Abstract
Islet transplantation is a safe and effective procedure; however, it depends on the critical step of isolating high-quality islets from a whole pancreas. Human islet isolation requires considerable experience and expertise and is frequently seen as an art rather than a science. Without scientific knowledge of pancreatic anatomy, however, real experience cannot be gained. In particular, an understanding of ductal anatomy is important to perform human islet isolation. This review is based on clinical experience performing more than 900 human islet isolations over 10 years and aims to highlight the importance of pancreatic anatomy and surgical techniques in islet processing.
Key words: pancreas anomaly, ventral pancreas, dorsal pancreas, pancreatic duct, annular pancreas, pancreas divisum, pancreatic lipomatosis, circumportal pancreas
Introduction
Due to its promising and minimally invasive therapy for patients with type 1 diabetes, the replacement of pancreatic β-cells by islet transplantation has gained much attention. Especially the initial success of Edmonton protocol has led to a considerable expansion of clinical islet transplant program worldwide.1 However, the overall long-term function of transplanted islets is not satisfactory enough to merit widespread clinical application; at 5-year after transplantation only 15% of islet recipients remains insulin independent.2 This outcome is quite similar to that of pancreas transplant alone (PTA) performed more than 30 years ago (i.e., the pre-cyclosporine era); insulin independent rate after PTA was reported as 31% at 1 year and 11% at 5 years.3 This can be seen as a 30 years lag between the two therapies. However, results with islet transplant have been significantly improved recently. Indeed a study from the University of Minnesota has shown high rates of islet engraftment with 50% insulin independent rates sustained at 5 years4—promising outcomes that begin to approximate outcomes of current PTA.5
Providing high quality human islets which survive and function for longer period likely contributes to further improvement of long-term outcomes. However, islet isolation procedure requires considerable experience and expertise in spite of the many advances in isolation technology. Paul E. Lacy, the father of islet transplantation, stated in his final public lecture, “You can't transplant islets unless you know how to isolate them.”6 The pancreas is indispensable ‘raw material’ for islet isolation. For islet specialists who may not be a surgeon but conduct islet isolation, it is of paramount importance to know basic anatomy of the pancreas. Nevertheless, important aspects of its anatomical structure have not as yet been discussed from points of view of islet isolation. This review aims to highlight pancreas anatomy and surgical techniques in islet processing. Important technical aspects are outlined from a surgeon's perspective. The pancreas procurement is out of scope of this review. The interested readers may refer to other articles.7,8
The Importance of Intraductal Distention of the Pancreas
Since 1967, the year in which Lacy and Kostianovsky described intraductal distention of the pancreas for isolating rat islets,9 the injection of collagenase enzyme blend though the cannulated pancreatic duct has become current standard technique in human islet isolation. The principles behind the intraductal injection are (1) mechanical disruption of interstitial matrix, (2) uniform distribution of the collagenase blend throughout the parenchyma and (3) enhancement of collagenase binding to collagen in the pancreas.10,11 Uniform distention of the pancreas is crucial because islets are not isolated from undistended segments.12 It is thus vital that islet specialist is well-trained in inserting a catheter into the duct and in identifying ductal anomalies.
Duct Systems in the Human Pancreas
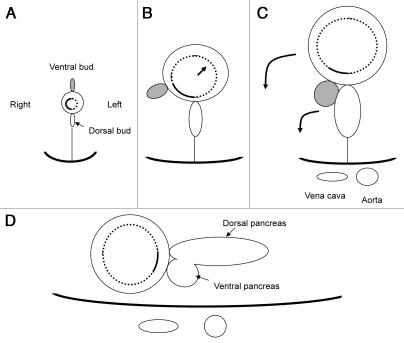
It is difficult to understand peculiarities of the ducts without knowledge of embryological development of the pancreas. The pancreas is derived from two separate anlagen in the foregut epithelium, one dorsal and the other ventral. Each of these two anlagen contains a duct, opening into the primitive duodenum. Due to dominant expansion of growth on the left side of the primitive duodenum, the ventral pancreas passively moves to the right and rotates posteriorly until it comes to lie to the left of the duodenum, subsequently fusing and merging to the dorsal pancreas (Fig. 1). Consequently, the duct systems of both portions become connected to each other. The dorsal pancreas contains the main duct in the tail and the body of the gland while the ventral duct (Wirsung duct) becomes the main conduit for pancreatic secretion terminating in the major duodenal papilla. The dorsal duct (Santorini duct), in some cases (∼45%), continues to drain through the minor duodenal papilla.13 In the remaining cases, Santorini duct is obliterated with no opening into the duodenum. When the ventral and dorsal ductal systems fail to fuse during embryological development, pancreatic juice is predominantly drained through the dorsal duct of Santorini. This malfusion, termed pancreas divisum, is the most frequent anatomic variant in the pancreas.14
Figure 1.
Hypothetical schema showing ‘rotation’ of duodenum and pancreas (transverse section). (A) A ventral pancreatic bud appears in the ventral wall of the primitive duodenum. (B) As a result of expansion of the left side of the duodenum, the ventral pancreas passively comes to the right. (C) The ventral pancreas fuses with the dorsal pancreas. (D) Final retroperitoneal position of duodenum and pancreas.
Surgical Techniques for Cannulation
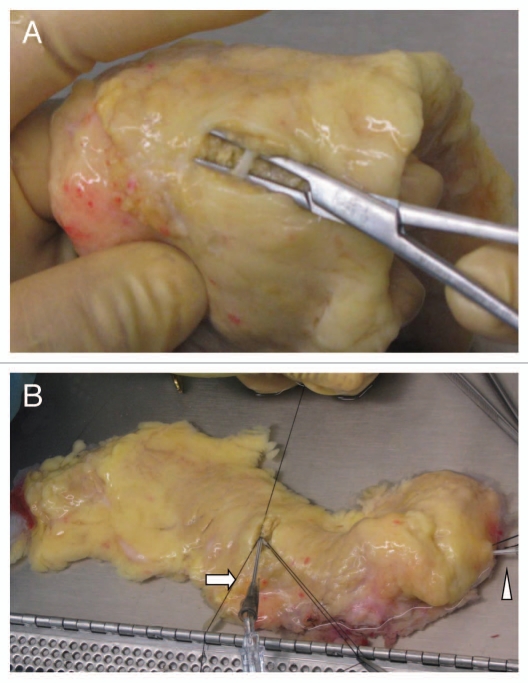
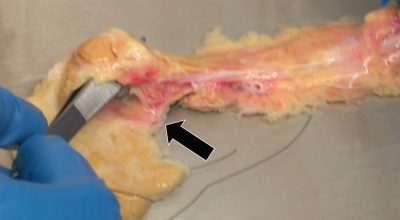
The pancreas is trimmed of the spleen and the duodenum prior to the cannulation procedure. Three catheters are inserted into the main pancreatic duct: one at the head (the first catheter) and two at the mid-body, one toward the tail (the second catheter) and the other toward the head (the third catheter). The catheter insertion technique begins with an identification of the orifice of Wirsung at the head. After the first catheter is inserted into the orifice of Wirsung, the catheter is secured with a 3-0 silk ligature around the ductal wall. Small amount (∼1 mL) of solution (e.g., Hanks' solution) is quickly injected through the first catheter using a syringe. This procedure may help to identify the course of the main duct at the mid-body especially if the duct runs shallowly (Sup. Video). Also this helps to see if there is communication between the dorsal and ventral ducts. The pancreatic parenchyma is then minimally cut with a knife at the mid-body and the main duct is exposed (Fig. 2A). The main duct is encircled using a hemostat, and two 3-0 silk ligatures are placed underneath the duct. A small hole is made on the wall of the duct using microscissors. Next, the second catheter is introduced through the hole into the duct directed toward the tail (Fig. 2B). Lastly, the third catheter is advanced into the duct toward the head in a similar fashion. The first catheter (one from the head) is usually not used for loading of enzyme solution. However this catheter can serve as a back-up for a failure of the third catheter insertion, and is necessary for distending the ventral pancreas in case of complete type of pancreas divisum, as will be discussed later.
Figure 2.
A catheter insertion at the mid-body. (A) The main pancreatic duct is exposed at the mid-body. (B) A 16 gauge catheter is inserted into the main duct toward the tail (arrow). Arrowhead points to a catheter inserted into the main duct from the head.
The advantages of the method described above are (1) minimizing leakage of enzyme solution during subsequent perfusion owing to minimal dissection of the parenchyma at the mid-body and (2) catheters are easily secured with a simple silk tie; no parenchymal suture is required. The disadvantage is that it may be technically difficult to insert the third catheter due to space limitation.
Islet specialists may employ a slightly different method. The pancreas is completely cut into half at the mid-body, i.e., head section and tail section. Two catheters are inserted into the main duct at the cut surface, one directed toward the tail and the other to the head. Parenchymal suture with a 3-0 silk needle should be placed for securing the catheter. The advantage of this method is that it is easy to identify the opening of the duct at the cut surface. The disadvantages are that some pancreatic parenchyma is sacrificed for securing catheters and that leakage of enzyme solution from the cut surface is not insignificant during subsequent perfusion.
A unique method was described by Arita et al.15 They inserted a long tube into the duct at the head, advancing to the tail. They injected enzyme solution with a syringe to distend the tail portion first, and then pulled the tube back to the head while injecting solution to allow the body and head being distended. By using this deep tubing method, they obtained a significantly higher amount of human islets when compared with conventional cannulation method, probably because of better distention of the pancreas. Potential problem with deep tubing is the failure to place a tube deeply enough in case of variations in the pancreatic duct such as pancreas divisum, tortuous duct and ansa pancreatica.16
Cannulation Method for Complete Type of Pancreas Divisum
Pancreas divisum is the most frequent congenital anomaly in the pancreas.14 Its estimated incidence varies widely depending on the methods of identification; 1.3–7.5% based on image series17,18 and 5–22% according to anatomical studies.19–21 It is important to know that pancreas divisum can not be discerned from the outside appearance of the gland.22 Our previous assessment of this anomaly during the intraductal distention of the pancreas revealed that pancreas divisum did not have any negative impact on quality of distention of the pancreas.21 The interpretation was that there are ‘micro’ communications in a ductal system between ventral and dorsal pancreata in most cases of pancreas divisum, thereby ventral pancreas can be distended satisfactory by injecting solution though the main duct at the mid-body. However, in our subsequent assessment of 390 deceased donor pancreata, we found 13 cases of pancreas divisum (3.3%) showing no communications at all between ventral and dorsal duct systems (complete type).
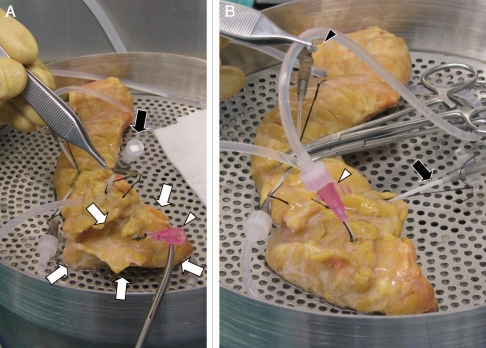
When pancreas divisum is suspected, i.e., the orifice of Wirsung duct is smaller than that of Santorini duct on visualization, the following modification of cannulation method is useful to distend the ventral pancreas. Firstly a smaller catheter (18–20 gauge) may be used instead of a regular size catheter (16 gauge) for the orifice of Wirsung due to its tiny size. This catheter from the Wirsung will not help to identify the course of the main duct at the mid-body in case of complete type of pancreas divisum; however, the catheter is necessary for distention of the ventral pancreas. For the identification of the main duct at the mid-body, another catheter inserted though the orifice of Santorini will be helpful. In Figure 3, an example of such a case is shown. This modification is not required for incomplete type of pancreas divisum.
Figure 3.
Intraductal distention in case of complete type of pancreas divisum. (A) The dorsal pancreas is being distended using two catheters inserted at the mid-body. The ventral pancreas (white arrows) is not distended. There is no fluid emptying from a small catheter (20 gauge) inserted into the orifice of Wirsung duct (white arrowhead), indicating no ductal communications between the ventral and dorsal systems. (B) The ventral pancreas is being distended through the Wirsung duct (white arrowhead). There is no fluid emptying from a catheter at the mid-body (black arrowhead), again indicating no ductal communications between the ventral and dorsal systems. Black arrow indicates a catheter inserted into the orifice of Santorini duct.
Trimming of the Pancreas
The pancreas is removed typically en bloc with the duodenum and spleen, and is transported to an islet isolation facility. Proximal and distal ends of the duodenum are sealed with a linear cutting stapler during the organ procurement to avoid contamination. Upon arrival at a facility, the pancreas is trimmed of the spleen, the duodenum, surrounding fat and major vessels. The pancreatic head, the intrapancreatic bile duct, and the duodenum, these three entities are traditionally thought to form a surgically inseparable unit because of their anatomical and embryological relations. Therefore, it is technically challenging for surgeons to remove, for example, the only pancreatic head while preserving the bile duct and duodenum.23 Although the same dose not hold true for islet specialists, there are several anatomical aspects which islet specialists should be aware of.
When dissecting the duodenum from the pancreas, special care should be taken to avoid rupture of the duodenal wall because leakage of the duodenal content compromises sterility of islet product.24 It is especially important to bear in mind that the duodenal diverticulum may be embedded inside of the pancreatic parenchyma, thus can not be identified from the outside appearance of the duodenum. Duodenal diverticula are frequently seen, occurring in 15% to 22% based on a postmortem study.25,26 They are typically located in the mesenteric border of the descending duodenum near the major papilla and the head of the pancreas. Figure 4 shows a dissected duodenum accompanied with a diverticulum.
Figure 4.
In this case, the duodenal diverticulum was not recognized from the outside appearance of the pancreas and duodenum.
The connection between the duodenum and pancreatic head is loose enough to allow blunt dissection. One exception is the duodenal wall around the minor papilla where the pancreatic tissue heavily infiltrates.27 Dissection plane of this area should be the muscular layer of the duodenum, not between the duodenal serosa and the pancreatic parenchyma (Fig. 5).
Figure 5.
Appearance of the duodenum after dissection of the pancreas. Arrows indicate an area around the minor duodenal papilla where the pancreatic tissue was invading to the duodenal wall.
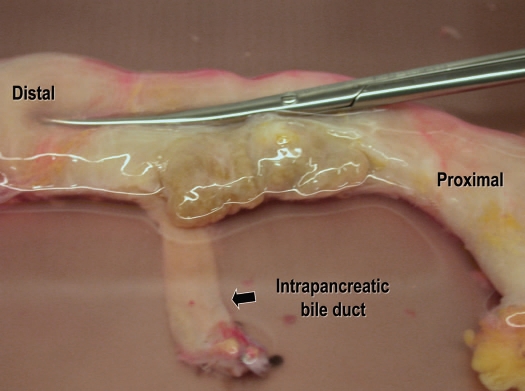
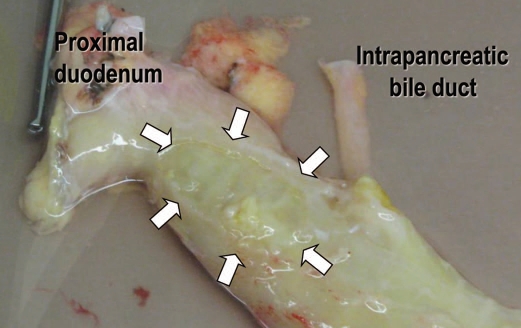
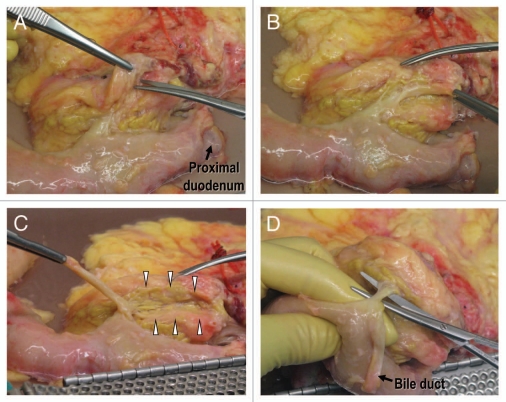
The classical description of the intrapancreatic bile duct is that it is buried into the pancreatic parenchyma. In order to expose the intrapancreatic bile duct, surgeons usually transect the posterior aspect of the pancreatic parenchyma.28,29 However, a fissure can be found to the right of the intrapancreatic bile duct when the pancreatoduodenal region is approached posteriorly.30 This fissure gives access to the bile duct without transection of the pancreatic parenchyma (Fig. 6). The pancreatic tissue covering the intrapancreatic bile duct is called ‘pancreatic lingula’.31 (Fig. 7). After skeletonization of the intrapancreatic bile duct, the confluence of the bile duct and the main pancreatic duct becomes easily identified. Dividing the main duct near the entrance to the duodenum makes it easy to identify the orifice of Wirsung duct during subsequent cannulation procedure. Finally, islet specialists should bear in mind the possible existence of a long common pancreatobiliary channel when dissecting the bile duct.32
Figure 6.
Posterior aspect of the pancreatoduodenal region. (A) There is a fusion cleft to the right of the intrapancreatic bile duct. (B) The intrapancreatic bile duct is exposed in its entire length without any injury to the pancreatic parenchyma. (C) The intrapancreatic bile duct is retracted by the forceps. Groove for passage of the bile duct can be seen (arrowheads). (D) The main pancreatic duct is exposed.
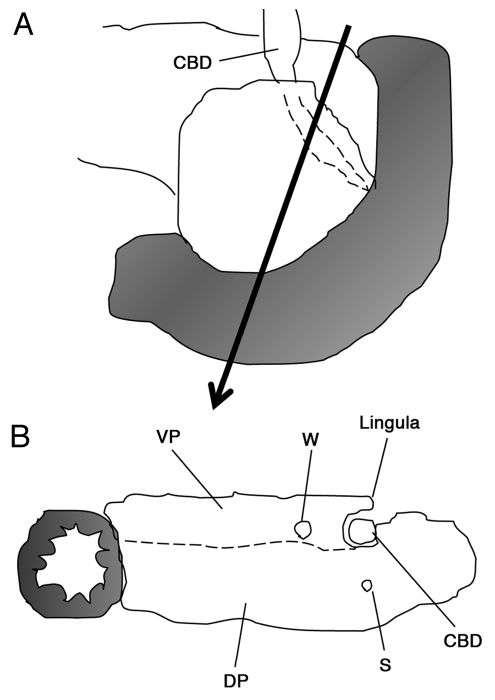
Figure 7.
Schema showing the pancreatic lingula. (A) Posterior aspect of the pancreatoduodenal region. (B) The intrapanceratic bile duct is covered by the pancreatic lingula. The lingula is derived from the ventral pancreas. A dotted line indicates a border between the ventral and dorsal pancreata, however, such a border can not be identified macroscopically. CBD, common bile duct; VP, ventral pancreas; DP, dorsal pancreas; W, Wirsung duct; S, Santorini duct.
Anatomical Orientation of the Pancreas
There is a tricky anatomical orientation hidden in the pancreas. The common mistake is misinterpretation of the anterior aspect of the body/tail as the posterior aspect. Ontogenetic twist at the neck of the pancreas likely contributes to this mistake.33 Figure 8 shows surface relationship between the head and the body/tail. One may recognize the smooth continuity of the surface from the anterior head to the posterior body as shown in Figure 8A. In order to place the pancreas in anatomical position, the body/tail should be twisted on its long axis by 180° anterior-inferiorly. According to Nagai, this twist at the neck is closely related with development of the omental bursa.33
Figure 8.
(A) The posterior aspect of the body/tail is facing the observer while the head portion is placed in anatomical position. Note a smooth surface transition from the head to body. (B) The body/tail is being twisted on its long axis by 180° anterior-inferiorly. The whole pancreas is now placed in anatomical position.
Congenital Anomaly
In addition to pancreas divisum, there are several congenital anomalies which islet specialists should be aware of.
Annular pancreas.
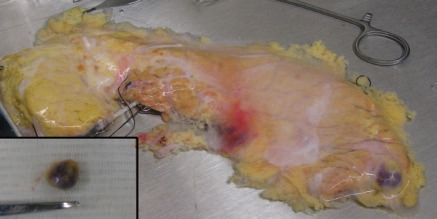
The encirclement of the second part of the duodenum by a ring-like band of pancreatic tissue is known as annular pancreas, which is a rare anomaly with an incidence of three in 20,000 autopsies.34 Two cases of a whole pancreas transplantation from an annular pancreas have been reported in the literature.35,36 Because of a technical challenge, individual with annular pancreas may be considered as a marginal donor.37 In contrast to a whole pancreas graft which involves with a segment of the duodenum, the technical challenges of islet isolation from an annular pancreas seem comparatively small. We reported a case of a successful transplantation of islets isolated from an annular pancreas38 (Fig. 9). Special care must be taken to avoid parenchymal injury when dissecting the duodenum from the pancreas.
Figure 9.
Annular pancreas. Forceps are inserted to show the passage of the duodenum.
Accessory spleen.
Accessory spleens are common, seen in 10% of population,39 and may be located anywhere in the abdominal cavity. The pancreas is the second most frequent location of accessory spleens with an incidence of one in every five accessory spleens.40 Most accessory spleens we found during islet isolation are solitary and located at the tail of the pancreas (Fig. 10). These spleens should not be removed prior to completion of intraductal distention. They are embedded deeply inside the pancreatic parenchyma, so it is difficult to extirpate them without parenchymal injury.
Figure 10.
An accessory spleen embedded into the tail of the pancreas. Inset: the accessory spleen was extirpated after intraductal distention of the pancreas.
Circumportal pancreas.
The encircling of the portal vein by pancreatic parenchyma, while normal in pigs,41 is a rare and not well recognized variant in humans. In 1987, complete pancreatic encasement of the portal vein was reported for the first time by Sugiura et al.42 At least 7 surgically confirmed cases have been reported in the literature.42–48 Leyendecker and Baginski introduced the term ‘circumportal pancreas’.49 Recent retrospective studies of image records revealed an unexpectedly high incidence of this variant.47 In some cases with circumportal pancreas, the main pancreatic duct was found to run posterior to the portal vein.46,49 Therefore, islet specialists should bear in mind this possibility in case of circumportal pancreas when cannulating. In our two cases presented previously,45,48 the main duct appeared to pass anterior to the portal vein (Fig. 11).
Figure 11.
Circumportal pancreas. The uncinate process is communicating with the posterior aspect of the pancreas body (arrow). Forceps are inserted to show the passage of the portal vein.
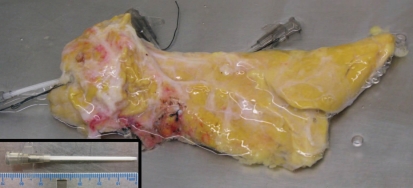
Short pancreas or agenesis of the dorsal pancreas.
Agenesis of the ventral pancreas is extremely rare, but complete or partial agenesis of the dorsal pancreas, also known as congenital short pancreas, has been reported more frequently.50 Because the dorsal pancreas represents approximately 90% of the pancreatic volume,51 thereby the majority of beta cells, complete agenesis of the dorsal pancreas is not suitable for islet isolation. Partial agenesis of the dorsal pancreas may be considered as a transplantable graft when absence of diabetes in donor is confirmed (Fig. 12). We have performed an islet isolation and transplantation using such a donor pancreas and obtained excellent clinical outcome.48
Figure 12.
Posterior view of congenital short pancreas. Inset: a length of catheter is shown.
Pancreatic Lipomatosis
Pancreatic lipomatosis is characterized by the replacement of exocrine pancreatic parenchyma with adipose tissue or fat infiltration of the pancreas. Several human conditions are associated with pancreatic lipomatosis, such as obesity, aging, obstruction of the pancreatic duct and congenital disorders.52–55 It is important to distinguish fat infiltration of the pancreas from fat deposition surrounding the pancreas. The latter is easily dissected from the pancreas without injury while in the former fat is diffusely and deeply infiltrating into the parenchyma. Moreover it is sometimes difficult to discern infiltrated fat from the parenchyma. Dissecting fats infiltrating into the pancreas leads to parenchymal injuries, resulting in leakage of enzyme solution during intraductal distention (Fig. 13).
Figure 13.
Heavily fat infiltrated pancreas. The parenchyma of the pancreas is hardly seen from the surface.
A fat infiltrated pancreas is not likely utilized for a whole organ transplantation since such a gland is associated with an increased risk of early graft loss.56 In contrast, a fat infiltrated pancreas seems to be preferred for islet isolation. The skew is highlighted by a study of Frank et al. who reported that 30 of 30 pancreata for a whole organ transplant were labeled as “none to low” in fat infiltration whereas 25 of 27 pancreata processed for islet isolation were categorized as “moderate to severe”.57
A link between gross fat content in the pancreas and islet isolation outcome was suggested in the mid 1990s.58 Subsequent histological study revealed a correlation between a higher fat content in the pancreas and successful islet isolation.59 These findings stimulated islet processing centers to analyze the impact of fat infiltration of the pancreas on islet isolation outcome. Although hampered by other confounding donor factors and by a great component of subjectivity in the assessment, most studies are in agreement with higher islet yield from a fat infiltrated pancreas.60,61 The cause of increased isolation success with fat infiltration has not been determined. Relatively less amounts of acinar tissue in a fat infiltrated pancreas, thereby less amounts of harmful endogenous enzymes released from acinar tissue, may be a favored condition for pancreas dissociation during islet isolation. Relatively high amounts of fat may affect enzymatic digestion of the pancreas. One study showed that caseinase activity of commercially available crude enzyme mixture was inhibited by free fatty acids.62
In our own experience, we have found in some cases that digestion of pancreas with heavy fat infiltration results in remarkably less volume of digested tissue with preserved islet mass, rendering subsequent purification procedure unnecessary.
Occult Cancer
Transmission of malignant tumor from a donor to a transplant recipient is rare event in solid organ transplants. A report from United Network Organ Sharing transplant data has given rate of 0.012% for deceased donor tumor transmission.63 There are three reports illustrating cancer transmission from a pancreas graft.64–66 Needless to say, in all three cases a primary tumor was undetectable in a pancreas graft at procurement. Avoidance of transmission of malignant tumor in relation to organ donation is highly desired in islet transplantation since the transplant is considered as a life-enhancing procedure rather than a life-saving procedure. Furthermore, complete removal of the graft would be technically challenging for intraportally distributed islets, and would likely necessitate total hepatectomy and liver transplantaiton. The process for islet purification with elimination of the majority of the exocrine pancreas provides a measure of comfort, but it should be emphasized that even purified islet preparations are far from being perfectly pure. It is also unclear how much of the partially digested exocrine pancreatic tissue actually survives intraportal implantation as it is exceptional to visualize pancreatic exocrine tissue on post-transplant liver biopsies.67 Bearing in mind that occult cancer may not be detected by procuring surgeon even with careful palpation of the pancreas, islet specialists should examine inside the pancreas after intraductal distention of the pancreas. Previously, we cut the pancreas into 7–9 pieces before digestion, but our routine practice since 2003 has been to cut the distended pancreas sagittally at ∼2 cm thickness and to carefully examine the cut surface of each piece.
It is worthwhile to note that pancreatic metastasis of renal cell carcinoma is unique in that development of lesion is generally late with a disease-free interval from nephrectomy to the recognition of metastatic disease being 10 years or more.68 Therefore, special care should be taken to assess potential donors who have a long history of disease free after resection of renal cell carcinoma.
Conclusion
Despite the many advances in technical aspects of human islet isolation, it still requires considerable experience and expertise, as is frequently seen as ‘an art rather than a science’. However, without scientific knowledge of anatomy of the pancreas, real experience can not be gained. Pancreas donation depends on public confidence and trust in all aspects of islet transplantation, so high standards of technical expertise in islet isolation must be met. The ability to isolate islets efficiently and safely without technical failure, such that most of islet preparations may subsequently be used for transplantation, is of paramount importance.
Acknowledgements
The authors would like to acknowledge the staff of the Clinical Islet Laboratory for technical help in islet preparation; to the members of the Clinical Islet Transplant Program for ongoing help with clinical care; to the organ procurement organizations across Canada for identifying donors; and to our colleagues in the Human Organ Procurement and Exchange program in Edmonton for assistance in organ procurement. The Clinical Islet Transplant Program receives funding from the Juvenile Diabetes Research Foundation, from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, and from the Immune Tolerance Network. The Program is further supported by Province Wide Services through Alberta Health and Wellness. Generous philanthropic support is provided by the Roberts Family, the North American Foundation for the Cure of Diabetes, the Alberta Building Trades Council and from the Diabetes Research Institute Foundation Canada.
Abbreviation
- PTA
pancreas transplant alone
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/13019
Supplementary Material
References
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 3.Gruessner RWG, Sutherland DER, Kandaswamy R, Gruessner AC. Over 500 solitary pancreas transplants in nonuremic patients with brittle diabetes mellitus. Transplantation. 2008;85:42–47. doi: 10.1097/01.tp.0000296820.46978.3f. [DOI] [PubMed] [Google Scholar]
- 4.Bellin M, Barton F, Hering B the investigators of the Collaborative Islet Transplant Registry, author. Induction immunosuppression with T-cell depleting antibodies facilitates longterm insulin independence after islet allotransplantation in type 1 diabetes. Xenotransplantation. 2009;16:541. [Google Scholar]
- 5.Danovitch GM, Cohen DJ, Weir MR, Stock PG, Bennett WM, Christensen LL, et al. Current status of kidney and pancreas transplantation in the United States 1994–2003. Am J Transplant. 2005;5:904–915. doi: 10.1111/j.1600-6135.2005.00835.x. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AMJ. A historical perspective on experimental and clinical islet transplantation. In: Shapiro AMJ, Shaw JAM, editors. Islet transplantation and beta cell replacement therapy. New York: Informa Healthcare USA, Inc; 2007. pp. 1–27. [Google Scholar]
- 7.Lee TC, Barshes NR, Brunicardi FC, Alejandro R, Ricordi C, Nguyen L, et al. Procurement of the human pancreas for pancreatic islet transplantation. Transplantation. 2004;78:481–483. doi: 10.1097/01.tp.0000128910.41921.4b. [DOI] [PubMed] [Google Scholar]
- 8.Barshes NR, Lee TC, Undell IW, O'Mahony CA, Brunicardi FC, Goss JA, et al. The surgical aspects of pancreas procurement for pancreatic islet transplantation. In: Shapiro AMJ, Shaw JAM, editors. Islet transplantation and beta cell replacement therapy. New York: Informa Healthcare USA, Inc; 2007. pp. 81–97. [Google Scholar]
- 9.Lacy PE, Kostianovsky M. Method for isolation of intact islets of Langerhans from rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 10.Kin T, Johnson PR, Shapiro AMJ, Lakey JRT. Factors influencing the collagenase digestion phase of human islet isolation. Transplantation. 2007;83:7–12. doi: 10.1097/01.tp.0000243169.09644.e6. [DOI] [PubMed] [Google Scholar]
- 11.Kin T. Islet isolation for clinical transplantation. In: Islam S, editor. The islets of Langerhans. Dordrecht: Springer Netherlands; 2010. pp. 791–798. [Google Scholar]
- 12.Goto M, Eich TM, Felldin M, Foss A, Kallen R, Salmela K, et al. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation. 2004;78:1367–1375. doi: 10.1097/01.tp.0000140882.53773.dc. [DOI] [PubMed] [Google Scholar]
- 13.Kamisawa T. Clinical significance of the minor duodenal papilla and accessory pancreatic duct. J Gastroenterol. 2004;39:605–615. doi: 10.1007/s00535-004-1390-1. [DOI] [PubMed] [Google Scholar]
- 14.Quest L, Lombard M. Pancreas divisum: opinio divisa. Gut. 2000;477:317–319. doi: 10.1136/gut.47.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arita S, Smith CV, Nagai T, Sakamoto Y, Ochiai M, Maruyama M, et al. Improved human islet isolation by a tube method for collagenase infusion. Transplantation. 1999;68:705–707. doi: 10.1097/00007890-199909150-00019. [DOI] [PubMed] [Google Scholar]
- 16.Delhaye M, Matos C, Deviere J. Acute relapsing pancreatitis. Congenital variants: diagnosis, treatment, outcome. JOP J Pancreas. 2001;2:373–381. [PubMed] [Google Scholar]
- 17.Satterfield ST, McCarthy JH, Geenen JE, Hogan WJ, Venu RP, Dodds WJ, et al. Clinical experience in 82 patients with pancreas divisum: preliminary results of manometry and endoscopic therapy. Pancreas. 1988;3:248–253. doi: 10.1097/00006676-198805000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Bernard JP, Sahel J, Giovannini M, Sarles H. Pancreas divisum is a probable cause of acute pancreatitis: a report of 137 cases. Pancreas. 1990;5:248–254. doi: 10.1097/00006676-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 19.MacCarty RL, Stephens DH, Brown AL, Jr, Carlson HC. Retrograde pancreatography in autopsy specimens. Am J Roentgenol. 1975;123:359–366. doi: 10.2214/ajr.123.2.359. [DOI] [PubMed] [Google Scholar]
- 20.Stimec B, Bulajic M, Korneti V, Milosavljevic T, Krstic R, Ugljesic M. Ductal morphometry of ventral pancreas in pancreas divisum. Comparison between clinical and anatomical results. Ital J Gastroenterol. 1996;28:76–80. [PubMed] [Google Scholar]
- 21.Kin T, Shapiro AMJ, Lakey JRT. Pancreas divisum: a study of the cadaveric donor pancreas for islet isolation. Pancreas. 2005;30:325–327. doi: 10.1097/01.mpa.0000160280.56828.3f. [DOI] [PubMed] [Google Scholar]
- 22.Warshaw AL. Pancreas divisum-really. Surgery. 2000;128:832–833. doi: 10.1067/msy.2000.109610. [DOI] [PubMed] [Google Scholar]
- 23.Beger HG, Büchler M. Duodenum-preserving resection of the head of the pancreas in chronic pancreatitis with inflammatory mass in the head. World J Surg. 1990;14:83–87. doi: 10.1007/BF01670550. [DOI] [PubMed] [Google Scholar]
- 24.Kin T, Rosichuk S, Shapiro AMJ, Lakey JRT. Detection of microbial contamination during human islet isolation. Cell Transplant. 2007;16:9–13. doi: 10.3727/000000007783464498. [DOI] [PubMed] [Google Scholar]
- 25.Ackermann W. Diverticula and variations of the duodenum. Ann Surg. 1943;117:403–413. doi: 10.1097/00000658-194303000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldwin WM. Duodenal diverticula in man. Anat Rec. 1911;5:121–139. [Google Scholar]
- 27.Kimura W, Nagai H. Study of surgical anatomy for duodenum-preserving resection of the head of the pancreas. Ann Surg. 1995;221:359–363. doi: 10.1097/00000658-199504000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondo S, Hirano S, Ambo Y, Tanaka E, Morikawa T, Okushiba S, et al. Pancreas-preserving biliary amputation with pancreatic diversion: a new surgical technique for complete resection of the intrapancreatic biliary system. Hepatogastroenterology. 2004;51:1255–1258. [PubMed] [Google Scholar]
- 29.Kolb A, Kleeff J, Frohlich B, Werner J, Friess H, Büchler MW. Resection of the intrapancreatic bile duct preserving the pancreas. J Hepatobiliary Pancreat Surg. 2009;16:31–34. doi: 10.1007/s00534-008-0013-2. [DOI] [PubMed] [Google Scholar]
- 30.Lytle WJ. The common bile-duct groove in the pancreas. Br J Surg. 1959;47:209–212. doi: 10.1002/bjs.18004720302. [DOI] [PubMed] [Google Scholar]
- 31.Smanio T. Varying relations of the common bile duct with the posterior face of the pancreatic head in Negroes and white persons. J Int Coll Surg. 1954;22:150–173. [PubMed] [Google Scholar]
- 32.Sugiyama M, Baba M, Atomi Y, Hanaoka H, Mizutani Y, Hachiya J. Diagnosis of anomalous pancreaticobiliary junction: value of magnetic resonance cholangiopancreatography. Surgery. 1998;123:391–397. [PubMed] [Google Scholar]
- 33.Nagai H. Configurational anatomy of the pancreas: its surgical relevance from ontogenetic and comparative-anatomical viewpoints. J Hepatobiliary Pancreat Surg. 2003;10:48–56. [PubMed] [Google Scholar]
- 34.Ravitch MM, Woods AC., Jr Annular pancreas. Ann Surg. 1950;132:1116–1127. doi: 10.1097/00000658-195012000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barone GW, Henry ML, Elkhammas EA, Tesi RJ, Ferguson RM. Whole-organ transplant of an annular pancreas. Transplantation. 1992;53:492–493. doi: 10.1097/00007890-199202010-00043. [DOI] [PubMed] [Google Scholar]
- 36.Romagnoli J, Papalois VE, Hakim NS. Transplantation of an annular pancreas with enteric drainage. Int Surg. 1998;83:36–37. [PubMed] [Google Scholar]
- 37.Salvalaggio PR, Davies DB, Fernandez LA, Kaufman DB. Outcomes of pancreas transplantation in the United States using cardiac-death donors. Am J Transplant. 2006;6:1059–1065. doi: 10.1111/j.1600-6143.2006.01310.x. [DOI] [PubMed] [Google Scholar]
- 38.Kin T, Shapiro AMJ, Ryan EA, Lakey JRT. Islet isolation and transplantation from an annular pancreas: a case report. JOP J Pancreas. 2005;6:274–276. [PubMed] [Google Scholar]
- 39.Halpert B, Gyorkey F. Lesions observed in accessory spleens of 311 patients. Am J Clin Pathol. 1959;32:165–168. doi: 10.1093/ajcp/32.2.165. [DOI] [PubMed] [Google Scholar]
- 40.Halpert B, Alden ZA. Accessory spleens in or at the tail of the pancreas: a survey of 2,700 additional necropsies. Arch Pathol. 1964;77:652–654. [PubMed] [Google Scholar]
- 41.Ferrer J, Scott WE, 3rd, Weegman BP, Suszynski TM, Sutherland DER, Hering BJ, et al. Pig pancreas anatomy: implications for pancreas procurement, preservation and islet isolation. Transplantation. 2008;86:1503–1510. doi: 10.1097/TP.0b013e31818bfda1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugiura Y, Shima S, Yonekawa H, Yoshizumi Y, Ohtsuka H, Ogata T. The hypertrophic uncinate process of the pancreas wrapping the superior mesenteric vein and artery—a case report. Jpn J Surg. 1987;17:182–185. doi: 10.1007/BF02470596. [DOI] [PubMed] [Google Scholar]
- 43.Hamanaka Y, Evans J, Sagar G, Neoptolemos JP. Complete pancreatic encasement of the proximal hepatic portal vein: a previously undescribed congenital anomaly. Br J Surg. 1997;84:785. [PubMed] [Google Scholar]
- 44.Marjanovic G, Obermaier R, Benz S, Bley T, Juettner E, Hopt UT, et al. Complete pancreatic encasement of the portal vein—surgical implications of an extremely rare anomaly. Langenbecks Arch Surg. 2007;392:489–491. doi: 10.1007/s00423-006-0123-8. [DOI] [PubMed] [Google Scholar]
- 45.Kin T, Shapiro AMJ. Circumportal pancreas and islet isolation. Surgery. 2009;146:126–127. doi: 10.1016/j.surg.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto Y, Ross AS, Traverso LW. Circumportal pancreas with retroportal main pancreatic duct. Pancreas. 2009;38:713–715. doi: 10.1097/MPA.0b013e3181a910ca. [DOI] [PubMed] [Google Scholar]
- 47.Karasaki H, Mizukami Y, Ishizaki A, Goto J, Yoshikawa D, Kino S, et al. Portal annular pancreas, a notable pancreatic malformation: frequency, morphology and implications for pancreatic surgery. Surgery. 2009;146:515–518. doi: 10.1016/j.surg.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Kin T, Shapiro AMJ. Partial dorsal agenesis accompanied with circumportal pancreas in a donor for islet transplantation. Islets. 2010;2:146–148. doi: 10.4161/isl.2.3.11715. [DOI] [PubMed] [Google Scholar]
- 49.Leyendecker JR, Baginski SG. Complete pancreatic encasement of the portal vein (circumportal pancreas): imaging findings and implications of a rare pancreatic anomaly. J Comput Assist Tomogr. 2008;32:61–64. doi: 10.1097/rct.0b013e3180557448. [DOI] [PubMed] [Google Scholar]
- 50.Chen R, Hussain K, Al-Ali M, Dattani MT, Hindmarsh P, Jones PM, et al. Neonatal and late-onset diabetes mellitus caused by failure of pancreatic development: report of 4 more cases and a review of the literature. Pediatrics. 2008;121:1541–1547. doi: 10.1542/peds.2007-3543. [DOI] [PubMed] [Google Scholar]
- 51.Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–371. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- 52.Olsen TS. Lipomatosis of the pancreas in autopsy material and its relation to age and overweight. Acta Pathol Microbiol Scand. 1978;86:367–373. doi: 10.1111/j.1699-0463.1978.tb02058.x. [DOI] [PubMed] [Google Scholar]
- 53.Walters MNI. Adipose atrophy of the exocrine pancreas. J Pathol Bacteriol. 1966;92:547–557. doi: 10.1002/path.1700920232. [DOI] [PubMed] [Google Scholar]
- 54.Dreiling DA, Elsbach P, Schaffner G, Schwartz IL. The effect of restriction of protein and total calories on pancreatic function in obese patients. Gastroenterology. 1962;42:686–690. [PubMed] [Google Scholar]
- 55.Dror Y, Freedman MH. Shwachman-Diamond syndrome. Br J Haematol. 2002;118:701–713. doi: 10.1046/j.1365-2141.2002.03585.x. [DOI] [PubMed] [Google Scholar]
- 56.Knight RJ, Bodian C, Rodriguez-Laiz G, Guy SR, Fishbein TM. Risk factors for intra-abdominal infection after pancreas transplantation. Am J Surg. 2000;179:99–102. doi: 10.1016/s0002-9610(00)00276-2. [DOI] [PubMed] [Google Scholar]
- 57.Frank A, Deng S, Huang XL, Velidedeoglu E, Bae YS, Liu CY, et al. Transplantation for type 1 diabetes: comparison of vascularized whole-organ pancreas with isolated pancreatic islets. Ann Surg. 2004;240:631–640. doi: 10.1097/01.sla.0000140754.26575.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lakey JRT, Warnock GL, Rajotte RV, Suarez-Almazor ME, Ao Z, Shapiro AMJ, et al. Variables in organ donors that affect the recovery of human islets of Langerhans. Transplantation. 1996;61:506–508. doi: 10.1097/00007890-199604150-00010. [DOI] [PubMed] [Google Scholar]
- 59.Mahler R, Franke FE, Hering BJ, Brandhorst D, Brandhorst H, Brendel MD, et al. Evidence for a significant correlation of donor pancreas morphology and the yield of isolated purified human islets. J Mol Med. 1999;77:87–89. doi: 10.1007/s001090050308. [DOI] [PubMed] [Google Scholar]
- 60.Nano R, Clissi B, Melzi R, Calori G, Maffi P, Antonioli B, et al. Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia. 2005;48:906–912. doi: 10.1007/s00125-005-1725-3. [DOI] [PubMed] [Google Scholar]
- 61.Ponte GM, Pileggi A, Messinger S, Alejandro A, Ichii H, Baidal DA, et al. Toward maximizing the success rates of human islet isolation: influence of donor and isolation factors. Cell Transplant. 2007;16:595–607. doi: 10.3727/000000007783465082. [DOI] [PubMed] [Google Scholar]
- 62.Rennert B, Melzig MF. Free fatty acids inhibit the activity of Clostridium histolyticum collagenase and human neutrophil elastase. Planta Med. 2002;68:767–769. doi: 10.1055/s-2002-34411. [DOI] [PubMed] [Google Scholar]
- 63.Kauffman HM, Cherikh WS, McBride MA, Maureen A, Cheng Y, et al. Deceased donors with a past history of malignancy: an Organ Procurement and Transplantation Network/United Network for Organ Sharing update. Transplantation. 2007;84:272–274. doi: 10.1097/01.tp.0000267919.93425.fb. [DOI] [PubMed] [Google Scholar]
- 64.Roza AM, Johnson C, Juckett M, Eckels D, Adams M. Adenocarcinoma arising in a transplanted pancreas. Transplantation. 2001;72:1156–1157. doi: 10.1097/00007890-200109270-00030. [DOI] [PubMed] [Google Scholar]
- 65.Wong C, Hold P, Mohteshamzadeh M, Dhanda R, Sells R. Occult donor malignancy in pancreas transplantation. Ren Fail. 2007;29:243–244. doi: 10.1080/08860220601100536. [DOI] [PubMed] [Google Scholar]
- 66.Perosa M, Crescentini F, Antunes I, Marchini G, Rosemberg S, Bezerra A, et al. Donor-derived malignancy in a pancreas graft. Transpl Int. 2010;23:5–6. doi: 10.1111/j.1432-2277.2009.00989.x. [DOI] [PubMed] [Google Scholar]
- 67.Toso C, Isse K, Demetris AJ, Dinyari P, Koh A, Imes S, et al. Histologic graft assessment after clinical islet transplantation. Transplantation. 2009;88:1286–1293. doi: 10.1097/TP.0b013e3181bc06b0. [DOI] [PubMed] [Google Scholar]
- 68.Faure JP, Tuech JJ, Richer JP, Pessaux P, Arnaud JP, Carretier M. Pancreatic metastasis of renal cell carcinoma: presentation, treatment and survival. J Urol. 2001;165:20–22. doi: 10.1097/00005392-200101000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.