Abstract
Ts65Dn mice, like individuals with Down syndrome (DS), demonstrate a functional dissociation between explicit and implicit forms of memory, showing selective impairment in explicit or declarative learning tasks. Here, we explored Ts65Dn explicit memory deficits further by evaluating the ability of these mice to assimilate the temporal and spatial contexts under which previously novel objects had been encountered. We found that Ts65Dn mice could in fact form contextual representations of objects over the course of a few hours, contrary to their inability to discriminate object novelty over a more prolonged period of 24 hours. These results suggest that Ts65Dn mice might have particular difficulties in declarative tasks requiring long-term memory, presenting an especially important putative therapeutic target for pre-clinical and clinical DS research.
Keywords: Ts65Dn, object recognition, medial temporal lobe, Down syndrome
Accumulating evidence suggests that declarative learning and memory systems associated with the medial temporal lobe (MTL) are disproportionately influenced in people with Down syndrome (DS), leading to severe shortcomings in information processing and in the acquisition of new knowledge [19]. As such, these findings reinvigorate the need to determine the dynamic range of cognitive deficits displayed by Ts65Dn mice, the foremost animal model of DS [15,23]. Specifically, do Ts65Dn have performance deficits in what are largely considered MTL-mediated tasks? Do they have behavioral signatures of episodic-like memory? If so, to what degree? In rodents, declarative memory can be delineated most parsimoniously along two domains: spatial and recognition memory [25]. Like individuals with DS, Ts65Dn mice are generally impaired in cognitive tasks requiring the use of spatial cues. Herein, they show navigation problems in the Morris water maze (i.e., increases in time necessary to reach the hidden platform) [15,23], difficulties in the radial arm maze task (i.e., increases in reference memory errors) [5,9,10,16], lower percentage alternation in a T-maze [14], and learning deficits during contextual fear conditioning (i.e., decreased time spent freezing in a shock-associated chamber) [17]. Their performance in the novel object recognition task is similarly affected, as they are unable to discriminate between familiar and novel items over a 24-h stretch [14]. Nonetheless, Ts65Dn mice do exhibit normal reactivity to spatial displacement and to object novelty within set object arrays over short time periods (~ 3 min), suggesting that they possess some rudimentary – albeit limited – ability to process object-based information [18].
The interwoven structures that comprise the MTL, namely the hippocampus and perirhinal, entorhinal and parahippocampal cortices, jointly oversee the proper encoding, consolidation and retrieval of information related to discrete events [25]. This processing ultimately gives way to recognition memory, itself consisting of two components: 1.) familiarity, a general awareness of the features of a stimulus, and 2.) recollection, knowledge of the stimulus in the context of other information, such as when, where, or under what circumstances the stimulus was encountered [25]. Considering that the “episodic” or recollective component of recognition memory is defined by an integrated conceptualization of the “what,” – “where” – and – “when” particulars of a situation, it has been difficult to establish whether active recollection (as opposed to familiarity) occurs in rodents as it does in humans. In other words, can rodents behaviorally demonstrate that they remember seeing a specific object in a specific place at a given time? To address this issue, Dere and colleagues recently developed a test that combines different versions of the novelty preference paradigm, dubbed the episodic-like memory task [11]. The authors took advantage of the fact that mice show innate preferences for exploring novel objects versus familiar ones, preferences for exploring familiar objects that have been moved versus familiar ones that have remained stationary, and preferences for exploring familiar objects that they have seen less recently versus those that they have seen more recently. Subsequently, we have used this task in the current study to evaluate episodic-like memory in Ts65Dn mice, asking whether these mice have the ability to form “what–where–when” representations over the course of a few hours (a model MTL function).
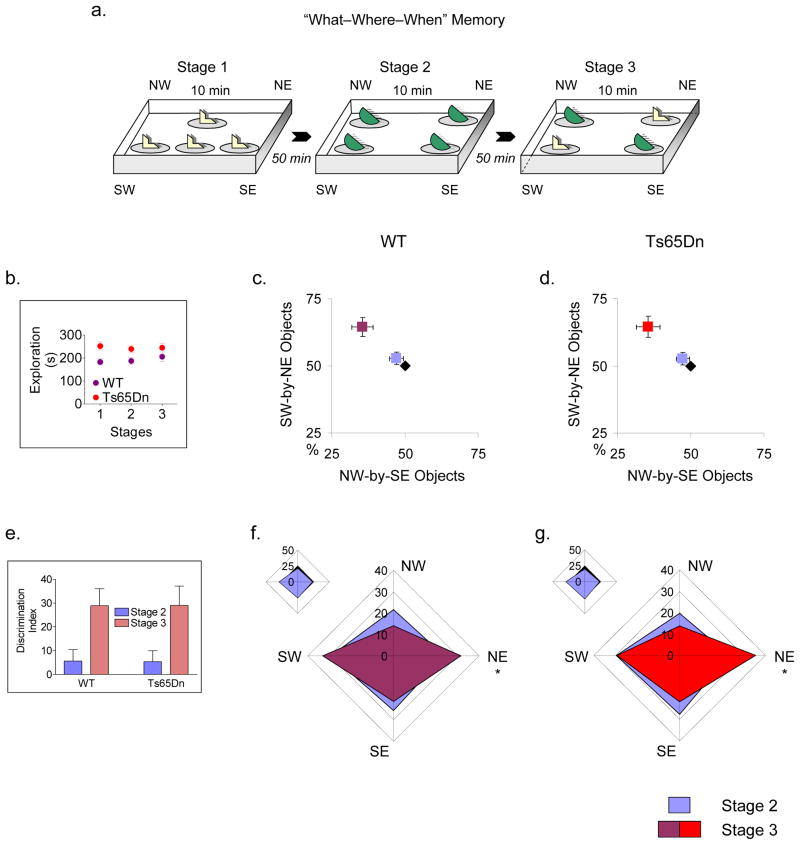
Ts65Dn mice were obtained by mating female carriers of the 1716 chromosome (B6EiC3H – a/ATs65Dn) with (C57BL/6JEi x C3H/HeJ)F1 (JAX # JR1875) males [8]. Ts65Dn mice were thus maintained on the B6/C3H background. Two cohorts of wild-type (WT) and Ts65Dn mice (n = 9 and 11, respectively; 4–6 months of age; male) were submitted to daily experimenter handling, and given an opportunity to habituate to a black acrylic, open field arena (48 cm X 38 cm wide X 27 cm). Thereafter, the animals were submitted to the Dere protocol – a task consisting of three 10 min stages separated by 50 min inter-session periods (Fig. 1a). In Stage 1, WT and Ts65Dn mice were placed into the open field containing 4 copies of a never-before-seen object arranged in a T-shaped configuration (one copy was located against the center of the northern wall, one was located against the center of the southern wall, and two were located at the southwest and southeast corners of the arena). In Stage 2, the mice were returned to the open field, but this time exposed to 4 copies of another never-before-seen object arranged in a square or quadratic configuration (one copy of each object occupied the northwest, northeast, southwest and southeast corners of the arena). In Stage 3, the animals were placed into the open field one final time and exposed to a mixed quadratic object array. Whereas 2 copies of the object from Stage 1 were positioned at the southwest (SW) and northeast (NE) corners of the apparatus, 2 copies of the object from Stage 2 were positioned at the northwest (NW) and southeast (SE) corners. Thus, 2 copies of the old familiar object were situated along the SW-NE diagonal of the open field (with one copy newly positioned at the NE spot), while 2 copies of the recent familiar object were situated along the NW-SE diagonal (at stationary spots). Per the Dere protocol, we operationally defined two main behaviors that functioned as mnemonic indices: (1) Recency preference – defined as an increase in the percentage time mice spent investigating old familiar objects versus recent familiar objects in Stage 3, suggestive of memory for the temporal order in which the objects were presented; and (2) Spatial preference – defined as an increase in the percentage time mice spent investigating the displaced old familiar object versus stationary recent familiar objects in Stage 3, in conjunction with increases in the amount of exploration directed at the NE location from Stage 2 to Stage 3. Please note that object exploration constituted any investigative behavior (i.e., head orientation, sniffing occurring within < 1.0 cm) or deliberate contact that occurred with each item. The objects were made from various non-porous materials (porcelain, metal, glass, etc.), and had different color schemes. All were generally consistent in height and volume, and were symmetrical on a horizontal plane. To control for odor cues, the open field arena and the objects were thoroughly cleaned with 90% ethanol, dried, and ventilated for a few minutes between mice.
Figure 1. Ts65Dn mice form “what-where-when” representations in the episodic-like memory task.
(a) Schematic of the episodic-like memory task. Here, the animals are simultaneously “asked” when and where they encountered two different never–before–seen objects (please see text for technical details). (b) WT (purple circles) and Ts65Dn (red circles) do not show any differences in total object exploration time between stages in the Dere protocol, suggesting that the cognitive performance of both groups of mice is not secondary to general changes in the amounts of explorative activity across the Dere sequence. (c,d) WT and Ts65Dn mice do not differentially explore SW-by-NE related objects (i.e., objects in the SW and NE corners of the arena) versus NW-by-SE related objects (i.e., objects in the NW and SE corners of the arena) in Stage 2 (blue squares), suggesting that they do not exhibit directional biases in the quadratic-shaped object configuration (for reference, a theoretically perfect split in exploration between both object sets is depicted with black diamonds). However, mice from both genotypes exhibit recency preferences in Stage 3 (WT, purple squares; Ts65Dn, red squares), spending almost twice as much time with old familiar objects versus recent familiar objects. (e) WT and Ts65Dn recency preferences calculated alternatively with discrimination indices (DI’s), where DI = [SW-by-NE Object Exploration Time/Total Exploration Time] – [NW-by-SE Object Exploration Time/Total Exploration Time] X 100. (f,g inserts) WT and Ts65Dn mice do not differentially explore individual, NW-NE-SW-SE objects in Stage 2 (blue plots). Notably, there is a symmetrical distribution of explorative activity (for reference, a theoretically perfect 25% split in exploration between individual objects is depicted with the black plot). (f,g) Animals from both genotypes polarize their exploration towards the NE-shifted old familiar object in Stage 3 (WT, purple plot; Ts65Dn, red plot; see Table 1 for a complete statistical breakdown of the episodic-like memory task). Please note that all experimental procedures were approved by the Stanford University Institutional Animal Care and Use Committee (IACUC), and were conducted in compliance with the NIH’s Guide for the Care and Use of Laboratory Animals.
By and large, WT and Ts65Dn mice showed similar topographies of exploration in the episodic-like memory task (Fig. 1b–g; Table 1). First, animals from both genotypes exhibited no significant differences in total object exploration between stages, particularly between Stages 1 and 2, suggesting that their motivations to explore the Stage 1 and Stage 2 associated objects were approximately equal. Likewise, there were no significant differences in the amounts of explorative activity aimed at the SW–by–NE related objects versus the NW–by–SE related objects (or in the amounts of explorative activity aimed at individual objects) in Stage 2, suggesting that neither diploid or partially trisomic mice had an a priori directional bias within the quadratic object configuration (Fig. 1b–g; Table 1). In light of this important control data, WT and Ts65Dn mice together showed behaviors consistent with the proper formation of “what–where–when” representations. For instance, animals from both genotypes exhibited a preference for old familiar objects (at the SW and NE spots) as opposed to recent familiar objects (at the NW and SE spots) in Stage 3, spending approximately twice as much time with Stage 1 associated items (Fig. 1c–e; Table 1). Moreover, both WT and Ts65Dn mice gravitated their exploration towards the NE–shifted old familiar object. Not only did the animals spend more time with the displaced old familiar object versus each of the recent familiar objects in Stage 3, but also increased the percentage time they spent with the NE-related item from Stage 2 to Stage 3 (Fig. 1f–g; Table 1).
Table 1.
Breakdown of WT and Ts65Dn Object Exploration in the Episodic-like Memory Task
| Values | WT | Ts65Dn |
|---|---|---|
| % Time Spent with SW-by-NE Objects, Stage 2 | 52.82 ± 2.374% | 52.72 ± 2.284% |
| % Time Spent with NW-by-SE Objects, Stage 2 | 47.18 ± 2.374%a | 47.28 ± 2.284%a |
| % Time Spent with SW-by-NE Objects, Stage 3 | 64.46 ± 3.585%b,c | 64.55 ± 4.024%b,c |
| % Time Spent with NW-by-SE Objects, Stage 3 | 35.54 ± 3.585% | 35.45 ± 4.024% |
| % Time Spent with NW Object, Stage 2 | 21.68 ± 2.194% | 19.78 ± 1.847% |
| % Time Spent with NE Object, Stage 2 | 23.59 ± 1.817% | 23.12 ± 3.712% |
| % Time Spent with SE Object, Stage 2 | 25.50 ± 3.134% | 27.51 ± 1.864% |
| % Time Spent with SW Object, Stage 2 | 29.24 ± 1.720%d | 29.59 ± 3.698%d |
| % Time Spent with NW Object, Stage 3 | 14.17 ± 1.385% | 13.76 ± 1.850% |
| % Time Spent with NE Object, Stage 3 | 31.42 ± 2.632%e | 35.46 ± 4.640%e |
| % Time Spent with SE Object, Stage 3 | 21.37 ± 3.139% | 21.69 ± 3.620% |
| % Time Spent with SW Object, Stage 3 | 33.03 ± 3.571%f,g | 29.09 ± 5.432%f,g |
| % Change in Time Spent with NE Object | 7.838 ± 2.711% | 12.33 ± 4.322% |
WT and Ts65Dn mice do not exhibit biases for SW-NE related objects versus NW-SE related objects in Stage 2: WT, DI = 5.645 ± 4.748; Ts65Dn, DI = 5.432 ± 4.568; DI > 0, p’s > 0.25 (within-group Student’s t-test).
WT and Ts65Dn mice show increases in time spent with SW-by-NE objects from Stage 2 to Stage 3: WT, t8 = 3.467, p < 0.005; Ts65Dn, t10 = 2.235, p < 0.025 (planned comparison paired t-test).
WT and Ts65Dn mice show significant preferences for “old familiar” objects versus “new familiar” objects in Stage 3: WT, DI = 28.91 ± 7.169; Ts65Dn, DI = 29.09 ± 8.047.
WT and Ts65Dn mice do not differentially explore individual objects (NW-NE-SE-SW) in Stage 2: WT, F3,24 = 1.494, p > 0.24; Ts65Dn, F3,30 = 1.695, p > 0.18 (repeated measures ANOVA).
WT and Ts65Dn mice show increases in time spent with the NE-related object from Stage 2 to Stage 3: WT, t8 = 2.891, p < 0.01; Ts65Dn, t10 = 2.854, p < 0.01 (planned comparison paired t-test).
WT and Ts65Dn mice differentially explore individual objects (NW-NE-SE-SW) in Stage 3: WT, F3,24 = 7.509, p < 0.001; Ts65Dn, F3,30 = 3.896, p < 0.02 (repeated measures ANOVA).
WT and Ts65Dn mice show greater exploration of the NE-related object versus the NW- and SE-related objects in Stage 3: all p’s < 0.05 (Fisher’s LSD post hoc test).
Investigation of cognitive dysfunction in DS has been greatly facilitated by the development of the Ts65Dn mouse, an animal model of DS which is trisomic for segments of mouse chromosome 16 (Mmu16) highly homologous to the long arm of human chromosome 21 (HSA21) [8]. Ts65Dn orthologues of HSA21 include App, Sod1, Grik1, Dscr1 and Dyrk1a, and extend along the length of Mmu16 to genes encoding myxovirus resistance-2 (Mx2) and zinc finger protein 295 (Znf295; approximately 17 Mb of DNA containing 108 of the 225 genes catalogued to HSA21) [1]. Given this genetic repertoire, Ts65Dn mice appear to faithfully recapitulate some of the most fundamental features of DS, showing craniofacial and cerebellar abnormalities [4,24] and Alzheimer–related degeneration later in life [15]. However, it continues to be an open question as to how well the cognitive profile of these mice parallels that of DS–affected individuals. A strong picture is nevertheless emerging. At a broad level, both people with DS and Ts65Dn mice demonstrate a functional dissociation between implicit and explicit (i.e., declarative) memory. That is, they can perform procedural or motor learning tasks but are impaired in tasks requiring the conscious recollection of information [14,27]. Moreover, both man and mouse exhibit deficits in working memory or executive function [12,13,28]. Working memory signifies a specialized short–term memory store comprised of a central executive which allocates attention and memory space to two rehearsal systems (one auditory, the other visual) thought to maintain rapidly–decaying memory traces for temporary and goal directed uses [2]. Not surprisingly, executive function is implicated in contingency–driven response planning, cognitive flexibility and behavioral inhibition [2]. Failures of executive function in DS appear to become more pronounced as individuals enter the preclinical stages of Alzheimer disease, and might point to the increasing vulnerability of the DS brain to frontal lobe pathology during aging [3,26].
Nadel and Pennington (2003) recently proposed the hippocampal hypothesis of DS, suggesting that cognitive impairment in DS is disproportionately influenced by hippocampal dysfunction. One prediction stemming from this hypothesis would be that people with DS and Ts65Dn mice exhibit a greater degree of impairment in the spatial versus recognition domain of declarative memory. For example, hippocampal function is known to decline during normal aging, and spatial memory deficits tend to precede those in object recognition [29]. Correspondingly, while partial experimental lesions of the hippocampus abolish spatial navigation in rodents, nearly full lesions are necessary to perturb novel object discrimination [6]. Similar to people with DS [19,21], Ts65Dn mice show severely debilitated allocentric spatial navigation irrespective of when learning and memory are tested – representing an inability to form viewer–independent cognitive maps [9,10,14,15,23]. On the other hand, difficulties with object recognition are more nuanced in man and mouse alike as expected. In the human condition, adults affected with DS have been shown to commit fewer errors in a delayed–non–match–to–sample (DNMS) task than in an analogous delayed–non–match–to–position (DNMP) task [20]. The present results, together with previous data, also indicate that Ts65Dn’s ability to discern object novelty is acutely intact but strained over longer time scales or when the mice are exposed to a larger number of never–before–seen objects [14,18]. Thus, the role of the hippocampus as a general mnemonic device might be preserved in DS at least in small part under situations that require less effortful processing, but not under those that entail an increasing memory load. By contrast, the hippocampus’ role as an allocentric spatial integrator might be more significantly compromised in the disorder.
In summary, Ts65Dn mice are able to construct episodic–like memories for objects and their spatial and temporal context over the span of a few hours. This ability is consistent with data showing that Ts65Dn react normally to object novelty and movement over short intervals of a few minutes [18], but contrasts with data showing that they cannot detect object novelty over 24 h (a typical time frame used to evaluate rodent long-term memory) [14]. Interestingly, people with DS also exhibit particular deficiencies in long-term declarative memory function. Indeed, children and young adults with DS are often subject to rapid forgetting of newly attained cognitive skills (e.g., understanding the notion of object permanence), a learning problem thought to result from failures to “stabilize” or consolidate information after initial acquisition [7,19,22,30]. The marked inability of Ts65Dn mice and DS-affected individuals to properly maintain declarative information over progressively longer time periods might prove to be an especially important target for future therapeutic strategies that hope to mitigate intellectual difficulties associated with DS.
Acknowledgments
We thank the National Science Foundation (F.F.), the National Institute of Health (F.F.), the Down syndrome Research and Treatment Foundation (C.C.G.), the Hillblom Foundation (C.C.G.), as well as the Stanford Down syndrome Center for their support (C.C.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akeson EC, Lambert JP, Narayanswami S, Gardiner K, Bechtel LJ, Davisson MT. Ts65Dn - localization of the translocation breakpoint and trisomic gene content in a mouse model for Down syndrome. Cytogenet Cell Genet. 2001;93:270–276. doi: 10.1159/000056997. [DOI] [PubMed] [Google Scholar]
- 2.Baddeley AD. Working Memory. London: Oxford University Press; 1986. [Google Scholar]
- 3.Ball SL, Holland AJ, Treppner P, Watson PC, Huppert FA. Executive dysfunction and its association with personality and behaviour changes in the development of Alzheimer's disease in mild to moderately learning disabled adults with Down syndrome. Br J Clin Psychol. 2007 doi: 10.1348/014466507X230967. (in press) [DOI] [PubMed] [Google Scholar]
- 4.Baxter LL, Moran TH, Richtmeier JT, Troncoso J, Reeves RH. Discovery and genetic localization of Down syndrome cerebellar phenotypes using the Ts65Dn mouse. Hum Mol Genet. 2000;9:195–202. doi: 10.1093/hmg/9.2.195. [DOI] [PubMed] [Google Scholar]
- 5.Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav Brain Res. 2003;139:47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- 6.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlesimo GA, Marotta L, Vicari S. Long-term memory in mental retardation: Evidence for a specific impairment in subjects with Down’s syndrome. Neuropsychologia. 1997;35:71–79. doi: 10.1016/s0028-3932(96)00055-3. [DOI] [PubMed] [Google Scholar]
- 8.Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome 16: A new model system for studying Down syndrome. Prog Clin Biol Res. 1990;360:263–280. [PubMed] [Google Scholar]
- 9.Demas GE, Nelson RJ, Krueger BK, Yarowsky PJ. Spatial memory deficits in segmental trisomic Ts65Dn mice. Behav Brain Res. 1996;82:85–92. doi: 10.1016/s0166-4328(97)81111-4. [DOI] [PubMed] [Google Scholar]
- 10.Demas GE, Nelson RJ, Krueger BK, Yarowsky PJ. Impaired spatial working and reference memory in segmental trisomy (Ts65Dn) mice. Behav Brain Res. 1998;90:199–201. doi: 10.1016/s0166-4328(97)00116-2. [DOI] [PubMed] [Google Scholar]
- 11.Dere E, Huston JP, De Souza Silva MA. Episodic-like memory in mice: Simultaneous assessment of object, place and temporal order memory. Brain Res Protoc. 2005;16:10–19. doi: 10.1016/j.brainresprot.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Dowdy-Sanders NC, Wenger GR. Working memory in the Ts65Dn mouse, a model for Down syndrome. Behavl Brain Res. 2006;168:349–352. doi: 10.1016/j.bbr.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Driscoll LL, Carroll JC, Moon J, Crnic LS, Levitsky DA, Strupp BJ. Impaired sustained attention and error-induced stereotypy in the aged Ts65Dn mouse: A mouse model of Down syndrome and Alzheimer’s disease. Behav Neurosci. 2004;118:1196–1205. doi: 10.1037/0735-7044.118.6.1196. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, Garner CC. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–413. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- 15.Holtzman DM, Santucci D, Kilbridge J, Chua-Couzens J, Fontana DJ, Daniels SE, Johnson RM, Chen K, Sun Y, Carlson E, Alleva E, Epstein CJ, Mobley WC. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proc Natl Acad Sci USA. 1996;93:13333–13338. doi: 10.1073/pnas.93.23.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter CL, Bimonte-Nelson HA, Nelson M, Eckman CB, Granholm AC. Behavioral and neurobiological markers of Alzheimer’s disease in Ts65Dn mice: effects of estrogen. Neurobiol Aging. 2004;25:873–884. doi: 10.1016/j.neurobiolaging.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Hyde LA, Frisone DF, Crnic LS. Ts65Dn mice, a model for Down syndrome, have deficits in context discrimination learning suggesting impaired hippocampal function. Behav Brain Res. 2001;118:53–60. doi: 10.1016/s0166-4328(00)00313-2. [DOI] [PubMed] [Google Scholar]
- 18.Hyde LA, Crnic LS. Reactivity to object and spatial novelty is normal in older Ts65Dn mice that model Down syndrome and Alzheimer’s disease. Brain Res. 2002;945:26–30. doi: 10.1016/s0006-8993(02)02500-3. [DOI] [PubMed] [Google Scholar]
- 19.Nadel L. Down’s syndrome: a genetic disorder in biobehavioral perspective. Genes Brain Behav. 2003;2:156–166. doi: 10.1034/j.1601-183x.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 20.Nelson L, Johnson JK, Freedman M, Lott I, Groot J, Chang M, Milgram NW, Head E. Learning and memory as a function of age in Down syndrome: A study using animal-based tasks. Prog Neuro-Psychopharmacol Biol Psychiatr. 2005;29:443– 453. doi: 10.1016/j.pnpbp.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Pennington BF, Moon J, Edgin J, Stedron J, Nadel L. The neuropsychology of Down syndrome: Evidence for hippocampal dysfunction. Child Dev. 2003;74:75–93. doi: 10.1111/1467-8624.00522. [DOI] [PubMed] [Google Scholar]
- 22.Rast M, Meltzoff AN. Memory and representation in young children with Down syndrome. Exploring deferred imitation and object permanence. Dev Psychopathol. 1995;7:393–407. doi: 10.1017/S0954579400006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT, Davisson MT. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- 24.Richtsmeier JT, Baxter LL, Reeves RH. Parallels of craniofacial maldevelopment in Down syndrome and Ts65Dn mice. Dev Dyn. 2000;217:137–145. doi: 10.1002/(SICI)1097-0177(200002)217:2<137::AID-DVDY1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 25.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 26.Vallar G, Papagno C. Preserved vocabulary acquisition in Down's syndrome: the role of phonological short-term memory. Cortex. 1993;29:467–483. doi: 10.1016/s0010-9452(13)80254-7. [DOI] [PubMed] [Google Scholar]
- 27.Vicari S, Bellucci S, Carlesimo GA. Implicit and explicit memory: a functional dissociation in persons with Down syndrome. Neuropsychologia. 2000;38:240–251. doi: 10.1016/s0028-3932(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 28.Vicari S, Carlesimo GA. Short-term memory deficits are not uniform in Down and Williams syndromes. Neuropsychol Rev. 2006;16:87–94. doi: 10.1007/s11065-006-9008-4. [DOI] [PubMed] [Google Scholar]
- 29.Walker LC, Kitt CA, Struble RJ, Wagster MV, Price DL, Cork LC. The neural basis of memory decline in aged monkeys. Neurobiol Aging. 1988;9:657–666. doi: 10.1016/s0197-4580(88)80130-1. [DOI] [PubMed] [Google Scholar]
- 30.Wishart J. The development of learning difficulties in children with Down syndrome. J Intellectual Disability Res. 1993;37:389–403. doi: 10.1111/j.1365-2788.1993.tb00882.x. [DOI] [PubMed] [Google Scholar]