husain I, Conn C, et al. Plk3 functionally links DNA damage to cell cycle arrest and apoptosis at least in part via the p53 pathway. J Biol Chem. 2001;276:43305–43312. doi: 10.1074/jbc.M106050200. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Bai J, Shen R, Brown SA, Komissarova E, Huang Y, et al. Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1alpha under hypoxic conditions. Cancer Res. 2008;68:4077–4085. doi: 10.1158/0008-5472.CAN-07-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 47.Caenepeel S, Charydczak G, Sudarsanam S, Hunter T, Manning G. The mouse kinome: Discovery and comparative genomics of all mouse protein kinases. Proc Natl Acad Sci USA. 2004;101:11707–11712. doi: 10.1073/pnas.0306880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Carcer G, Escobar B, Higuero AM, Garcia L, Anson A, Perez G, et al. Plk5, a polo box domain-only protein with specific roles in neuron differentiation and glioblastoma suppression. Mol Cell Biol. 2011;31:1225–1239. doi: 10.1128/MCB.00607-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andrysik Z, Bernstein WZ, Deng L, Myer DL, Li YQ, Tischfield JA, et al. The novel mouse Polo-like kinase 5 responds to DNA damage and localizes in the nucleolus. Nucleic Acids Res. 2010;38:2931–2943. doi: 10.1093/nar/gkq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carvalho-Santos Z, Machado P, Branco P, Tavares-Cadete F, Rodrigues-Martins A, Pereira-Leal JB, et al. Stepwise evolution of the centriole-assembly pathway. J Cell Sci. 2010;123:1414–1426. doi: 10.1242/jcs.064931. [DOI] [PubMed] [Google Scholar]
- 51.Winkles JA, Alberts GF. Differential regulation of polo-like kinase 1, 2, 3 and 4 gene expression in mammalian cells and tissues. Oncogene. 2005;24:260–266. doi: 10.1038/sj.onc.1208219. [DOI] [PubMed] [Google Scholar]
- 52.Yuan J, Horlin A, Hock B, Stutte HJ, Rubsamen-Waigmann H, Strebhardt K. Polo-like kinase, a novel marker for cellular proliferation. Am J Pathol. 1997;150:1165–1172. [PMC free article] [PubMed] [Google Scholar]
- 53.Ando K, Ozaki T, Yamamoto H, Furuya K, Hosoda M, Hayashi S, et al. Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. J Biol Chem. 2004;279:25549–25561. doi: 10.1074/jbc.M314182200. [DOI] [PubMed] [Google Scholar]
- 54.Seeburg DP, Pak D, Sheng M. Polo-like kinases in the nervous system. Oncogene. 2005;24:292–298. doi: 10.1038/sj.onc.1208277. [DOI] [PubMed] [Google Scholar]
- 55.Kauselmann G, Weiler M, Wulff P, Jessberger S, Konietzko U, Scafidi J, et al. The polo-like protein kinases Fnk and Snk associate with a Ca(2+)- and integrin-binding protein and are regulated dynamically with synaptic plasticity. EMBO J. 1999;18:5528–5539. doi: 10.1093/emboj/18.20.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ang XL, Seeburg DP, Sheng M, Harper JW. Regulation of Postsynaptic RapGAP SPAR by Polo-like Kinase 2 and the SCFÎ2-TRCP Ubiquitin Ligase in Hippocampal Neurons. J Biol Chem. 2008;283:29424–29432. doi: 10.1074/jbc.M802475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DT, Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58:571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pak DT, Sheng M. Targeted protein degradation and synapse remodeling by an inducible protein kinase. Science. 2003;302:1368–1373. doi: 10.1126/science.1082475. [DOI] [PubMed] [Google Scholar]
- 59.Evers DM, Matta JA, Hoe HS, Zarkowsky D, Lee SH, Isaac JT, et al. Plk2 attachment to NSF induces homeostatic removal of GluA2 during chronic over-excitation. Nat Neurosci. 2011;13:1199–1207. doi: 10.1038/nn.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Draghetti C, Salvat C, Zanoguera F, Curchod ML, Vignaud C, Peixoto H, et al. Functional whole-genome analysis identifies Polo-like kinase 2 and poliovirus receptor as essential for neuronal differentiation upstream of the negative regulator alphaBcrystallin. J Biol Chem. 2009;284:32053–32065. doi: 10.1074/jbc.M109.009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Inglis KJ, Chereau D, Brigham EF, Chiou SS, SchÃbel S, Frigon NL, et al. Polo-like Kinase 2 (PLK2) phosphorylates Î-Synuclein at serine 129 in central nervous system. J Biol Chem. 2009;284:2598–2602. doi: 10.1074/jbc.C800206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee KS, Grenfell TZ, Yarm FR, Erikson RL. Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc Natl Acad Sci USA. 1998;95:9301–9306. doi: 10.1073/pnas.95.16.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song S, Grenfell TZ, Garfield S, Erikson RL, Lee KS. Essential function of the polo box of Cdc5 in subcellular localization and induction of cytokinetic structures. Mol Cell Biol. 2000;20:286–298. doi: 10.1128/mcb.20.1.286-298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jang YJ, Lin CY, Ma S, Erikson RL. Functional studies on the role of the C-terminal domain of mammalian polo-like kinase. Proc Natl Acad Sci USA. 2002;99:1984–1989. doi: 10.1073/pnas.042689299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elia AE, Cantley LC, Yaffe MB. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science. 2003;299:1228–1231. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- 66.Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, et al. The molecular basis for phospho-dependent substrate targeting and regulation of Plks by the Polo-box domain. Cell. 2003;115:83–95. doi: 10.1016/s0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Alvarez B, de Carcer G, Ibanez S, Bragado-Nilsson E, Montoya G. Molecular and structural basis of polo-like kinase 1 substrate recognition: Implications in centrosomal localization. Proc Natl Acad Sci USA. 2007;104:3107–112. doi: 10.1073/pnas.0609131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Archambault V, D'Avino PP, Deery MJ, Lilley KS, Glover DM. Sequestration of Polo kinase to micro-tubules by phosphopriming-independent binding to Map205 is relieved by phosphorylation at a CDK site in mitosis. Genes Dev. 2008;22:2707–2720. doi: 10.1101/gad.486808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leung GC, Hudson JW, Kozarova A, Davidson A, Dennis JW, Sicheri F. The Sak polo-box comprises a structural domain sufficient for mitotic subcellular localization. Nat Struct Biol. 2002;9:719–724. doi: 10.1038/nsb848. [DOI] [PubMed] [Google Scholar]
- 71.Swallow CJ, Ko MA, Siddiqui NU, Hudson JW, Dennis JW. Sak/Plk4 and mitotic fidelity. Oncogene. 2005;24:306–312. doi: 10.1038/sj.onc.1208275. [DOI] [PubMed] [Google Scholar]
- 72.Perez de Castro I, de Carcer G, Malumbres M. A census of mitotic cancer genes: new insights into tumor cell biology and cancer therapy. Carcinogenesis. 2007;28:899–912. doi: 10.1093/carcin/bgm019. [DOI] [PubMed] [Google Scholar]
- 73.Perez de Castro I, de Carcer G, Montoya G, Malumbres M. Emerging cancer therapeutic opportunities by inhibiting mitotic kinases. Curr Opin Pharmacol. 2008;8:375–383. doi: 10.1016/j.coph.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 74.de Carcer G, Perez de Castro I, Malumbres M. Targeting cell cycle kinases for cancer therapy. Curr Med Chem. 2007;14:969–985. doi: 10.2174/092986707780362925. [DOI] [PubMed] [Google Scholar]
- 75.Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10:825–841. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 76.Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov. 2010;9:643–660. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- 77.Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 78.Syed N, Smith P, Sullivan A, Spender LC, Dyer M, Karran L, et al. Transcriptional silencing of Polo-like kinase 2 (SNK/PLK2) is a frequent event in B-cell malignancies. Blood. 2006;107:250–256. doi: 10.1182/blood-2005-03-1194. [DOI] [PubMed] [Google Scholar]
- 79.Smith P, Syed N, Crook T. Epigenetic inactivation implies a tumor suppressor function in hematologic malignancies for Polo-like kinase 2 but not Polo-like kinase 3. Cell Cycle. 2006;5:1262–1264. doi: 10.4161/cc.5.12.2813. [DOI] [PubMed] [Google Scholar]
- 80.Benetatos L, Dasoula A, Hatzimichael E, Syed N, Voukelatou M, Dranitsaris G, et al. Polo-like kinase 2 (SNK/PLK2) is a novel epigenetically regulated gene in acute myeloid leukemia and myelodysplastic syndromes: genetic and epigenetic interactions. Ann Hematol. 2011 doi: 10.1007/s00277-011-1193-4. [DOI] [PubMed] [Google Scholar]
- 81.Syed N, Coley HM, Sehouli J, Koensgen D, Mustea A, Szlosarek P, et al. Polo-like kinase Plk2 is an epigenetic determinant of chemosensitivity and clinical outcomes in ovarian cancer. Cancer Res. 2011;71:3317–3327. doi: 10.1158/0008-5472.CAN-10-2048. [DOI] [PubMed] [Google Scholar]
- 82.Pellegrino R, Calvisi DF, Ladu S, Ehemann V, Staniscia T, Evert M, et al. Oncogenic and tumor suppressive roles of polo-like kinases in human hepatocellular carcinoma. Hepatology. 2010;51:857–868. doi: 10.1002/hep.23467. [DOI] [PubMed] [Google Scholar]
- 83.Lu LY, Wood JL, Minter-Dykhouse K, Ye L, Saunders TL, Yu X, et al. Polo-like kinase 1 is essential for early embryonic development and tumor suppression. Mol Cell Biol. 2008;28:6870–6876. doi: 10.1128/MCB.00392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]