Abstract
Study design
This was designed as an experimental study.
Objectives
Locomotor training is one of the most effective strategies currently available for facilitating recovery of function after an incomplete spinal cord injury (SCI). However, there is still controversy regarding the timing of treatment initiation for maximal recovery benefits. To address this issue, the present study compares the effects of exercise initiated in the acute and secondary phase of SCI.
Setting
Texas A&M University, College Station, TX, USA.
Methods
Rats received a moderate spinal contusion injury and began an exercise program 1 (D1-EX) or 8 days (D8-EX) later. They were individually placed into transparent exercise balls for 60 min per day, for 14 consecutive days. Control rats were placed in exercise balls that were rendered immobile. Motor and sensory recovery was assessed for 28 days after injury.
Results
The D1-EX rats recovered significantly more locomotor function (BBB scale) than controls and D8-EX rats. Moreover, analyses revealed that rats in the D8-EX group had significantly lower tactile reactivity thresholds compared with control and D1-EX rats, and symptoms of allodynia were not reversed by exercise. Rats in the D8-EX group also had significantly larger areas of damage across spinal sections caudal to the injury center compared with the D1-EX group.
Conclusion
These results indicate that implementing an exercise regimen in the acute phase of SCI maximizes the potential for recovery of function.
Keywords: spinal cord injury, contusion, exercise, recovery of function, allodynia, temporal modulation
Introduction
Exercise training is one of the most successful approaches for rehabilitation after an incomplete spinal cord injury (SCI). In the clinic, treadmill training is associated with significant improvements in locomotor function, as well as reduced muscle atrophy and improved psychological well-being.1,2 Similar beneficial effects of exercise are seen in animal models of SCI. In the laboratory, researchers encourage locomotion with treadmill training and running wheels. These studies suggest that training after a spinal injury significantly extends the period of spontaneous recovery of function, and increases motor abilities in the chronic phases of injury.3,4 Exercise also benefits sensory recovery. Hutchinson et al.5 found that treadmill training induced complete recovery from early symptoms of tactile allodynia after SCI. In addition to facilitating motor recovery, therefore, exercise has the potential to prevent the sensory dysfunction inherent to a contusion injury.
The timing of initiation of exercise therapy is thought to be a critical variable for recovery, with an early onset of rehabilitation thought to be most beneficial. Researchers have observed that, although exercise produces significant improvements in the acute phases of injury, recovery curves plateau, as training continues into the more chronic injury phase.6,7 Heng and de Leon4 also hypothesized that a failure to increase successful weight-bearing steps, with treadmill training after a severe contusion injury, may be because of the 1 month delay between injury and the onset of training. Yet, others have found that short delays in the initiation of training do not undermine the effectiveness of therapy,8 and some suggest that long delays may be beneficial.9 Karjacic et al.9 found that the timing of training (day 4 versus 12 after injury) did not affect acquisition of a forelimb reaching task after a unilateral cervical dorsolateral lesion, but early training (day 4) impaired the subsequent acquisition of a ladder-crossing task. The effects of timing of therapy, therefore, appear to be controversial, and may depend on both injury model and behavioral/physiological outcome. To further understand how exercise affects recovery, we compared the effects of the immediate and delayed implementations of an exercise program after a contusion injury. We found that exercise was most beneficial when initiated soon after injury.
Subjects and methods
Experimental animals
The subjects were male Sprague–Dawley rats (Harlan, Houston, TX, USA). They were approximately 90–110 days old (350–400 g). Before surgery, they were housed two per cage in Plexiglass bins and were individually housed after surgery. Food and water were available ad libitum. After surgery, rats were weighed on the days that they were assessed for locomotor function, and were checked daily for signs of autophagia and spasticity. Bladders were manually expressed until rats regained bladder control, which was operationally defined as three consecutive days, with an empty bladder at the time of expression. The rats were maintained on a 12 h light/dark cycle and tested during the last 6 h of the light cycle.
This experiment was reviewed and approved by the institutional animal care committee at the Texas A&M University, and all the National Institutes of Health guidelines for the care and use of animal subjects were followed.
Surgery
Rats received a contusion injury using the MASCIS device. The surgical procedures have been described in detail in Grau et al.10 Briefly, the dorsal spinous processes at T12–T13 were removed, and the spinal tissue exposed while leaving the dura intact. The vertebral column was fixed in the MASCIS device, and a moderate injury was produced by allowing the 10 g impactor to drop to 12.5 mm. The wound was then closed using Michel clips (FST, Foster City, CA, USA).
After surgery, the rats were placed in a recovery room, maintained at 26.6 °C for 24 h. To help prevent infection, they were treated with 100 000 units kg−1 penicillin G potassium (Pfizerpen) immediately after surgery and again 2 days later. To compensate for fluid loss, rats were given 2.5 ml of saline (i.p.) after surgery. The Michel clips were removed 14 days after surgery.
Exercise training
Rats were handled and acclimated in Clear Run-about Balls (29.21 cm diameter) for 4 days before surgery. All rats spent 60 min per day in the exercise balls during this acclimation period. They were able to roll in the balls in a small, sound attenuated and completely dark room (90 sq. ft). Acclimation and exercise training started within the first 90 min of the beginning of the dark cycle of rats (2000–2130 h).
At 24 h after surgery, baseline motor function was measured with the BBB scale,11 an objective scoring procedure that assesses hindlimb movement, paw placement and coordination during locomotion. The rats were then assigned to one of three exercise conditions (n = 6 per group): no exercise (no-EX), day 1 exercise (D1-EX) or day 8 exercise (D8-EX). Rats in no-EX were individually placed in an exercise ball contained in a wooden box that prevented any rolling. Rats in D1-EX and D8-EX conditions were individually placed into an exercise ball, and were allowed unrestricted movement within their ball and around the room. Rats in no-EX and D1-EX received training on days 1–14 following surgery. The D8-EX rats were trained on days 8–21 after injury.
Assessment of motor recovery
Recovery of hindlimb stepping was assessed for 28 days after injury. Locomotor behavior was scored, using the BBB scale, on days 1–7, 9, 11, 13, 15, 18, 21 and 28. Each rat was placed in an open field and observed for 4 min.
Assessment of sensory function and pain reactivity
Baseline nociceptive and tactile reactivities of rats were measured on day 1 after surgery. Nociceptive reactivity was assessed with radiant heat, as described in prior studies.12 Rats were placed in the restraining tubes, with their tail positioned in a 0.5 cm deep groove, cut into an aluminum block. They acclimated to the tubes for 15 min before testing. Thermal thresholds were then assessed two times using the tail flick test. The average of the two trials, recording the latency to flick the tail away from the radiant heat, was used as an index of nociceptive reactivity.
Tactile reactivity was assessed using von Frey stimuli (Semmes-Weinstein Anesthesiometer; Stoelting, Chicago, IL, USA) formed from nylon monofilaments and applied to the plantar surface of the paw. For this test, progressively stronger von Frey filaments were applied sequentially at approximately 2 s intervals until subjects exhibited a paw withdrawal response. If a response was not observed, testing was terminated at a force of 300 g. Each rat was tested twice on each foot in a counterbalanced ABBA order. The test sequences were spaced at 2 min apart.
Assessments of thermal and tactile reactivities were also conducted on days 8 and 28 after injury. Assessments were performed during the last 6 h of the light cycle and before exercise training.
Histology
At the end of behavioral testing, rats were deeply anesthetized (100 mgkg−1 pentobarbital, i.p.) and perfused with 4% paraformaldehyde. A 1 cm long segment of the spinal cord (including lesion center) was taken and prepared for cryostat sectioning. The tissue was sectioned coronally in 20 μm sections and every tenth slice was preserved for staining. All sections were stained with cresyl violet for Nissl substance and luxol fast blue for myelin.13
The total cross-sectional area of the cord and spared tissue was assessed at the lesion center using MicrobrightField software (MBF Biosciences, Williston, VT, USA). Sections ± 600, 1200 and 1800 μm from the lesion center were also traced and analyzed. Four indices of lesion magnitude were derived: damage area, area of residual gray and white matter, and width. Areas of damage were assessed by tracing around the boundaries of cystic formations and areas of dense gliosis. Nissl-stained areas that contained neurons and glia of approximately normal densities denoted residual gray matter. White matter was judged spared in myelin-stained areas lacking dense immune cell infiltration and swollen fibers. The width of each section was also determined by measuring the distance between the most lateral points of sections along the transverse plane. These analyses yielded six parameters for each section: white and gray matter area, spared tissue (white + gray), damaged tissue area, net area (white + gray + damage) and section width.
To control for variability in section area across subjects, we applied a correction factor that is derived from the standard undamaged cord sections. This correction factor is based on section widths and is multiplied by all area measurements to standardize area across analyses.10 By standardizing area, we were able to estimate the degree to which tissue is ‘missing,’ and derive an index of the relative lesion (missing + damaged) in each section that is comparable across the sections. We also computed the relative percent of gray and white matter remaining throughout the lesion extent, relative to intact controls.
Data analyses
Analysis of variance and trend analysis were used to assess differences in recovery across groups. Mixed design analyses of variance were also used to analyze continuous independent variables (for example, rostral–caudal histological sections). Significant group differences were further analyzed with Duncan’s new multiple range tests.
Results
Immediate implementation of exercise therapy improves locomotor function
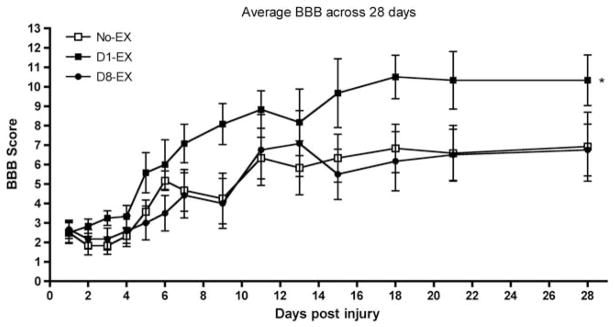
The day 1 BBB scores did not differ across the groups (F(2, 15) <1.0, P>0.05). Mean BBB scores ranged from 2.20±0.56 for the D8-EX group to 2.50±0.94 for the no-EX group. As shown in Figure 1, however, there was an effect of exercise on recovery of locomotor function across the 28-day assessment period, which yielded a significant linear relation between exercise condition and recovery scores (F(1) = 5.67, P<0.05). Rats in the D1-EX group recovered significantly more locomotor function than those in the no-EX and D8-EX groups. Initiation of exercise at 8 days after injury did not facilitate locomotor recovery; BBB scores were similar for D8-EX and no-EX rats.
Figure 1.
There was a significant effect of exercise on recovery of locomotor function across the 28-day assessment period. Subjects in the D1-EX group recovered significantly more locomotor function than those in the no-EX and D8-EX groups (P<0.05). Locomotor scores reached a plateau for all groups by 16 days, after injury, which suggests that training in the exercise balls did not extend the period of spontaneous recovery, often observed after SCI.14
Delayed exercise training does not reverse allodynia
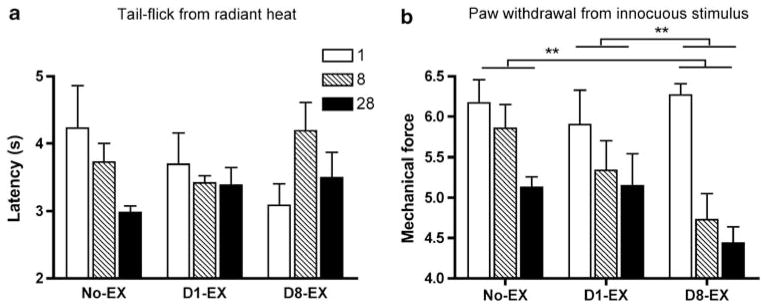
Baseline motor responses to the radiant heat stimulus did not differ across groups on day 1 after injury (F(2, 15) = 1.14, 2.06, respectively, P>0.05). There was also no effect of exercise condition on the latency to tail-flick, with the radiant heat stimulus at 8 or 28 days after injury (F(2, 15) = 2.96, P>0.05, Figure 2a). There was, however, a significant effect of condition on hindpaw tactile reactivity. Analysis of tactile reactivity revealed a main effect of exercise (F(2, 15) = 4.27, P<0.05, Figure 2b). The D8-EX rats showed significantly lower hindpaw tactile reactivity thresholds than both control and D1-EX rats at 8 days, and exercise did not reverse the allodynia observed.
Figure 2.
There was no effect of exercise condition on the latency to tail flick, with the radiant heat stimulus at 8 or 28 days after injury (a). However, analysis of hindpaw tactile reactivity revealed a main effect of condition after injury (b). The average change reactivity (± s.e.m.) with von Frey stimulation is depicted for days 1, 8, and 28. Subjects in the D8-EX group developed significantly lower tactile reactivity thresholds than both the control and D1-EX subjects by the time of assessment at day 8. This allodynic response was not reversed by exercise. **P<0.05.
No effects of exercise on weight gain, autophagia and bladder control after injury
There was no effect of exercise condition on weight gain after injury (F(2, 14) <1.0, P>0.05). Similarly, the incidence of autophagia and the time to recovery of bladder function did not differ across groups (all F(2, 15) <1.54, P>0.05).
Immediate exercise decreases tissue loss caudal to the injury site
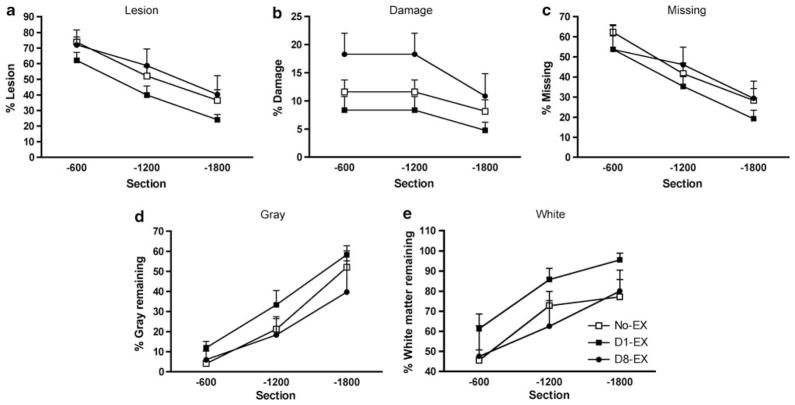
There was no effect of exercise condition on lesion size at the injury epicenter or rostral to the injury site. An analysis of variance, however, revealed significant differences in the area of damage caudal to the injury center (F (2, 12) = 4.39, P<0.05). The D8-EX rats had significantly larger areas of damage across the caudal spinal sections compared with the D1-EX rats (P<0.05). Independent analyses did not reveal significant differences among the groups for relative lesion (missing + damage), missing, area of gray and white tissue indices (P>0.05 in all analyses, Figure 3).
Figure 3.
This figure depicts the effects of exercise on lesion indices and tissue sparing in spinal sections that were 600–1800 μm caudal to the lesion epicenter. Although relative lesion (damage + missing) areas did not differ across groups (a), subjects in the D8-EX group had significantly larger areas of damage across spinal sections that were 600–1800 μm caudal to the injury center compared with the D1-EX subjects (b). Independent analyses did not reveal significant differences among the groups for missing (c), area of gray (d) and white tissue indices (e). However, although not significant, subjects in the D1-EX condition had less missing and lesioned tissue compared with the no-EX and D8-EX groups (c and a, respectively), and had more gray and white matter remaining caudal to the lesion center (d and e, respectively).
Discussion
The current data indicate that exercise is most beneficial when it is initiated in the acute phase of injury. Rats that began exercise on the first day after SCI exhibited substantial gains in locomotor function, when compared with both controls and those that began the same therapy 8 days later. These data concur with previous observations of a ‘window of plasticity’ for beneficial effects of exercise on recovery of function.6,7 The temporal constraints seen in these studies suggest that exercise may facilitate recovery by encouraging locomotion during the period that the spinal cord is inherently plastic.14 It has been suggested that rodents may ‘auto train’ themselves by locomoting around their cages in the early weeks after injury.15 Exposure to an exercise program in the acute phase of injury may potentiate this ‘auto training’ by encouraging locomotion in a period in which the acutely injured subject is less active. Indeed in traumatic brain studies, complete disuse of an impaired forelimb in the early phase of injury is associated with decreased functional recovery and increased vulnerability to neuronal tissue loss, with subsequent overuse of the impaired limb. Conversely, extreme overuse of the impaired forelimb in the early stage of injury also increases neuronal tissue loss and decreases functional recovery.16 These data suggest that the intensity of exercise in the acute phase of injury significantly modulates recovery; mild rehabilitative training in the early stage of injury may be beneficial, but either extreme overuse or complete disuse may disrupt functional recovery.16 Our study demonstrated a benefit of early training, but did not manipulate or measure relative exercise intensity or duration. Future studies will need to address this issue. Comparisons between the molecular changes inherent to ‘trained’ and ‘untrained’ subjects in the acute phase of SCI will also further our understanding of the conditions facilitating recovery of function.
Whereas exercise training initiated immediately after injury appeared to facilitate the recovery of motor function, delayed initiation of training was associated with the development of allodynic symptoms. Rats that began training 8 days after injury, displayed increased reactivity to innocuous mechanical stimulation of the hindpaws. Unfortunately, exposure to exercise therapy did not reverse this allodynia in the chronic phase of injury. This is in contrast with reports of reversal of cutaneous allodynia after treadmill training.5 Although exercise per se has the potential to reverse allodynia,5 the timing of initiation of therapy may be critical.
The analyses of lesion size also suggest that exercise is altering the molecular environment of the injured cord and, thereby, affecting recovery. Again, these effects appear to be temporally modulated. Supporting previous studies, there was no effect of exercise on lesion size at the center of the injury.5,17 Analyses of lesion size caudal to the injury epicenter, however, revealed that subjects in the D8-EX group had larger areas of damage relative to the D1-EX subjects. Although not significant in the small samples, initiation of exercise immediately after injury also appeared to protect the amount of white and gray matter remaining caudal to the lesion, relative to the D8-EX animals (Figure 3). These data concur with findings of exercise-induced sparing of tissue when analyses extend to the rostral and caudal tissue surrounding the lesion center.3 These protective effects of exercise on lesion size may depend on brain-derived neurotrophic factor (BDNF) activity. Indeed, in vivo models have demonstrated that BDNF reduces the size of the necrotic zone after SCI.18 Spinal cord injury significantly reduces BDNF levels,19 whereas post-injury exercise appears to reinstate spinal BDNF concentrations.3,7,20 Reinstating levels of BDNF in the acute phase of injury may be neuroprotective.
Clinical implications
Our data suggest that early implementation of exercise therapy facilitates the recovery of locomotor function. These data reinforce earlier findings, suggesting that initiating therapy soon after injury can have a beneficial effect. The data also underscore the need for further understanding of interactions between temporal changes in the molecular environment of the injured spinal cord and molecular changes induced by exercise. Clearly, the timing of interventions will be critical for maximizing functional recovery in the clinical setting.
Acknowledgments
We thank Kyle Baumbauer, Sandra Garraway, Kevin Hoy, Russell Huie, Kuan H Lee and Denise Puga for their comments on an earlier draft of this paper. A portion of the data from this study has been previously presented in abstract form.
Sponsorship: this study was supported by HD058412 and Mission Connect, a project of the TIRR foundation.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hicks AL, Adams MM, Martin Ginis K, Giangregorio L, Latimer A, Phillips SM, et al. Long-term body-weight-supported treadmill training and subsequent follow-up in persons wth chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord. 2005;43:291–298. doi: 10.1038/sj.sc.3101710. [DOI] [PubMed] [Google Scholar]
- 2.Giangregorio LM, Webber CE, Phillips SM, Hicks AL, Craven BC, Bugaresti JM, et al. Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl Physiol Nutr Metab. 2006;31:283–291. doi: 10.1139/h05-036. [DOI] [PubMed] [Google Scholar]
- 3.Berrocal Y, Pearse DD, Singh A, Andrade CM, McBroom JS, Puentes R, et al. Social and environmental enrichment improves sensory and motor recovery after severe contusive spinal cord injury in the rat. J Neurotrauma. 2007;24:1761–1772. doi: 10.1089/neu.2007.0327. [DOI] [PubMed] [Google Scholar]
- 4.Heng C, de Leon RD. Treadmill training enhances the recovery of normal stepping patterns in spinal cord contused rats. Exp Neurol. 2009;216:139–147. doi: 10.1016/j.expneurol.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- 6.Norrie BA, Nevett-Duchcherer JM, Gorassini MA. Reduced functional recovery by delaying motor training after spinal cord injury. J Neurophys. 2005;94:255–264. doi: 10.1152/jn.00970.2004. [DOI] [PubMed] [Google Scholar]
- 7.Engesser-Cesar C, Ichiyama RM, Nefas AL, Hill MA, Edgerton VR, Cotman CW, et al. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Euro J Neurosci. 2007;25:1931–1939. doi: 10.1111/j.1460-9568.2007.05469.x. [DOI] [PubMed] [Google Scholar]
- 8.Dupont-Versteegden EE, Houlé JD, Dennis RA, Zhang J, Knox M, Wagoner G, et al. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- 9.Krajacic A, Ghosh M, Puentas R, Pearse DD, Fouad K. Advantages of delaying the onset of rehabilitative reaching training in rats with incomplete spinal cord injury. Euro J Neurosci. 2009;29:641–651. doi: 10.1111/j.1460-9568.2008.06600.x. [DOI] [PubMed] [Google Scholar]
- 10.Grau JW, Washburn SN, Hook MA, Ferguson AR, Crown ED, Garcia G, et al. Uncontrollable stimulation undermines recovery after spinal cord injury. J Neurotrauma. 2004;21:1795–1817. doi: 10.1089/neu.2004.21.1795. [DOI] [PubMed] [Google Scholar]
- 11.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 12.Crown ED, King TE, Meagher MW, Grau JW. Shock-induced hyperalgesia: III. Role of the bed nucleus of the stria terminalis and amygdaloid nuclei. Behav Neurosci. 2000;114:561–573. [PubMed] [Google Scholar]
- 13.Behrmann DL, Bresnahan JC, Beattie MS, Shah BR. Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. J Neurotrauma. 1992;9:197–217. doi: 10.1089/neu.1992.9.197. [DOI] [PubMed] [Google Scholar]
- 14.Hook MA, Ferguson AR, Garcia G, Washburn SN, Koehly LM, Grau JW. Monitoring recovery after injury: procedures for deriving the optimal test window. J Neurotrauma. 2004;21:109–118. doi: 10.1089/089771504772695995. [DOI] [PubMed] [Google Scholar]
- 15.Caudle KL, Brown EH, Shum-Siu A, Whelan AE, Burke DA, Maddie MA, et al. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2009. Wheelchair restriction in a rat model of spinal cord injury rehabilitation. 2009. Program No. 176.15. [Google Scholar]
- 16.Leasure JL, Schallert T. Consequences of forced disuse of the impaired forelimb after unilateral cortical injury. Behav Brain Res. 2004;150:83–91. doi: 10.1016/S0166-4328(03)00254-7. [DOI] [PubMed] [Google Scholar]
- 17.Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary wheel running improves recovery from a moderate spinal cord injury. J Neurotrauma. 2005;22:157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- 18.Novikova L, Novikov L, Kellerth JO. Brain-derived neurotrophic factor reduces necrotic zone and supports neuronal survival after spinal cord hemisection in adult rats. Neurosci Lett. 1996;220:203–206. doi: 10.1016/s0304-3940(96)13267-5. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda O, Murakami M, Ino H, Yamazaki M, Nemoto T, Koda M, et al. Acute up-regulation of brain-derived neurotrophic factor expression resulting from experimentally induced injury in the rat spinal cord. Acta Neuropath. 2001;102:239–245. doi: 10.1007/s004010000357. [DOI] [PubMed] [Google Scholar]
- 20.Gómez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophys. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]