Abstract
The orexigenic effect of ghrelin is mediated by neuropeptide Y (NPY) and agouti-related protein (AgRP) in the hypothalamic arcuate nucleus (ARC). Recent evidence also indicates that ghrelin promotes feeding through a mechanism involving activation of hypothalamic AMP-activated protein kinase (AMPK) and inactivation of acetyl-CoA carboxylase and fatty acid synthase (FAS). This results in decreased hypothalamic levels of malonyl-CoA, increased carnitine palmitoyltransferase 1 (CPT1) activity, and mitochondrial production of reactive oxygen species. We evaluated whether these molecular events are part of a unique signaling cascade or whether they represent alternative pathways mediating the orexigenic effect of ghrelin. Moreover, we examined the gender dependency of these mechanisms, because recent evidence has proposed that ghrelin orexigenic effect is reduced in female rats. We studied in both genders the effect of ghrelin on the expression of AgRP and NPY, as well as their transcription factors: cAMP response-element binding protein (CREB and its phosphorylated form, pCREB), forkhead box O1 (FoxO1 and its phosphorylated form, pFoxO1), and brain-specific homeobox transcription factor (BSX). In addition, to establish a mechanistic link between ghrelin, fatty acid metabolism, and neuropeptides, we evaluated the effect of ghrelin after blockage of hypothalamic fatty acid β oxidation, by using the CPT1 inhibitor etomoxir. Ghrelin-induced changes in the AMPK-CPT1 pathway are associated with increased levels of AgRP and NPY mRNA expression through modulation of BSX, pCREB, and FoxO1, as well as decreased expression of endoplasmic reticulum (ER) stress markers in a gender-independent manner. In addition, blockage of hypothalamic fatty acid β oxidation prevents the ghrelin-promoting action on AgRP and NPY mRNA expression, also in a gender-independent manner. Notably, this effect is associated with decreased BSX expression and reduced food intake. Overall, our data suggest that BSX integrates changes in neuronal metabolic status with ARC-derived neuropeptides in a gender-independent manner.—Lage, R., Vázquez, M. J., Varela, L., Saha, A. K., Vidal-Puig, A., Nogueiras, R., Diéguez, C., López, M. Ghrelin effects on neuropeptides in the rat hypothalamus depend on fatty acid metabolism actions on BSX but not on gender.
Keywords: AMPK, CREB, dimorphism, FoxO1
Ghrelin, a hormone produced in the stomach with orexigenic properties (1,2,3,4,5,6,7,8), has been proposed as a potential antiobesity therapeutic target (9, 10). This is supported by evidence gathered during the past decade showing that ghrelin is an important signal in both rodents and humans to prepare for meal initiation (11,12,13). Furthermore, ghrelin modulates peripheral metabolism, weight gain, and adiposity (2, 14,15,16,17,18).
The orexigenic effects of ghrelin are exerted through the growth hormone secretagogue receptor 1a (GHS-R1a) (19), which is expressed in agouti-related peptide/neuropeptide Y (AgRP/NPY) neurons in the arcuate nucleus of the hypothalamus (ARC) (20, 21). In keeping with this orexigenic role, male rats either fed or deprived of food showed increased AgRP and NPY expression in the ARC when treated centrally with ghrelin (3, 4, 7, 8). The physiological relevance of both neuropeptides as mediators of ghrelin effects was firmly established by assessing the response to ghrelin in AgRP- and NPY-knockout (KO) mice. These experiments revealed that although AgRP-KO and NPY-KO male mice exhibited the expected hyperphagic response to ghrelin, the double AgRP/NPY-KO failed to show any response, indicating the existence of redundancy between these 2 neuropeptides as mediators of ghrelin orexigenic action (22). The molecular mechanisms linking ghrelin with the induction of agrp and npy gene expression have recently been identified. It was reported that the brain-specific homeobox transcription factor (BSX) regulates ghrelin’s stimulatory effect on agrp and npy gene expression in male rats (23, 24). Moreover, it has been recently reported that BSX interacts with 2 other transcription factors: the forkhead box O1 (FoxO1) and the phosphorylated cAMP response-element binding protein (pCREB), to modulate the expression of agrp and npy genes (25, 26), respectively. It is unclear whether ghrelin action on those neuropeptides is mediated by alterations in the level of pCREB and FoxO1.
Besides ARC-derived neuropeptides, we and others have recently demonstrated that, in male rats, the orexigenic effect of ghrelin requires the modulation of AMP-activated protein kinase (AMPK), a key upstream regulator of lipid metabolism (5,6,7,8). The physiological orexigenic effect of ghrelin requires AMPK-induced inhibition of acetyl-CoA carboxylase (ACC) and fatty acid synthase in the ventromedial nucleus of the hypothalamus. The net result of this effect is the decrease in the hypothalamic levels of malonyl-CoA and subsequent increase in carnitine palmitoyltransferase 1 (CPT1) activity, which leads to robust changes in hypothalamic mitochondrial respiration and production of reactive oxygen species (ROS) (7, 8).
Despite these results, the link between ghrelin-induced modulation of neuronal lipid metabolism and the orexigenic changes in neuropeptide expression remains uncertain. In this study we demonstrate that ghrelin-elicited changes in the AMPK-CPT1 pathway are associated with increased levels of AgRP and NPY mRNA expression in the ARC and that this effect is dependent on the transcription factors BSX, FoxO1, and pCREB. Notably, we also show that this effect is present in both male and female rats, indicating that this is a robust gender-independent physiological mechanism controlling feeding. These results are particularly relevant because the vast majority of data regarding ghrelin orexigenic effects are limited to males, and not many studies have examined the effect of ghrelin in females. In fact, the current assumption is that ghrelin orexigenic effect is reduced in female rats as a consequence of the action of ovarian estrogens (27). Furthermore, the gender issue is important given that 1) circulating ghrelin levels and the expression of the GHS-R are influenced by gender (28); 2) there are sex differences in the expression and regulation of pCREB in specific neuronal populations of the central nervous system (29, 30); 3) the synthesis and release of AgRP and NPY, as well as some of its biological effects, are influenced by gender and gonadal status (31,32,33); and finally, 4) differences in lipid metabolism in peripheral tissues are greatly dependent on the gender and gonadal status (34).
MATERIALS AND METHODS
Animals
We used adult male and female Sprague-Dawley (200–250 g) rats. Rats were housed in a temperature-controlled room, with a 12-h light-dark cycle (lights from 8:00 AM to 8:00 PM). All experiments and procedures involved in this study were reviewed and approved by the Ethics Committee of the University of Santiago de Compostela, in accordance with EU normative regulations for the use of experimental animals.
Implantation of intracerebroventricular (i.c.v.) cannulae
Chronic i.c.v. cannulae were implanted under ketamine/xylazine anesthesia as described previously (7, 18, 24, 35). The correct location of the cannulae in the lateral ventricle was confirmed by methylene blue staining. Animals were individually caged and allowed to recover for 1 wk before experimentation. During the postoperative recovery period, the rats were handled regularly under nonstressful conditions.
I.c.v. treatments
For the ghrelin acute experiments, male and female rats received an i.c.v. administration of either vehicle (5 μl of saline) or ghrelin (5 μg = 1.5 nmol) in a total volume of 5 μl (Bachem, Bubendorf, Switzerland) (7, 18, 24). For the experiments with etomoxir, rats received an i.c.v. injection of vehicle (5 μl of saline) or etomoxir (10 μg in a total volume of 5 μl; Sigma, St. Louis, MO, USA) prior to ghrelin administration. We used 8 rats/group, and the experiments were repeated at least twice; animals were treated at 9:00 AM (1 h after start of light cycle), when they were satiated. Rats were killed by cervical dislocation. The hypothalamus (for Western blotting and enzymatic activity analysis) or the whole brain (for studies of in situ hybridization) were dissected and stored at −80°C until further processing.
Western blotting
Hypothalamic total protein lysates were subjected to SDS-PAGE, electrotransferred on a PVDF membrane, and probed with the following antibodies: ACC, pACCα-Ser79, AMPKα1, and AMPKα2 (Upstate, Temecula, CA, USA); C/EBP homologous protein (CHOP-10), CREB, FoxO1, pCREB-Ser129, CPT1m, CPT1l, phosphorylated eukaryotic translation initiation factor 2 α (peIF2α-Ser52), and pFoxO1-Ser256 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); CPT1c (Proteintech, Chicago, IL, USA); pAMPKα-Thr172 (Cell Signaling, Danvers, MA, USA); and β-actin (Sigma), as described previously (7, 18, 35). We used 8 rats/group. Values are expressed in relation to β-actin levels.
In situ hybridization
Coronal brain sections (16 μm) were probed with specific antisense oligonucleotides against AgRP, NPY, and BSX mRNAs (Table 1). In situ hybridizations were performed as described previously (7, 18, 24, 35, 36). We used 8 rats/group. We used 16 to 20 sections for each animal (4–5 slides; 4 sections/slide). The mean of these 16–20 values was used as the final densitometry value for each animal.
TABLE 1.
Antisense oligonucleotides for in situ hybridization analysis
| mRNA | GenBank accession no. | Sequence |
|---|---|---|
| AgRP | AF206017 | 5′-CGACGCGGAGAACGAGACTCGCGGTTCTGTGGATCTAGCACCTCTGCC-3′ |
| BSX | XM_001064837 | 5′-CCTCAACGGCTTGGGCTTGTGTAGCAGAATGTCC-3′ |
| NPY | M20373 | 5′-AGATGAGATGTGGGGGGAAACTAGGAAAAGTCAGGAGAGCAAGTTTCATT-3′ |
CPT1 activity assay
The CPT1 activity was measured in the supernatant, using methods described by Bieber et al.(37) and Zammit and Newsholm (38), as described previously (7, 18). We used 8 rats/group.
Statistical analysis
Data are expressed as means ± se. Statistical significance was determined by Student’s t test or ANOVA and post hoc 2-tailed Bonferroni test. Values of P < 0.05 were considered significant.
RESULTS
Central administration of ghrelin increases food intake and AgRP and NPY mRNA expression in male and female rats
It is well established that ghrelin administration induces a powerful acute orexigenic effect in male rats (1,2,3,4,5,6,7,8). However, the orexigenic effects of ghrelin in female rats are controversial (27). To determine whether ghrelin administration increases food intake in female rats, we centrally administered the same single dose of ghrelin to fed, satiated male and female rats. Our data showed that i.c.v. administration of ghrelin induced a marked increase in feeding in a gender-independent manner (Fig. 1A, B). Next, we evaluated whether ghrelin orexigenic action was associated with changes in ARC-derived neuropeptides. Our data showed that i.c.v. administration of ghrelin increased the mRNA expression of AgRP (Fig. 1C, E) and NPY (Fig. 1D, F) in male and female rats.
Figure 1.
Central administration of ghrelin increases food intake and AgRP and NPY mRNA expression in male and female rats. A, B) Cumulative food intake of male (A) and female (B) rats treated with vehicle (open diamonds) or ghrelin (solid circles). C–F) Expression of AgRP (C, E) and NPY (D, F) mRNA in ARC of male and female rats after i.c.v. treatment with vehicle (open bars) or ghrelin (solid bars). ***P < 0.001 vs. vehicle. 3V, third ventricle.
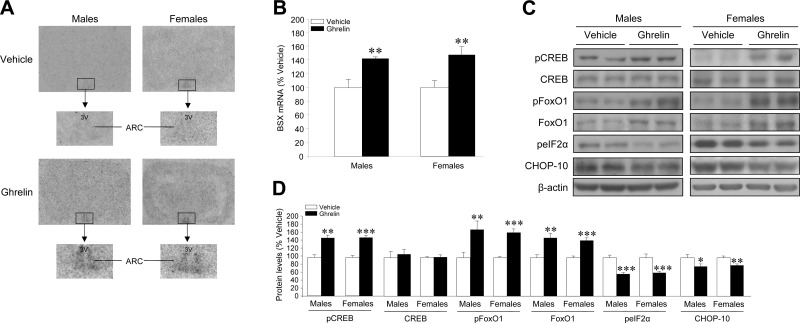
Central administration of ghrelin increases BSX mRNA expression and FoxO1, pFoxO1, and pCREB protein expression in male and female rats
It was recently reported that the transcription factor BSX interacts with pCREB and FoxO1 and plays an essential role in controlling the expression of npy and agrp genes, respectively, in the ARC of male rats (23, 24). Our data show that acute ghrelin administration increased the mRNA expression of BSX in the ARC in a similar extent in male and female rats (Fig. 2A, B). Furthermore, our results showed that acute ghrelin administration increased pCREB, FoxO1, and pFoxO1 hypothalamic protein levels (Fig. 2C, D) in male and female rats, in parallel to the increased mRNA levels of AgRP, NPY, and BSX. Current evidence has shown that in pancreatic β cells, ghrelin treatment increases both FoxO1 and its phosphorylated (and inactive) form pFoxO1, which elicits a protective effect against lipotoxicity, as shown by inhibition of endoplasmic reticulum stress (ER stress) and reduced levels of CHOP-10 (39, 40). In keeping with these observations, our data show that ghrelin decreased the hypothalamic levels of CHOP-10 and the phosphorylated form of its upstream regulator, peIF2α (Figs. 2C, D).
Figure 2.
Central administration of ghrelin increases BSX mRNA expression and FoxO1, pFoxO1, and pCREB protein expression in male and female rats. BSX mRNA levels in ARC (A, B) and pCREB, CREB, pFoxO1, FoxO1, CHOP-10, and peIF2α hypothalamic protein levels (C, D) of male and female rats after i.c.v. treatment with vehicle (open bars) or ghrelin (solid bars). *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle. 3V, third ventricle.
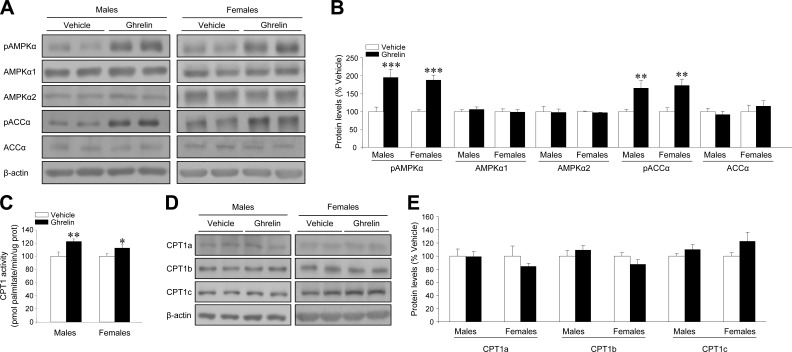
Central administration of ghrelin inactivates fatty acid synthesis de novo and stimulates CPT1 activity in the hypothalamus of male and female rats
Current data from several groups, including ours, have demonstrated that fatty acid metabolism is a target of ghrelin actions in the hypothalamus, mediating its orexigenic actions in male rats (5,6,7,8). However, no data have been reported about the effect of ghrelin on fatty acid metabolism in the hypothalamus of female rats. Our data show that ghrelin administration induced a similar increase in AMPKα (pAMPKα) and ACCα (pACCα) phosphorylation levels, with no changes in the nonphosphorylated isoforms (AMPKα1, AMPKα2, and ACCα, respectively) in the hypothalamus of male and female rats (Fig. 3A, B). As a result of the inactivation of fatty acid synthesis de novo and the subsequent decrease in the levels of malonyl-CoA (data not shown) (7), CPT1 activity was increased in the hypothalamus of both male and female rats (Fig. 3C). No changes were detected in the hypothalamic protein levels of CPT1a, CPT1b, and CPT1c (Fig. 3D, E), suggesting that the stimulatory action of ghrelin on CPT1 activity is not related to increased CPT1 expression.
Figure 3.
Central administration of ghrelin inactivates fatty acid synthesis de novo and stimulates CPT1 activity in the hypothalamus of male and female rats. pAMPKα, AMPKα1, AMPKα2, pACCα, and ACCα levels (A, B); hypothalamic CPT1 activity (C); and hypothalamic CPT1a, CPT1b, and CPT1c protein levels (D, E) of male and female rats after i.c.v. treatment with vehicle (open bars) or ghrelin (solid bars). *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle.
Orexigenic effect of ghrelin is mediated by stimulation of hypothalamic CPT1 activity in male and female rats
Recent data from our group and others have demonstrated that the orexigenic effect of ghrelin is associated with CPT1 activation in the hypothalamus of male rats (7, 8). To determine whether the effect of ghrelin on food intake involved the activation of CPT1 in female rats, we investigated the effect of etomoxir, an inhibitor of CPT1 (7, 8), on ghrelin orexigenic effect. The selected dose of etomoxir (10 μg) induced no anorectic effect per se (Fig. 4A) but significantly inhibited hypothalamic CPT1 activity (Fig. 4B). Our data showed that i.c.v. injection of etomoxir decreased the orexigenic effect of ghrelin in male (Fig. 4C) and female (Fig. 4D) rats. Etomoxir action blunting ghrelin-induced feeding was longer lasting in female rats (up until 6 h) than in male rats (up until 4 h).
Figure 4.
Orexigenic effect of ghrelin is mediated by stimulation of hypothalamic CPT1 activity in male and female rats. A, B) Cumulative food intake (A) and hypothalamic CPT1 activity (B) after i.c.v. treatment with vehicle (open bars) or the CPT1 inhibitor etomoxir (shaded and solid bars). *P < 0.05 vs. vehicle. C, D) Cumulative food intake after i.c.v. administration of vehicle or etomoxir prior to i.c.v. administration of vehicle or ghrelin in male (C) and female (D) rats. Open diamonds, vehicle/vehicle; solid squares, vehicle/etomoxir; solid circles, ghrelin/vehicle; open triangles, ghrelin/etomoxir. *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle/vehicle; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. ghrelin/vehicle.
Inhibition of hypothalamic CPT1 activity prevents the ghrelin-induced increase in AgRP, NPY, and BSX mRNA expression in the hypothalamus of male and female rats
Having shown that inhibition of CPT1 activity by using etomoxir decreased ghrelin’s orexigenic effect in both genders, we investigated the effect of this treatment on the mRNA expression levels of AgRP, NPY, and BSX in the ARC of male and female rats. Our results showed that administration of etomoxir prevented the ghrelin-induced increase in AgRP (Figs. 5A, B and 6A, B), NPY (Figs. 5C, D and 6C, D) and BSX (Figs. 5E, F and 6E, F) in male (Fig. 5) and female (Fig. 6) rats. These data demonstrate that ghrelin’s effects on AgRP, NPY, and their upstream transcription factor BSX are mediated by a CPT1-dependent mechanism. Overall, these results suggest that BSX integrates ghrelin-induced changes in the fatty acid metabolism pathway with neuropeptide expression in a gender-independent manner.
Figure 5.
Inhibition of hypothalamic CPT1 activity prevents ghrelin-induced increase in AgRP, NPY, and BSX mRNA expression in the hypothalamus of male rats. AgRP (A, B), NPY (C, D), and BSX (E, F) mRNA levels in ARC of male rats after i.c.v. administration of vehicle (open bars) or the CPT1 inhibitor etomoxir (solid bars) prior to i.c.v. administration of vehicle or ghrelin. **P < 0.01, ***P < 0.001 vs. vehicle/vehicle; #P < 0.05, ##P < 0.01, ###P < 0.001 for ghrelin/etomoxir vs. ghrelin/vehicle. 3V, third ventricle.
Figure 6.
Inhibition of hypothalamic CPT1 activity prevents ghrelin-induced increase in AgRP, NPY, and BSX mRNA expression in the hypothalamus of female rats. AgRP (A, B), NPY (C, D), and BSX (E, F) mRNA levels in ARC of female rats after i.c.v. administration of vehicle (open bars) or the CPT1 inhibitor etomoxir (solid bars) prior to i.c.v. administration of vehicle or ghrelin. **P < 0.01, ***P < 0.001 vs. vehicle/vehicle; #P < 0.05, ##P < 0.01 for ghrelin/etomoxir vs. ghrelin/vehicle. 3V, third ventricle.
DISCUSSION
In this study, we demonstrate for the first time that ghrelin-induced changes in the AMPK-CPT1 pathway elicit increased levels of AgRP and NPY mRNA expression in the ARC, through modulation of the transcription factors BSX, FoxO1, and pCREB in male and female rats. These data first suggest that BSX integrates changes in neuronal metabolic status with neuropeptide networks in the ARC and very importantly that this link is a gender-independent physiological mechanism controlling feeding.
Besides the well-established stimulatory action of ghrelin on AgRP/NPY neurons in the ARC (3, 4, 7, 8, 22), current evidence has demonstrated that the ghrelin orexigenic effect is mediated by the selective modulation of hypothalamic fatty acid metabolism (5,6,7,8). Although it is clear that the above-described pathways are both bona fide components of ghrelin signaling, the mechanistic link between them is still unclear. It has been recently reported that ghrelin-induced activation of hypothalamic fatty acid oxidation leads to robust changes in hypothalamic mitochondrial respiration and production of ROS, which are buffered by uncoupling protein 2 (UCP2) (8). This mechanism is critical for ghrelin-induced electric activation of AgRP/NPY neurons, as well for ghrelin-dependent gene transcription events in those cells. Thus, ghrelin-induced up-regulation of agrp, npy, ucp2, cpt1, and nuclear respiratory factor 1 (nrf1) gene expression is blunted in UCP2-KO mice (8). Although these results provide an interesting mechanism, linking ghrelin-induced changes in fatty acid metabolism and neuropeptide expression, how alterations in mitochondrial function lead to nuclear transcriptional events remains unresolved. In this study, we show compelling evidence demonstrating that BSX, a recently discovered transcription factor that regulates both AgRP and NPY expression (23, 24), connects ghrelin-promoted activation of hypothalamic fatty acid β oxidation with AgRP and NPY expression in the ARC. More important, we demonstrate that this association is a physiological gender-independent mechanism modulating feeding. In fact, pharmacological blockage of hypothalamic fatty acid β oxidation, by using the CPT1 inhibitor etomoxir, blocks the ghrelin-induced effect on BSX and subsequently on AgRP and NPY mRNA expression in the ARC of both male and female rats. Additional work, using loss of function experiment, such as BSX-KO mice (23), is necessary to confirm the existence of alternative pathways to BSX linking ghrelin fatty acid metabolism and neuropeptide expression. In fact, considering that BSX-KO mice do not totally lack food-deprivation-induced response in AgRP and NPY mRNA levels (23), the involvement of alternative additional transcription factors cannot be excluded, albeit their role is likely to be of lesser importance.
We also show for the first time that FoxO1 and pCREB, 2 well-established transcription factors modulating agrp and npy expression, respectively (23, 25, 26, 41), are stimulated after central ghrelin administration, also in a gender-independent manner. Recent evidence has demonstrated that peripheral signals, such as leptin and insulin, regulate agrp and pomc (proopiomelanocortin) gene expression through modulation of the balance between FoxO1 (active form) and phosphorylated FoxO1 (pFoxO1, inactive form). Thus, leptin and insulin promote pFoxO1 and prevent its translocation to the nucleus (41), which results in increased expression of pomc(42,43,44) and decreased expression of agrp(26). Contrary to leptin and insulin, we show that central ghrelin administration increased both hypothalamic FoxO1 and pFoxO1 in a similar extent. In keeping with previous literature, increased levels of FoxO1 would be the mechanism leading to increased expression of the agrp gene by ghrelin (26); however, the physiological relevance of increased pFoxO1 is intriguing. Current evidence has shown that in pancreatic β cells, ghrelin treatment increases both FoxO1 and pFoxO1. Increased levels of pFoxO1 after ghrelin treatment elicit a protective effect against lipotoxicity, as shown by inhibition of ER stress and reduced levels of CHOP-10 (39, 40). Similar results showing ghrelin’s protective effects against lipotoxicity-induced apoptosis and ER stress has been also reported in an ischemic heart model (45). Consistent with these observations, our data show that ghrelin reduces the hypothalamic levels of CHOP-10 and the phosphorylated form of its upstream regulator peIF2α. Considering that hypothalamic ER stress has recently been proposed as a pathophysiological mechanism under leptin resistance and obesity (46,47,48,49), and that central ghrelin administration increases hypothalamic ROS (8), which are well-recognized ER stress inducers (49,50,51,52,53), it is tempting to speculate that ghrelin-induced increase in hypothalamic pFoxO1 might be a compensatory mechanism leading to protection against ER stress, a hypothesis that will require further investigation.
Another particular question that remains unresolved is whether BSX, pCREB, and FoxO1 mediate just ghrelin effects or whether their role is extensible to other peripheral hormonal signals modulating hypothalamic fatty acid metabolism, such as leptin, insulin, glucagon-like peptide, adiponectin, resistin, glucocorticoids, thyroid hormones, and endocannabinoids (54,55,56) In this sense, recent evidence from our group has demonstrated that BSX integrates leptin signaling in the ARC and may play a major role in the pathogenesis of leptin resistance (24). Considering that, similarly to ghrelin, leptin effects on feeding are mediated by the modulation of hypothalamic lipid metabolism (5, 57, 58), it is tempting to speculate that BSX may also be a mediator of those effects. In this sense, it has been currently published that acute central administration of palmitate induces leptin resistance in the central nervous system (59). Further work will be required to address the possible implication of BSX in fatty acid-induced leptin resistance.
Ovarian steroids play a major role in the regulation of feeding and energy balance by modulating neuropeptide expression, synaptic plasticity, and leptin effects on hypothalamic networks (27, 32, 33, 60,61,62,63,64). However, one particular black box about the effects of ghrelin on energy balance is that the vast majority of data have been generated by using male rats and mice, but not many studies have examined the effects of ghrelin in females (27). In fact, the current assumption is that ghrelin orexigenic effect is reduced in female rats as a consequence of the action of ovarian estrogens. In this sense, Clegg et al.(27) have elegantly examined the effect of ghrelin in female rats and mice. They reported that ovariectomized (OVX) female rats (as well as male rats) were significantly more sensitive than intact female rats to the orexigenic effects of both centrally and systemically administered ghrelin and that this effect is attenuated by estradiol administration (27). Our findings indicate that the whole molecular network under ghrelin orexigenic actions (inhibition of fatty acid synthesis, activation of fatty acid oxidation, and increased pCREB, FoxO1, BSX, AgRP, and NPY expression) is common in both males and females. These discrepancies are likely due to the different rat strain (Long Evans vs. Sprague-Dawley, as well as their physical parameters) experimental models: OVX (total lack of circulating estrogens) vs. intact female rats and time of the injection (1 h after lights on vs. 6 h before lights off). Finally, and very importantly, the eating-stimulatory effect of ghrelin varies across different phases of the ovarian cycle in intact rats. Cleeg et al.(27) reported that central ghrelin had no reliable overall effect when cycle day was not taken into account; however, when cycle day was considered, central ghrelin increased eating during diestrus 1 and diestus 2 but not during proestrus or estrus. In any case, the lack of differences that we report agrees with data obtained from ghrelin-KO and GHS-R-KO mice, where a similar resistance to high-fat-diet-induced obesity was found in both genders (15, 16). Moreover, these data indicate that the physiological response of hypothalamic fatty acid metabolism is more conserved than in peripheral tissues, where gender and gonadal status markedly modulates lipid metabolism (34). Altogether, this evidence demonstrates that ghrelin elicits feeding in a gender-independent manner, through a physiological mechanism involving the modulation of hypothalamic fatty acid metabolism, FoxO1, pCREB, BSX, and neuropeptide expression in the ARC.
In summary, here we show that the orexigenic action of ghrelin is associated with the specific FoxO1, pCREB, and BSX-mediated integration of hypothalamic fatty acid metabolism with AgRP and NPY expression in the ARC. Our data also confirm FoxO1, pCREB, and BSX as gender-independent mediators of food intake of relevance for the understanding and treatment of obesity. Finally, our preliminary data showing that ghrelin influences the expression of 2 hypothalamic ER stress markers, such as CHOP-10 and peIF2α, merits further investigation in order to assess its biological relevance.
Acknowledgments
The authors thank Pablo B. Martínez de Morentin for his technical assistance. This work has been supported by grants from Fondo Investigationes Sanitarias (M.L.: PI061700 and PS09/01880), Ministerio de Educacion y Ciencia (C.D.: BFU2008; M.L.: RyC-2007-00211; R.N.: RyC-2008-02219), the European Union (C.D., M.L., and R.N.: Health-F2-2008-223713, Reprobesity; AVP FP7MITIN and LSHM-CT-2005-018734, Hepadip, http://www.hepadip. org), the U.K. Medical Research Council (A.V.P.), The Wellcome Trust (A.V.P.), and the U.S. Public Health Service (A.K.S.: K-19514 and DK-67509). CIBER de Fisiopatología de la Obesidad y Nutrición is an initiative of Instituto de Salud Carlos III.
References
- Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Tschop M., Smiley D. L., Heiman M. L. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Nakazato M., Murakami N., Date Y., Kojima M., Matsuo H., Kangawa K., Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Kamegai J., Tamura H., Shimizu T., Ishii S., Sugihara H., Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- Andersson U., Filipsson K., Abbott C. R., Woods A., Smith K., Bloom S. R., Carling D., Small C. J. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Kola B., Farkas I., Christ-Crain M., Wittmann G., Lolli F., Amin F., Harvey-White J., Liposits Z., Kunos G., Grossman A. B., Fekete C., Korbonits M. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López M., Lage R., Saha A. K., Pérez-Tilve D., Vàzquez M. J., Varela L., Sangiao-Alvarellos S., Tovar S., Raghay K., Rodríguez-Cuenca S., Deoliveira R. M., Castañeda T., Datta R., Dong J. Z., Culler M., Sleeman M. W., Álvarez C. V., Gallego R., Lelliott C. J., Carling D., Tschop M. H., Diéguez C., Vidal-Puig A. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008;7:389–399. doi: 10.1016/j.cmet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Andrews Z. B., Liu Z. W., Walllingford N., Erion D. M., Borok E., Friedman J. M., Tschop M. H., Shanabrough M., Cline G., Shulman G. I., Coppola A., Gao X. B., Horvath T. L., Diano S. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster-Schubert K. E., Cummings D. E. Emerging therapeutic strategies for obesity. Endocr Rev. 2006;27:779–793. doi: 10.1210/er.2006-0041. [DOI] [PubMed] [Google Scholar]
- Zorrilla E. P., Iwasaki S., Moss J. A., Chang J., Otsuji J., Inoue K., Meijler M. M., Janda K. D. Vaccination against weight gain. Proc Natl Acad Sci U S A. 2006;103:13226–13231. doi: 10.1073/pnas.0605376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren A. M., Seal L. J., Cohen M. A., Brynes A. E., Frost G. S., Murphy K. G., Dhillo W. S., Ghatei M. A., Bloom S. R. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- Cummings D. E., Purnell J. Q., Frayo R. S., Schmidova K., Wisse B. E., Weigle D. S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Gutierrez J. A., Solenberg P. J., Pfluger P. T., Czyzyk T. A., Willency J. A., Schurmann A., Joost H. G., Jandacek R. J., Hale J. E., Heiman M. L., Tschop M. H. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop M., Weyer C., Tataranni P. A., Devanarayan V., Ravussin E., Heiman M. L. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- Wortley K. E., Del Rincon J. P., Murray J. D., Garcia K., Iida K., Thorner M. O., Sleeman M. W. Absence of ghrelin protects against early-onset obesity. J Clin Invest. 2005;115:3573–3578. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman J. M., Nakano Y., Coppari R., Balthasar N., Marcus J. N., Lee C. E., Jones J. E., Deysher A. E., Waxman A. R., White R. D., Williams T. D., Lachey J. L., Seeley R. J., Lowell B. B., Elmquist J. K. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theander-Carrillo C., Wiedmer P., Cettour-Rose P., Nogueiras R., Perez-Tilve D., Pfluger P., Castaneda T. R., Muzzin P., Schurmann A., Szanto I., Tschop M. H., Rohner-Jeanrenaud F. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest. 2006;116:1983–1993. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiao-Alvarellos S., Vazquez M. J., Varela L., Nogueiras R., Saha A. K., Cordido F., Lopez M., Dieguez C. Central ghrelin regulates peripheral lipid metabolism in a growth hormone-independent fashion. Endocrinology. 2009;150:4562–4574. doi: 10.1210/en.2009-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Wang P., Zheng H., Smith R. G. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum G. S., Lapointe M., Beaudet A., Howard A. D. Expression of growth hormone secretagogue-receptors by growth hormone- releasing hormone neurons in the mediobasal hypothalamus. Endocrinology. 1998;139:4420–4423. doi: 10.1210/endo.139.10.6330. [DOI] [PubMed] [Google Scholar]
- Zigman J. M., Jones J. E., Lee C. E., Saper C. B., Elmquist J. K. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. Y., Trumbauer M. E., Chen A. S., Weingarth D. T., Adams J. R., Frazier E. G., Shen Z., Marsh D. J., Feighner S. D., Guan X. M., Ye Z., Nargund R. P., Smith R. G., Van Der Ploeg L. H., Howard A. D., MacNeil D. J., Qian S. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y (NPY) and Agouti-related protein (AgRP) Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- Sakkou M., Wiedmer P., Anlag K., Hamm A., Seuntjens E., Ettwiller L., Tschop M. H., Treier M. A role for brain-specific homeobox factor bsx in the control of hyperphagia and locomotory behavior. Cell Metab. 2007;5:450–463. doi: 10.1016/j.cmet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Nogueiras R., López M., Lage R., Pérez-Tilve D., Pfluger P., Mendieta-Zeron H., Sakkou M., Wiedmer P., Benoit S., Datta R., Dong J. Z., Culler M., Sleeman M., Vidal-Puig A., Horvath T., Treier M., Diéguez C., Tschop M. H. BSX, a novel hypothalamic factor linking feeding with locomotor activity, is regulated by energy availability. Endocrinology. 2008;149:3009–3015. doi: 10.1210/en.2007-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Albergine M., Ippolito D. L., Beavo J. A. Downregulation of fasting-induced cAMP response element-mediated gene induction by leptin in neuropeptide Y neurons of the arcuate nucleus. J Neurosci. 2001;21:1238–1246. doi: 10.1523/JNEUROSCI.21-04-01238.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Feng Y., Ido K. Y., Chua S. C., Xu A. W., Barsh G. S., Rossetti L., Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- Clegg D. J., Brown L. M., Zigman J. M., Kemp C. J., Strader A. D., Benoit S. C., Woods S. C., Mangiaracina M., Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- Kamegai J., Wakabayashi I., Kineman R. D., Frohman L. A. Growth hormone-releasing hormone receptor (GHRH-R) and growth hormone secretagogue receptor (GHS-R) mRNA levels during postnatal development in male and female rats. J Neuroendocrinol. 1999;11:299–306. doi: 10.1046/j.1365-2826.1999.00330.x. [DOI] [PubMed] [Google Scholar]
- Mogi K., Funabashi T., Mitsushima D., Hagiwara H., Kimura F. Sex difference in the response of melanin-concentrating hormone neurons in the lateral hypothalamic area to glucose, as revealed by the expression of phosphorylated cyclic adenosine 3′,5′-monophosphate response element-binding protein. Endocrinology. 2005;146:3325–3333. doi: 10.1210/en.2005-0078. [DOI] [PubMed] [Google Scholar]
- Nazarian A., Sun W. L., Zhou L., Kemen L. M., Jenab S., Quinones-Jenab V. Sex differences in basal and cocaine-induced alterations in PKA and CREB proteins in the nucleus accumbens. Psychopharmacol (Berl) 2009;203:641–650. doi: 10.1007/s00213-008-1411-5. [DOI] [PubMed] [Google Scholar]
- Archer Z. A., Findlay P. A., McMillen S. R., Rhind S. M., Adam C. L. Effects of nutritional status and gonadal steroids on expression of appetite-regulatory genes in the hypothalamic arcuate nucleus of sheep. J Endocrinol. 2004;182:409–419. doi: 10.1677/joe.0.1820409. [DOI] [PubMed] [Google Scholar]
- Santollo J., Eckel L. A. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav Brain Res. 2008;191:173–177. doi: 10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson L. E., Pierce A. A., Xu A. W. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc Natl Acad Sci U S A. 2009;106:15932–15937. doi: 10.1073/pnas.0904747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaak E. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care. 2001;4:499–502. doi: 10.1097/00075197-200111000-00006. [DOI] [PubMed] [Google Scholar]
- López M., Lelliott C. J., Tovar S., Kimber W., Gallego R., Virtue S., Blount M., Vázquez M. J., Finer N., Powles T., O'Rahilly S., Saha A. K., Diéguez C., Vidal-Puig A. J. Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes. 2006;55:1327–1336. doi: 10.2337/db05-1356. [DOI] [PubMed] [Google Scholar]
- Chakravarthy M. V., Zhu Y., López M., Yin L., Wozniak D. W., Coleman T., Hu Z., Wolfgang M., Vidal-Puig A., Lane M. D., Semenkovich C. F. Brain fatty acid synthase activates PPAR-alpha to maintain energy homeostasis. J Clin Invest. 2007;117:2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber L. L., Abraham T., Helmrath T. A rapid spectrophotometric assay for carnitine palmitoyltransferase. Anal Biochem. 1972;50:509–518. doi: 10.1016/0003-2697(72)90061-9. [DOI] [PubMed] [Google Scholar]
- Zammit V. A., Newsholme E. A. Activities of enzymes of fat and ketone-body metabolism and effects of starvation on blood concentrations of glucose and fat fuels in teleost and elasmobranch fish. Biochem J. 1979;184:313–322. doi: 10.1042/bj1840313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Liu Y., Chen Y., Cao C., Xiang Y., Zhang D., Han L., Zhao H., Liu G. Inhibition of Foxo1 mediates protective effects of ghrelin against lipotoxicity in MIN6 pancreatic beta-cells. Peptides. 2010;31:307–314. doi: 10.1016/j.peptides.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Wang W., Zhang D., Zhao H., Chen Y., Liu Y., Cao C., Han L., Liu G. Ghrelin inhibits cell apoptosis induced by lipotoxicity in pancreatic beta-cell line [E-pub ahead of print] Regul Pept. 2010 doi: 10.1016/j.regpep.2009.12.017. doi: 10.1016/j.regpep.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Kim M. S., Pak Y. K., Jang P. G., Namkoong C., Choi Y. S., Won J. C., Kim K. S., Kim S. W., Kim H. S., Park J. Y., Kim Y. B., Lee K. U. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- Plum L., Lin H. V., Dutia R., Tanaka J., Aizawa K. S., Matsumoto M., Kim A. J., Cawley N. X., Paik J. H., Loh Y. P., DePinho R. A., Wardlaw S. L., Accili D. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt B. F., Husch A., Rother E., Ernst M. B., Wunderlich F. T., Hampel B., Klockener T., Alessi D., Kloppenburg P., Bruning J. C. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Ernst M. B., Wunderlich C. M., Hess S., Paehler M., Mesaros A., Koralov S. B., Kleinridders A., Husch A., Munzberg H., Hampel B., Alber J., Kloppenburg P., Bruning J. C., Wunderlich F. T. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J Neurosci. 2009;29:11582–11593. doi: 10.1523/JNEUROSCI.5712-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. G., Teng X., Liu Y., Cai Y., Zhou Y. B., Duan X. H., Song J. Q., Shi Y., Tang C. S., Yin X. H., Qi Y. F. Inhibition of endoplasm reticulum stress by ghrelin protects against ischemia/reperfusion injury in rat heart. Peptides. 2009;30:1109–1116. doi: 10.1016/j.peptides.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi T., Sasaki M., Miyahara T., Hashimoto C., Matsuo S., Yoshii M., Ozawa K. Endoplasmic reticulum stress induces leptin resistance. Mol Pharmacol. 2008;74:1610–1619. doi: 10.1124/mol.108.050070. [DOI] [PubMed] [Google Scholar]
- Ozcan L., Ergin A. S., Lu A., Chung J., Sarkar S., Nie D., Myers M. G., Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Martínez de Morentin P. B., Varela L., Ferno J., Nogueiras R., Diéguez C., López M. Hypothalamic lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:350–361. doi: 10.1016/j.bbalip.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Tagawa Y., Hiramatsu N., Kasai A., Hayakawa K., Okamura M., Yao J., Kitamura M. Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP) Free Radic Biol Med. 2008;45:50–59. doi: 10.1016/j.freeradbiomed.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Santos C. X., Tanaka L. Y., Wosniak J., Laurindo F. R. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- Lee G. H., Kim H. K., Chae S. W., Kim D. S., Ha K. C., Cuddy M., Kress C., Reed J. C., Kim H. R., Chae H. J. Bax inhibitor-1 regulates endoplasmic reticulum stress-associated reactive oxygen species and heme oxygenase-1 expression. J Biol Chem. 2007;282:21618–21628. doi: 10.1074/jbc.M700053200. [DOI] [PubMed] [Google Scholar]
- Medina-Gomez G., Yetukuri L., Velagapudi V., Campbell M., Blount M., Jimenez-Linan M., Ros M., Oresic M., Vidal-Puig A. Adaptation and failure of pancreatic beta cells in murine models with different degrees of metabolic syndrome. Dis Model Mech. 2009;2:582–592. doi: 10.1242/dmm.003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Alquier T., Carling D., Hardie D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kola B., Boscaro M., Rutter G. A., Grossman A. B., Korbonits M. Expanding role of AMPK in endocrinology. Trends Endocrinol Metab. 2006;17:205–215. doi: 10.1016/j.tem.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Lage R., Diéguez C., Vidal-Puig A., López M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008;14:539–549. doi: 10.1016/j.molmed.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y., Alquier T., Furukawa N., Kim Y. B., Lee A., Xue B., Mu J., Foufelle F., Ferre P., Birnbaum M. J., Stuck B. J., Kahn B. B. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Gao S., Kinzig K. P., Aja S., Scott K. A., Keung W., Kelly S., Strynadka K., Chohnan S., Smith W. W., Tamashiro K. L., Ladenheim E. E., Ronnett G. V., Tu Y., Birnbaum M. J., Lopaschuk G. D., Moran T. H. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc Natl Acad Sci U S A. 2007;104:17358–17363. doi: 10.1073/pnas.0708385104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinridders A., Schenten D., Konner A. C., Belgardt B. F., Mauer J., Okamura T., Wunderlich F. T., Medzhitov R., Bruning J. C. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H., Ohtani K., Kato Y., Tanaka Y., Mori M. Withdrawal of [corrected] estrogen increases hypothalamic neuropeptide Y (NPY) mRNA expression in ovariectomized obese rat. Neurosci Lett. 1996;204:81–84. doi: 10.1016/0304-3940(96)12322-3. [DOI] [PubMed] [Google Scholar]
- Mystkowski P., Seeley R. J., Hahn T. M., Baskin D. G., Havel P. J., Matsumoto A. M., Wilkinson C. W., Peacock-Kinzig K., Blake K. A., Schwartz M. W. Hypothalamic melanin-concentrating hormone and estrogen-induced weight loss. J Neurosci. 2000;20:8637–8642. doi: 10.1523/JNEUROSCI.20-22-08637.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor M., Delemarre-van de Waal H. A. Are POMC neurons targets for sex steroids in the arcuate nucleus of the rat? Neuroreport. 2001;12:3989–3991. doi: 10.1097/00001756-200112210-00027. [DOI] [PubMed] [Google Scholar]
- Gao Q., Mezei G., Nie Y., Rao Y., Choi C. S., Bechmann I., Leranth C., Toran-Allerand D., Priest C. A., Roberts J. L., Gao X. B., Mobbs C., Shulman G. I., Diano S., Horvath T. L. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- Gao Q., Horvath T. L. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab. 2008;294:E817–E826. doi: 10.1152/ajpendo.00733.2007. [DOI] [PubMed] [Google Scholar]