Abstract
Anopheles mosquitoes are important vectors of malaria and lymphatic filariasis (LF), which are major public health diseases in Nigeria. Malaria is caused by infection with a protozoan parasite of the genus Plasmodium and LF by the parasitic worm Wuchereria bancrofti. Updating our knowledge of the Anopheles species is vital in planning and implementing evidence based vector control programs. To present a comprehensive report on the spatial distribution and composition of these vectors, all published data available were collated into a database. Details recorded for each source were the locality, latitude/longitude, time/period of study, species, abundance, sampling/collection methods, morphological and molecular species identification methods, insecticide resistance status, including evidence of the kdr allele, and P. falciparum sporozoite rate and W. bancrofti microfilaria prevalence. This collation resulted in a total of 110 publications, encompassing 484,747 Anopheles mosquitoes in 632 spatially unique descriptions at 142 georeferenced locations being identified across Nigeria from 1900 to 2010. Overall, the highest number of vector species reported included An. gambiae complex (65.2%), An. funestus complex (17.3%), An. gambiae s.s. (6.5%). An. arabiensis (5.0%) and An. funestus s.s. (2.5%), with the molecular forms An. gambiae M and S identified at 120 locations. A variety of sampling/collection and species identification methods were used with an increase in molecular techniques in recent decades. Insecticide resistance to pyrethroids and organochlorines was found in the main Anopheles species across 45 locations. Presence of P. falciparum and W. bancrofti varied between species with the highest sporozoite rates found in An. gambiae s.s, An. funestus s.s. and An. moucheti, and the highest microfilaria prevalence in An. gambiae s.l., An. arabiensis, and An. gambiae s.s. This comprehensive geo-referenced database provides an essential baseline on Anopheles vectors and will be an important resource for malaria and LF vector control programmes in Nigeria.
Introduction
Malaria and lymphatic filariasis (LF) are the two most important vector borne parasitic diseases worldwide [1], [2]. In Africa these diseases are both primarily transmitted by Anopheles species. Nigeria has the largest burden of malaria and lymphatic filariasis in Africa – yet very little is known about the distribution of Anopheles mosquitoes that act as vectors for both diseases, how the species interact, overlap or differ across the country [1], [2]. Knowledge of the geographical distribution of the different species, their ecological parameters, role in transmission, and susceptibility to insecticide-based interventions is critical if malaria and LF are to be controlled and eliminated in the next decade [3].
The World Health Organization's (WHO) Position Statement on Integrated Vector Management to control malaria and lymphatic filariasis promotes integrated vector management (IVM) to improve the cost effectiveness of vector-control operations, and to strengthen the capacity of programmes, partnerships and intersectoral collaboration in their efforts to control vector-borne diseases [3]. There is overlapping geographical distribution of malaria and LF in large areas of Africa, and where Anopheles mosquitoes transmit both the malarial and lymphatic filariasis parasites, scaling up vector-control methods such of insecticide-treated mosquito nets (ITNs) and implementing indoor residual spraying (IRS) for malaria control can effectively reduce transmission of LF [3]–[5].
Malaria is caused by infection with a protozoan parasite of the genus Plasmodium and is endemic in 106 countries and responsible for about 225 million clinical cases and 781,000 deaths annually [1]. The short-term goal of WHO's Global Malaria Programme is to reduce the burden of malaria until it is no longer a public-health problem, while the long-term goal is to reduce the global incidence to zero by progressively eliminating the disease in endemic countries [3]. The two main components of this programme are vector control and appropriate case-management through diagnosis and treatment [3], [6]. Malaria vector control involves a two pronged approach: (1) use of ITNs and/or long lasting insecticide nets (LLINs); and (2) IRS with insecticides [3], [6]–[7].
International funding for malaria control has risen steeply in the past decade and has led to rapid scale-up of ITNs in Africa through support from various donors, including the Global Fund, World Bank, UNITAID, UNICEF, DfID, USAID and Canadian Red Cross, as well as funds from governments and other development agencies [8], [9]. While this increasing trend in vector control is encouraging, there is still a need to reach more households given that in recent years, an estimated 42% of households in Africa owned at least one ITN, and only 10% received IRS [1], [3]. By the end of 2009 a total of 19,300,000 ITNs and/or LLINs had been distributed in Nigeria and 330,000 people were protected by IRS but this was still significantly below the WHO target for 2010. Only 15% of the Nigerian population (100% at risk) owned an ITN [1]. Also critical is the need for monitoring and evaluation systems to assess the impact and efficacy of these interventions. Current vector control methods involving ITNs and IRS primarily use pyrethroid insecticides although bendiocarb and DDT (Dichloro-Diphenyl-Trichloroethane) are used in some areas [10]. This widespread use of a single class of insecticide could give rise to the development of insecticide resistance in the mosquito vectors and lead to a major public health problem given the limited availability of alternative insecticides [10]–[12]. In Nigeria the entire population (154,728,895) is at risk of malaria and in 2009 there were 4,295,686 confirmed cases, 658,732 inpatient malaria cases and 7,522 malaria attributed deaths [National/Nigeria Malaria report; 1]. The National Malaria Control Programme (NMCP) of Nigeria has various intervention policies and strategies. These include the following: distribution of ITNs or LLINs; IRS with insecticides; intermittent preventive treatment (IPT) during pregnancy; and malaria case management.
Lymphatic filariasis is caused by parasitic worms that are transmitted by mosquitoes. About 90% of infections are caused by Wuchereria bancrofti, while most of the remainder are caused by Brugia malayi. In Africa, the major vectors of W. bancrofti are mosquitoes of the genera Anopheles and Culex [2]. The Global Program to Eliminate Lymphatic Filariasis (GPELF) was launched in 2000 with the aim of (1) interrupting transmission; and (2) reducing morbidity and preventing disability [2], [3]. Interrupting transmission between mosquitoes and humans is possible through mass drug administration (MDA), using once-yearly treatment with a single dose of albendazole plus either ivermectin or diethylcarbamazine (DEC) for 4–6 years [2], [3].
The GPELF has scaled up rapidly and by the end of 2009, 52 out of 81 endemic countries were implementing MDA, and 2.7 billion treatments had been delivered to 695 million people [3]. Although significant progress has been made in the use of MDA for LF control, the role of vector control is an increasingly important issue for meeting the challenges of eliminating the disease [3], [13]. Vector control is recommended as a possible strategy for controlling LF in some countries where LF and Loa loa are co-endemic and where the burden of LF is heaviest e.g, Nigeria, Democratic Republic of the Congo, India, Indonesia and Bangladesh [3]. Reducing mosquito populations through the use of ITNs and other insecticide treated materials, as well as IRS, may help accelerate or sustain the interruption of transmission. In the year 2000, the WHO African Region launched the Programme for Elimination of Lymphatic Filariasis, and started MDA in four countries (Ghana, Nigeria, Togo and the United Republic of Tanzania). This programme has since expanded, and in 2009 a total of 19 African countries had started MDA implementation [2]. However MDA coverage in Nigeria is still less than 100% of the geographical area, which is of concern given that the country has the highest burden of the disease in Africa [2].
Several maps of malaria and LF vector spatial distributions in Africa have been produced, however, most are at continental or sub-regional scale with limited specific data available for further use by national programmes and researchers aiming to better understand the epidemiology of the diseases [14]–[19]. National vector spatial distribution maps and small databases have been developed for Ghana [20], Kenya [21], and Mali [22], [23]. However, to the best of our knowledge, no country has specifically developed a national database for all Anopheles vectors which includes extensive historical data and specific information on locations, methodologies, insecticide resistance and parasite prevalence available in the public domain.
In Nigeria, recent studies have identified mosquitoes of the An. gambiae (principally An. gambiae s.s. and An. arabiensis) and Anopheles funestus complexes as the main vectors of malaria and LF [18], [24]. An. melas is found in the coastal areas and is involved in malaria transmission [25]–[27]. Most entomological studies have focused on small area/district based collections except for a few studies [26], [28]–[31]. The most extensive data available are by Service [32] who described the distribution of 29 distinct Anopheles species across the country in the 1960s, and more recently by Awolola et al. [33] who developed a Malaria Entomological Profile for Nigeria.
The aim of this study was to compile a national database on the Anopheles vectors of malaria and LF, including related information on the location, time/period of study, species abundance, sampling and collection methods, morphological and molecular species identification methods, insecticide resistance status, including evidence of the kdr allele, and P. falciparum sporozoite rate and W. bancrofti microfilaria (mf) prevalence. This database will provide an essential baseline on Anopheles vectors and will be an important resource for malaria and LF vector control and elimination programmes in Nigeria.
Methods
Study area
Nigeria is a federal constitutional republic comprising thirty-six states and its Federal Capital Territory, Abuja. The states are grouped into six geopolitical zones (northwest, northeast, north central, southwest, southsouth and southeast) [34], [35]. The main latitude and longitude of Nigeria is 10° North and 8° East respectively [36]. Nigeria is approximately 923,768 sq. km and it is located in West Africa and shares borders with Benin in the west, Chad and Cameroon in the east, and Niger in the north [34]. Its coast in the south lies on the Gulf of Guinea on the Atlantic Ocean. There are two main seasons: the wet and the dry season. Most of the rainfall in Nigeria occurs between June and September. Nigeria is broadly grouped into two zones: forests and savanna [35], [37]. The following vegetation types are recognized in the country: the mangrove and fresh water swamps, the rain forest, the Guinea savanna, the Sudan savanna and the Sahel in a south-north transect. Between the rain forest and the Guinea savanna is a modified vegetation transition consisting of light deciduous forest and derived savanna [34].
Data collection/collation
A systematic collation of primary empirical occurrence data for Anopheles mosquitoes in Nigeria, in published articles, was carried out to develop a comprehensive geo-referenced database of the distribution of Anopheles mosquitoes. The search was conducted using electronic searches in online bibliographic archives i.e. PubMed, SCOPUS and The Walter Reed Biosystematics Unit Culicidae Systematic Literature Database. Search terms, and combinations thereof, included Nigeria, Anopheles, mosquito, vectors, malaria, lymphatic filariasis. All articles with information on Anopheles were included. Many articles were identified through this method and many of the references were obtained from the references listed within articles, and then from the references within those articles and so on. Articles that could not be obtained online were sourced from three main libraries: College of Medicine Library, University of Ibadan; Liverpool School of Tropical Medicine Library, and the National Institute of Health Library. The references of articles obtained were also searched for additional sources of information.
For each article the following information was recorded: the locality, latitude and longitude, time/period of study, year project was initiated, species, number of specimens recorded, collection method (animal baits, human baits, indoor/outdoor resting collections, bednet trap, exit traps, human landing catches, and pyrethrum spray catches), stage of collection (adult or larval), morphological identification method (cytogenetic analysis of polytene chromosome, cross mating, morphology), molecular identification method, insecticide resistance status, insecticide tested, presence or absence of the kdr allele, P. falciparum sporozoite rate, W. bancrofti microfilaria prevalence and the reference. Only articles containing information related to the aim of this study were included in the final analysis.
The locations (i.e. mosquito collection sites) were geo-referenced using the latitude and longitude coordinates obtained by cross-checking the names with data from the GEOnet Names Server [36], Directory of Cities and Towns in the World [38] databases. Some site locations were also obtained from the research articles, as provided by the authors, while others were obtained from the WHO Malaria Entomological Profile for Nigeria [33] and other internet sources. Degree/minutes/seconds were converted into decimal degrees. It is acknowledged that there are limitations in using geographical coordinates obtained retrospectively; however, we have listed the main source of coordinates in the absence of such data. All the relevant information was entered into an Excel spreadsheet and data analysis was performed using SPSS (Version 15 for Windows, SPSS Inc., Chicago, IL). All data were mapped using the geographical information systems software ArcGIS 9.2 (ESRI, Redlands, CA). The overall species distributions and levels of insecticide resistance of the main Anopheles species were mapped and compared between two time periods i.e. before and after 2000. The total number of studies carried out was also compared between the six geopolitical zones and between time periods. All other data were tabulated or graphed to highlight differences in sampling, collection and identification methodologies, information related to insecticides and parasite prevalence. The mean, minimum and maximum sporozoite and microfilariae prevalence rate was calculated using SPSS software and was calculated according to species.
Results
Mosquito species and distribution maps
In total, 110 publications reporting on a total of 484,747 Anopheles mosquitoes from 632 spatially unique descriptions at 142 geo-referenced locations across Nigeria were identified between 1900 and 2010 (full database available in Table S1). Overall, the vector species most often reported included An. gambiae complex (65.2%), An. funestus complex (17.3%), An. gambiae s.s. (6.5%) An. arabiensis (5. 0%) and An. funestus s.s. (2.5%). Other species (4.5%) included An. coustani, An. hancocki, An. leesoni, An. nili, An. melas, An. moucheti, An. rivulorum and An. wellcomei.
For the time period 1900–1999, a total of 420 species-specific data points (n = 422,137 Anopheles mosquitoes) were recorded across 66 geo-referenced locations from 63 references, while for the time period 2000–2010, a total of 212 data points (n = 62,610 Anopheles mosquito) were recorded across 82 geo-referenced locations from 48 references. A higher proportion of data is recorded for the An. gambiae and An. funestus complexes in 1900–1999, while more data is recorded for An. gambiae s.s. and An. funestus s.s. in 2000–2010.
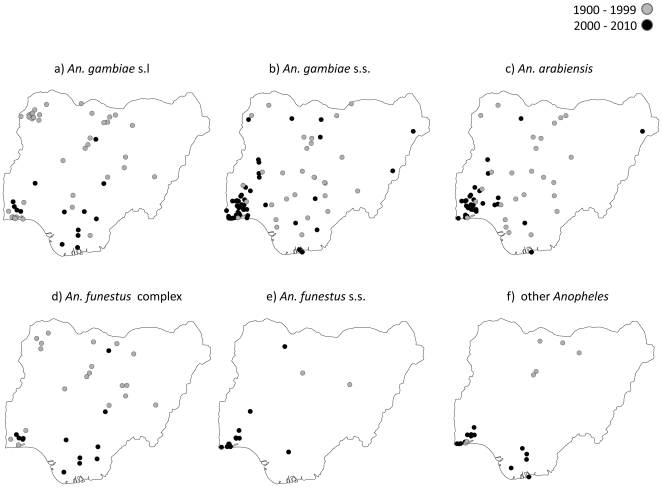
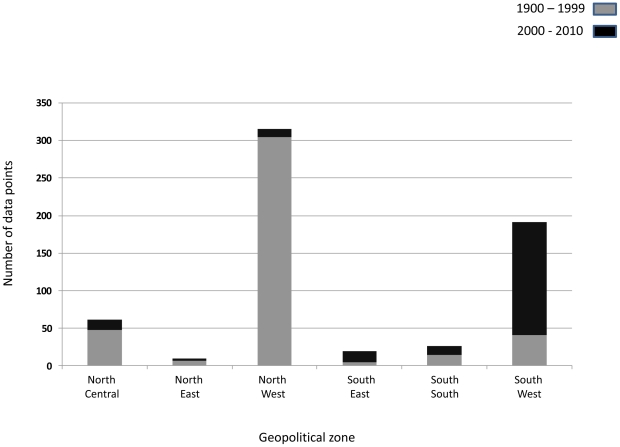
The distribution of each species and locations across the two different time periods is summarised in Table 1 and Figure 1a–f. Of the total 632 data points recorded, 50% (316/632) and 30.5% (193/632) were from the northwest zone and southwest zone respectively (Figure 2). The proportion of studies that were carried out in the north central, south south, southeast and northeast zones of the country was 10.1%, 4.3%, 3.5% and 1.6% respectively. The majority of the studies in the northwest zone were carried out before, rather than after the year 2000: 72.6% (305/420) versus 5.2% (11/212). However, the reverse was the case in the southwest zone, where the majority of the studies was carried out after, rather than before the year 2000: 71.7% (152/212) versus 9.8% (41/420).
Table 1. The number and proportion of Anopheles species found in studies between 1900 and 2010.
| 1900–1999 | 2000–2010 | Total | ||||
| Species(n = data points) | N | % | N | % | N | % |
| An. gambiae s.l (181) | 302677 | 71.7 | 13472 | 21.5 | 316149 | 65.2 |
| An. gambiae s.s. (156) | 8546 | 2.0 | 22760 | 36.4 | 31306 | 6.5 |
| An. arabiensis (122) | 19529 | 4.6 | 4634 | 7.4 | 24163 | 5.0 |
| An. funestus complex (95) | 79998 | 19.0 | 4064 | 6.5 | 84062 | 17.3 |
| An. funestus s.s. (21) | 2382 | 0.6 | 4946 | 1.7 | 7328 | 2.5 |
| Other species (57) | 9005 | 2.1 | 12734 | 20.3 | 21739 | 4.5 |
| Total (632) | 422,137 | 100.0 | 62,610 | 100.0 | 484,747 | 100.0 |
Figure 1. Distribution of Anopheles species a. An. gambiae s.l. b. An. gambiae s.s. c. An. arabiensis d. An. funestus complex e. An. funestus s.s. f. Other Anopheles.
Figure 2. Number of data points across six geopolitical zones by time period.
The maps in Figure 1a–f show the spatial and temporal differences of each main species, and highlight the focus in the southern regions of country in the past decade. Figure 1d–e also shows that very few studies have been carried out on the An. funestus complex and An. funestus s.s. The number of data points for all species by each year and time period are summarised in Table S2 and S3.
The total number of An. gambiae s.s. molecular forms M and S form collected throughout the study period was 4784 and 5224 (Table 2) across 59 and 61 geo-referenced locations, respectively. For the time period 1900–1999, a total of 170 and 244 species specific data points of An. gambiae M form and S form were collected (Table S4), compared with the time period 2000–2010, when a total of 4614 and 4980 data points of An. gambiae M form and S form were collected, respectively (Table S5). Figure 3 a–c shows the distribution of the molecular forms and the percentage contribution of each molecular form.
Table 2. The number and proportion of An. gambiae s.s. molecular forms found in studies between 1900 and 2010.
| 1900–1999 | 2000–2010 | Total | ||||
| An. gambiae s.s(n = data points) | N | % | N | % | N | % |
| M form (61) | 170 | 41.1 | 4614 | 48.1 | 4784 | 47.8 |
| S form (59) | 244 | 58.9 | 4980 | 51.9 | 5224 | 52.2 |
| Total (120) | 414 | 100.0 | 9594 | 100.0 | 10008 | 100.0 |
Figure 3. a. Distribution of An. gambiae s.s. molecular forms. b. Percent of M form c. Percent of S form.
Mosquito sampling and collection methods
Overall, the most used method of collecting Anopheles mosquitoes was via the adult stage. Chronologically (based on the year each project was initiated) adult collection represented 87.5% (7/8), 100% (3/3), 67.1% (47/70), 96.1% (221/230), 33.9% (41/121) and 36.2% (54/149) of collections made between 1900–1920, 1921–1940, 1941–1960, 1961–1980, 1981–2000 and 2001–2010 respectively. Larval collections represented 12.5% (1/8), 0% (0/3), 4.3% (3/70), 0.9% (2/230), 48.8% (59/121) and 34.2% (51/149) for the same time periods.
1900–1999
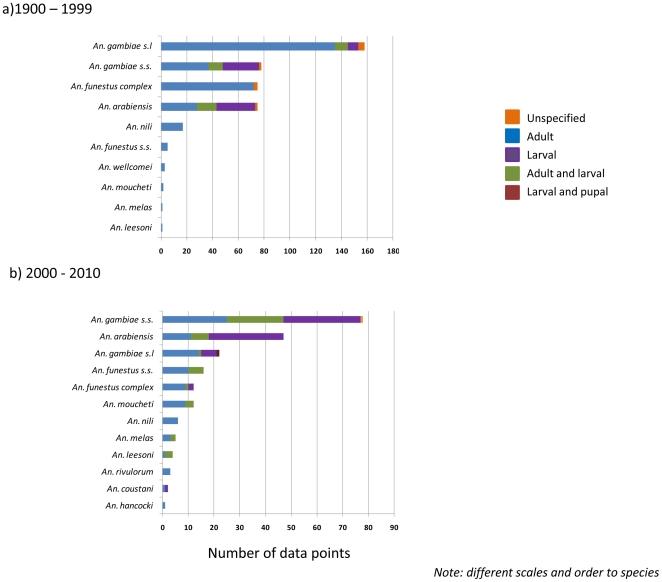
The main methods of collecting Anopheles mosquitoes included adult collections (72.9%; 306/420) and larval collections (15.7%; 65/420), while a small proportion used larval and adult collections combined (8.6%; 36/420) or did not specify the specific methods (2.9%; 12/420) (Table 3). In general, the frequency of methods used was similar between the main species (Figure 4a).
Table 3. Summary of Anopheles species collection methods in studies between 1900 and 1999.
| Species | Adult collection | Larval collection | Adult+larval collection | Unspecified | Total |
| An. gambiae s.l | 135 | 8 | 10 | 5 | 158 |
| An. gambiae s.s. | 37 | 28 | 11 | 2 | 78 |
| An. arabiensis | 28 | 30 | 15 | 2 | 75 |
| An. funestus complex | 77 | 0 | 0 | 3 | 80 |
| An. funestus s.s. | 5 | 0 | 0 | 0 | 5 |
| An. leesoni | 1 | 0 | 0 | 0 | 1 |
| An. melas | 1 | 0 | 0 | 0 | 1 |
| An. moucheti | 2 | 0 | 0 | 0 | 2 |
| An. nili | 17 | 0 | 0 | 0 | 17 |
| An. wellcomei | 3 | 0 | 0 | 0 | 3 |
| Total(n = data points) | 304 | 65 | 36 | 12 | 420 |
Figure 4. Summary of Anopheles species collection methods by time period a 1900–1999 b. 2000–2010.
Adult collections were made primarily from pyrethrum spray catches (PSC) (17.6%), human landing catches (HLC) (12.9%), indoor resting (IR) collections (8.6%), exit trap collections (ETC) (6.9%) and animal baits (1.7%). Some collections were made using a combination of methods e.g. net catch/human bait, pyrethrum spray catch/exit trap, human landing catch/pyrethrum spray catch.
Larval collections were made from potential breeding sites, which included gutters, abandoned road sides, standing waters, vehicle tracks, tires, shallow wells, ponds, swamps, drains, rivers, small streams, irrigation ditches, hoof prints, domestic containers and empty cans (Table S1). Some collections were made using a combination of larval and adult collection methods, e.g. pyrethrum spray catches/larval, indoor resting collections/larval, human landing catches/exit trap and indoor resting/human landing catch/pyrethrum spray catches/larval collections.
2000–2010
The main methods of collecting Anopheles mosquitoes included adult collections (45.3%; 96/212), larval collections (32.5%; 69/212), and larval and adult collections combined (21.2%; 45/212), while a small proportion used larval and pupal collections (0.5%; 1/212), or did not specify the methods (0.5%; 1/212), as shown in Table 4. Compared with data collection method in the 1900–1999 time period, overall there was a decrease in adult collections and an increase in the number of larval collections (Figure 4b).
Table 4. Summary of Anopheles species collection methods in studies between 2000 and 2010.
| Species | Adult collection | Larval collection | Larval+pupal collections | Adult+larval collection | Unspecified | Total |
| An. gambiae s.l | 15 | 6 | 1 | 1 | 0 | 23 |
| An. gambiae s.s. | 25 | 30 | 0 | 22 | 1 | 78 |
| An. arabiensis | 11 | 29 | 0 | 7 | 0 | 47 |
| An. funestus complex | 11 | 3 | 0 | 1 | 0 | 15 |
| An. funestus s.s. | 10 | 0 | 0 | 6 | 0 | 16 |
| An. leesoni | 1 | 0 | 0 | 3 | 0 | 4 |
| An. melas | 3 | 0 | 0 | 2 | 0 | 5 |
| An. moucheti | 9 | 0 | 0 | 3 | 0 | 12 |
| An. nili | 6 | 0 | 0 | 0 | 0 | 6 |
| An. rivulorum | 3 | 0 | 0 | 0 | 0 | 3 |
| An. coustani | 1 | 1 | 0 | 0 | 0 | 2 |
| An. hancocki | 1 | 0 | 0 | 0 | 0 | 1 |
| Total(n = data points) | 96 | 69 | 1 | 45 | 1 | 212 |
Adult collections were made primarily from human landing catches (18.4%), indoor resting collections (9.0%), CDC light traps (5.2%) and pyrethrum spray catches (5.2%). Some methods were also used in combination: human landing collections/pyrethrum spray catches/indoor resting collections, indoor resting/human landing catch.
Similar to the 1900–1999 time period, larval collections were made from a variety of potential breeding sites (Table S1), and some studies used a combination of adult and larval methods, e.g. human landing catch/larval/pyrethrum spray catches, larval/indoor resting, human landing catch/indoor resting/larval, larval/outdoor baited scarecrow, and larval/pupal collections.
Species identification methods
Chronologically (based on the year each project was initiated), the method of species identification was not specified in studies initiated between 1900–1960 (n = 81). For 1961–1980 period, cytogenetic analysis of polytene chromosome represented 28.7% (66/230) of identification method employed. Morphological identification and use of PCR was the most used method of identification in studies carried out between 1981–2000 and 2001–2010 representing 60.3% (73/281) and 38.3% (57/149) respectively.
1900–1999
The methods of species identification were based on cytogenetic analysis of polytene chromosome (16.0%; 67/420), morphology and PCR (14.0%; 59/420), PCR (6.0%; 25/420), cross mating techniques (1.9%; 8/420) and morphology alone (0.7%; 3/420) (Figure 5a). The method of species identification was not specified in studies initiated between 1900 and 1963 representing 61.4% (258/420) of sites. The earliest study that identified species based on cytogenetic analysis of chromosomes was in a study initiated in 1969 [39]; cross mating techniques was first used in a study initiated in 1963 [40], morphology was first specified in a study initiated in 1965 [41] while use of PCR was first mentioned in a study initiated in 1997 [29], [30].
Figure 5. Summary of Anopheles species identification methods by time period a 1900–1999 b. 2000–2010.
The morphological keys used included those described in [42]–[45]. The molecular methods - polymerase chain reaction (PCR) assays for the vector species complex and molecular forms - were those of [46] for An. gambiae complex; [47] for distinguishing the molecular forms of An. gambiae s.s. and [48] for An. funestus complex.
For An. gambiae s.l. and the An. funestus complex, the species identification method was not specified for 93.0% (147/158) and 95.0% (76/80) of sites, respectively. The PCR method was not used at any site for the An. gambiae complex or other species, and only once for the An. funestus complex. In contrast, for An. gambiae s.s. and An. arabiensis the PCR method of [46] was used for species identification and the method of [47] for An. gambiae s.s molecular form identification. For the An. gambiae s.s. data, a total of 10 (n = 78) collection sites had information on both the M form and the S form.
2000–2010
The methods of species identification were based on morphology and PCR (47.6%; 101/212), morphology alone (21.7%; 46/212), and PCR alone (9.9%; 21/212) (Figure 5b). The method of species identification was not specified in 20.8% (44/212) of sites. The morphological keys used included [43]–[45], [49]–[58]. The molecular PCR methods for the identification of vector species complex and molecular forms were those of [46]–[48], [59]–[61] (Table S1).
For An. gambiae s.l. and An. funestus complex, morphological methods were used at 69.6% (16/23) and 73.3% (11/15) of sites, and not specified for 26.1 (6/23) and 20% (3/15) of sites respectively (Figure 5b). For An. gambiae s.s., An. arabiensis and An. funestus s.s., a combination of PCR and morphological identification was primarily used, while for other species morphological methods were primarily used (An. coustani, An. leesoni, An. melas, An. moucheti, An. nili, An. hancocki and An. rivulorum).
Insecticide resistance
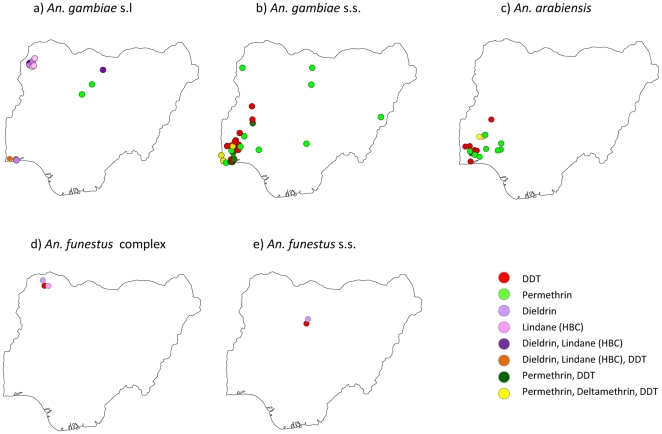
Insecticide resistance to pyrethroids and organochlorines was recorded in An. gambiae s.l., An. gambiae s.s., An. arabiensis, An. funestus complex and An. funestus s.s. across 45 geo-referenced locations (Table 5). The specific locations where resistance was found for each species, is available in Table S6 and highlighted in Figures 6a–g. The species most tested for insecticide resistance were An. gambiae s.s. 47.1% (33/70), An. arabiensis 25.7% (18/70) and An. gambiae s.l. 18.6% (13/70).
Table 5. Insecticides resistance recorded in Anopheles species.
| Insecticide | An. gambiae s.l | An. gambiae s.s. | An. arabiensis | An. funestus complex | An. funestus s.s. | Total |
| BHC | 3 | 0 | 0 | 1 | 0 | 4 |
| DDT | 1 | 12 | 6 | 2 | 1 | 22 |
| Deltamethrin, Permethrin and DDT | 0 | 4 | 0 | 0 | 0 | 4 |
| Dieldrin | 4 | 0 | 0 | 1 | 1 | 6 |
| Dieldrin and BHC | 3 | 0 | 0 | 0 | 0 | 3 |
| Dieldrin, Lindane, DDT | 1 | 0 | 0 | 0 | 0 | 1 |
| Permethrin | 1 | 12 | 10 | 0 | 0 | 23 |
| Permethrin and DDT | 0 | 5 | 2 | 0 | 0 | 7 |
| Total (n = data points) | 13 | 33 | 18 | 3 | 2 | 70 |
Figure 6. Distribution of insecticide resistance a. An. gambiae s.l. b. An. gambiae s.s. c. An. arabiensis d. An. funestus complex e. An. funestus s.s.
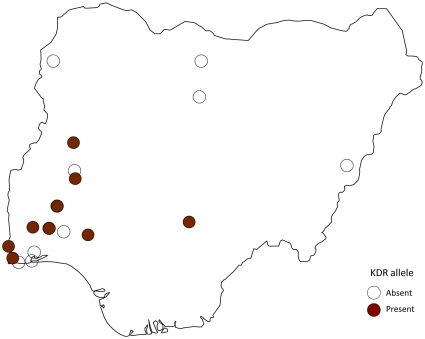
Data were recorded for individual insecticides as well as a combination of insecticides. These were predominantly permethrin (32.9%; 23/70), DDT (31.4%; 22/70), and permethrin and DDT (10.0%; 70), as well as dieldrin (8.6%; 6/70), deltamethrin and permethrin and DDT (5.7%; 4/70), lindane (BHC) (5.7%; 4/70), dieldrin and lindane (BHC) (4.3%; 3/70), and dieldrin and lindane (BHC) and DDT (1.4%; 1/70) (Table 5). For An. gambiae s.s., resistance to DDT and permethrin was mainly reported, and the kdr allele was tested 26 times and found to be present in ten locations predominantly in the southwestern region of the country (Figure 7). For An. arabiensis, resistance to permethrin, and for An. gambiae s.l resistance to lindane (BHC), and dieldrin were mainly reported (Table S1).
Figure 7. Distribution of kdr alleles.
Plasmodium falciparum sporozoite rate
In total, P. falciparum sporozoite rates were recorded at 106 data points and geo-referenced locations from 20 references, and most of these were carried out in the last decade. Overall, the sporozoite rate varied by Anopheles species (Table 6) and location, with most data recorded in the southwestern region of the country (Figure 8a). Approximately 35% (30/86) of data points were recorded in two of the studies [62], [26].
Table 6. Mean sporozoite rate recorded for the various Anopheles species (data point = 106).
| Species(n = data points) | Mean sporozoite rate (%) | Min sporozoite rate (%) | Max sporozoite rate (%) |
| An. gambiae s.l (32) | 2.81 | 0.4 | 10 |
| An. gambiae s.s (20) | 19.4 | 0 | 91 |
| An. arabiensis (11) | 2.34 | 0 | 6.1 |
| An. funestus complex (10) | 1.99 | 0 | 5.35 |
| An. funestus s.s (13) | 11.79 | 0 | 50 |
| An. leesoni (1) | 0 | 0 | 0 |
| An. melas (5) | 2.57 | 0.4 | 6.5 |
| An. moucheti (7) | 5.22 | 0 | 21.9 |
| An. nili (2) | 0 | 0 | 0 |
| An. rivulorum (3) | 0 | 0 | 0 |
| An hancocki (1) | 0 | 0 | 0 |
| An. wellcomei (1) | 0 | 0 | 0 |
Figure 8. Distribution of a. sporozoite rates and b. LF microfilariae.
The mean sporozoite rate in An. gambiae s.l. was 4.3% and ranged from 0.4% (Sokoto [63]) to 10% (Lagos [64]). For An. gambiae s.s. the mean rate was 19.4%, and ranged from 0 (Bama [65]) to 91% (Alimosho [62]). For An. arabiensis the mean rate was 2.3% and ranged from 0 (Amuwo-Odofin [62] and Lemu surburb of Lagos [25]) to 6.1% (Kaduna area [39]). For the An. funestus complex, the mean rate was 2.1% and ranged from 0 (Sokoto [63]) to 5.35% (Kaduna area [66]). For An. funestus s.s. the mean rate was 11.8% and ranged from 0 (Agege) to 50% (Amuwo-Odofin) [62]. For An. melas was 2.57% and ranged from 0.4 (Bonny [26]) to 6.5% (Moba [27]). For An. moucheti the mean rate was 5.2%, and ranged from 0 (Remo north and Odogbolu LGA [67]) to 21.9% (Mushin [62]). For other species, no sporozoites were recorded for An. leesoni, An. nili, An. rivulorum, An. hancocki and An. wellcomei (Table 6).
Lymphatic filariasis
The prevalence of LF microfilariae (mf) in Anopheles species was recorded for 21 data points, across ten geo-referenced locations and eight references (Table 7 and Figure 8b). The species found to be infected with mf included An. gambiae s.l., An. gambiae s.s., An. arabiensis and An. funestus complex. The mean mf prevalence in An. gambiae s.l was 5.64% and ranged from 0 (Seri [68] and Ibadan [69]) to 21.7% (Igwun Basin [70]). For An. gambiae s.s. and An. arabiensis mf was found only in one site and was 9.2% (Jos Plateau) and 11.1% (Jos Plateau) respectively [24]. For An. funestus complex the mean mf prevalence was 3.15% and ranged from 0% (Ibadan [69]) to 6.6% (Amassoma [71]). The data from two articles [68], [72] had to be excluded from the analysis because the general data on mf prevalence were combined from various sites and/or species. The infection rate of mosquitoes containing W. bancrofti was reported to be 1.0% for An. funestus complex and 3.55% for An. gambiae s.l but the particular site was not specified [68]. Also [71] gave the prevalence of mf to be 4.9% and 4.7% in the untreated and ivermectin treated village respectively, but the percentage contribution of each species was not given. These two papers both presented results from studies carried out in Plateau and Nassarawa states (Table S1).
Table 7. Prevalence of W. bancrofti microfilariae in various Anopheles species.
| Species (data points) | Mean mf prevalence (%) | Min mf prevalence (%) | Max mf prevalence (%) |
| An. gambiae s.l (10) | 5.64 | 0 | 21.7 |
| An. gambiae s.s (1) | 9.2 | 9.2 | 9.2 |
| An. arabiensis (1) | 11.1 | 11.1 | 11.1 |
| An. funestus complex (9) | 3.15 | 0 | 6.6 |
Discussion
This collation of data from 110 publications has produced one of the most comprehensive geo-referenced databases on Anopheles vectors of malaria and filariasis available for any country in the world. Importantly, it has been carried out for Nigeria, which has the largest burden of both diseases in Africa, and is a priority country for international donors (the Global Fund, World Bank, UNITAID, UNICEF, DFID, USAID and Canadian Red Cross) supporting large-scale control programmes [9]. The data span more than 100 years and show changes in the geographical focus of research sites, field and laboratory methodologies, and they highlight critical gaps in our knowledge on Anopheles species distributions, transmission dynamics, species and parasite interactions, level and impact of insecticide resistance.
The results reported by the various articles show that the most abundant vectors are the An. gambiae complex, An. funestus complex, An. gambiae s.s., An. arabiensis and An funestus s.s., with these vectors existing in sympatry in most locations. The earliest study found was the expedition by the Liverpool School of Tropical Medicine [73]. In the earlier reports An. gambiae s.l. was referred to as “A. costalis” [74], [75]. In the early days (and early reports) the term “An. gambiae” encompassed the species complex [40], [63], [66], [76]–[91]. In subsequent reports An. gambiae s.s. was referred to as “species A” [92], [93] and An. arabiensis was referred to as “species B” [92], [93].
The total number of mosquitoes collected is under represented as the numbers of mosquitoes collected were not available in some studies. As the species abundance was not specified in some studies, the totals for each species was carried out independently and did not interfere with any of the calculations reported here. In general, there is more information provided on the An. gambiae s.s. and An. funestus s.s. collected in studies initiated in the 2000s than in those from earlier years. Also, more information was available on Anopheles gambiae s.l., which represented over 60% of the overall species composition compared to about 19.8% for An. funestus (17.5% of the studies were on An. funestus complex while 2.3% were on An. funestus s.s.). Clearly, research on the composition and distribution of the An. funestus group is important, as it is an important vector contributing to the transmission of both LF and malaria in Nigeria [26], [68], [93], [94]. One main reason for the lack of research on An. funestus may be that this vector is refractory to colonization, and very difficult to find in the field, and its larvae are very difficult to find at low densities due to their tendency to stay submerged for long periods [43]. However, new techniques for egg laying and colonising are being developed, which will facilitate research opportunities in the future [95].
Of particular interest is how the molecular forms of An. gambiae s.s. varied across the country. In most studies the An. gambiae M and S molecular forms were found in sympatry, although the ratio varied from location to location [30], [31], [96]–[101]. At the 120 sites where M and/or S forms were reported, pure populations of the S form were recorded at 7 sites, all located in the northern region (Guinea and Sudan savanna ecological zones) [30], [31], [102], [103]. Pure populations of the M form were reported at 17 sites, 14 of them located in the southern region (mangrove, forest, and transitional ecological zones) [27], [30], [101], [104], [105]. The other 3 sites with pure populations of the M form were from the north central (Guinea savanna) part of the country [101]. Geographical and ecological differences in the chromosomal and molecular forms of An. gambiae s.s. and their potential role in disease transmission have been examined in regions of West Africa [17], [20], [106] with the An. gambiae S form broadly associated with malaria distributions, and the An. gambiae M form with LF distributions. However, the role of these two sibling species in the transmission of malaria and/or LF and how they interact is largely unknown.
The series of mosquito vector distribution maps produced in this study highlight that studies in the 20th Century were carried out predominately in the northern region of the country, while studies carried out more recently have focussed mainly in the southern region. The reason for this shift may be related to limited infrastructure and an overall lack of trained staff, vector ecologists and medical entomologists, which are common problems across Africa [6], [7], [107], [108]. It may also be due to the limited accessibility of remote locations, as the majority of studies were carried out in proximity to cities and towns in two main zones, northwest and southwest, leaving large gaps in the eastern regions of the country. Furthermore, very few studies were carried out in the same locations in different years or seasons: this lack of systematic sampling/collections restricts analysis and comparisons on multiple fronts, for instance of changes in species composition and abundance over time. The data collated here can serve as a baseline for future work that includes such same-site collections and allows for comparisons within and between populations. Currently, this is particularly important given the widespread vector control interventions taking place across the country [9], [35].
There was great variability in the reporting of the mosquito sampling and collection methods used over time. For example, between 1900 and 1999, PSC, HLC, IR and ETC were the methods most frequently used either singly or in combination for adult mosquito collections, whereas in studies carried out between 2000 and 2010, the CDC light trap was mainly used; there was no record of ETC use during this period. Surprisingly, the expensive and labour intensive method of the HLC [109] was used consistently throughout the study period from 1900 to 2010, which is probably due to a lack of a suitable substitute for this important metric. However, recent tent traps have been tested and calibrated to the HLC and their use as an alternative tool is being trialled in different locations across Africa [109], [110]. The variability in the use of different sampling and collection methods calls for simpler and more standardized methods; the value of this has been previously emphasized [111]–[113].
Similarly, there were changes in species identification methods over time. Earlier studies used more cross mating techniques [40], [114], morphological [28], [41], [115], [116] and cytogenetic methods[39], [93], [117]–[122] for species identification, however, by the 2000s cross mating techniques and cytogenetic methods were nonexistent. Most of the missing data were from earlier studies when there were few options for species identification, For example, most of the missing data were from articles published between 1900 and 1963, when cross mating and morphological methods were predominately used. However it was interesting to note that taxonomic keys [49]–[52] were used for species identification in a study carried out between 2002 and 2003 [123]. In recent decades the emphasis has shifted to molecular techniques including PCR assays that target specific regions of repeat gene families, such as the ribosomal RNA (rRNA) gene family. A diagnostic assay for the identification of the An. gambiae complex based on IGS and ITS sequence differences was developed and applied routinely [46]. Similar techniques were also developed for An. funestus s.l. [48], and An. moucheti s.l. [61], [124], along with more advancements allowing the simultaneous identification the An. gambiae complex species and An gambiae s.s. M and S molecular forms [46], [47], [59].
In contrast, there were no major changes in the methods used for the detection of insecticide resistance in Nigeria. In most of the studies, bioassays and molecular assays to detect resistance alleles (specifically the kdr mutation) were used. Bioassays are the best indicators of the presence of resistance in a field population and are widely used across Africa [7], [11], [125], [126]. However, molecular and biochemical techniques are important to verify bioassay results of resistance in wild populations and gain an understanding of the underlying mechanisms of resistance [11], [12], [127]. In Nigeria only one study used bioassay and microarray analysis [105], and another used bioassays, molecular assays to detect resistance alleles, and biochemical analysis and microarray analysis to characterize pyrethroid resistance mechanism in colony breed An. gambiae s.s. [128]. The reason for the heavy reliance on bioassays and molecular assays for resistance monitoring is probably because the biochemical assays and microarray analysis require qualified personnel and specialized and costly equipment [10].
Insecticide resistance in the An. gambiae complex, An. gambiae s.s., An.arabiensis, An. funestus complex and An. funestus s.s. to various insecticides (DDT, permethrin, dieldrin, deltamethrin and lindane (BHC)) was widespread across the southwestern region of the country. Resistance was most frequently reported in An. gambiae s.s. to permethrin and DDT, which is of major concern given that this vector was found to have among the highest malaria sporozoite and LF microfilaria rates in Nigeria, permethrin is currently the only class of insecticide used for ITNs, and permethrin and DDT are widely used for IRS [10]. Vector control can be hampered by the occurrence of insecticide resistance and as such monitoring of insecticide resistance regularly across a wide geographical area is critical [7], [11], [12], [129]–[132]. The findings also highlight the importance of a consensus standardization of the number and location of surveillance sites and the frequency with which resistance monitoring should occur [11]. To date very little has been done in the northwestern region of Nigeria, especially, in the area where The Garki Project was carried out in the 1960–70s [93]. This is disappointing given that it was one of the largest and most comprehensive control programmes to be carried out in Africa at the time, and vector and resistance monitoring may have provided some invaluable insights into the long term effects of large scale vector control programmes.
In general, there were few studies that focussed on malaria and LF parasites. The sporozoite rates recorded emphasize the role of An. gambiae s.s. and An. funestus s.s. as very efficient malaria vectors. In areas of the southern part of the country when the two species were studied in sympatry, An. gambiae s.s. had higher sporozoite rates than An. funestus s.s. At Bungudu-Gusau in the northern part of the country, however, An. funestus s.s. had a sporozoite rate of 2.3% compared to 0.46% recorded in An. gambiae s.s. [26]. The sporozoite rates recorded for An. arabiensis were also lower than those for An. gambiae s.s. in areas where they were studied at the same time [26], [67], [133]. These findings call for a widespread systematic sampling across the country. For LF, the results show that An. gambiae s.l., An. gambiae s.s., An. arabiensis and An. funestus complex are important vectors. Of the eight studies [24], [68]–[71], [73], [75], [134] carried out to determine the prevalence of LF only one study [75] reported co-infection of Plasmodium parasites and LF in the mosquitoes that were trapped. Annett et al [73] confirmed the role played by Anopheles mosquitoes as malaria vectors but it was not stated whether co-infection of malaria parasites and LF was studied. The few records found make it difficult for comparisons to be made between studies, locations and species. This becomes alarming as Nigeria bears the greatest potential burden of LF in Africa, with 80 million people (19% of the total population) at risk [135].
As this study has restated that LF and malaria are transmitted by the same vectors in Nigeria, both diseases can be jointly controlled since the two diseases share a large proportion of their target population, and the national programmes have similar goals and strategies [3], [136]. The result from all the articles used in the database [24]–[31],[33],[40],[41],[61]–[94],[96]–[105],[114]–[124],[133]–[134],[137]–[177] emphasizes the need for a detailed understanding of the distribution, species composition, behaviour and insecticide susceptibility levels of local vectors in order to successfully control the diseases. It reveals the areas where a dearth of data exists and emphasizes the importance of collecting data systematically, so that the impact of the interventions can be measured. These considerations are important in the light of the goal of the WHO's Global Malaria Programme and the WHO's Global Programme to Eliminate Lymphatic Filariasis to eliminate the diseases as a public health problem [3]. The findings in this research shows that better linkages and partnerships between entomologists, parasitologists, national control programmes, international donors and other stakeholders in the country is needed, in order to carry out meaningful research and control on the vectors and their disease transmission across the country.
Supporting Information
Nigeria Anopheles vector database.
(XLSX)
The number of data points by each species and each year from 1900–1999.
(XLSX)
The number of data points by each species and each year from 2000–2010.
(XLSX)
Number of specimen of Anopeles gambiae s.s. M form and S form from 1900–1999.
(XLSX)
Number of specimen of Anopeles gambiae s.s. M form and S form from 2000–2010.
(XLSX)
Localities and species recording resistance and the frequency.
(XLSX)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.WHO. World malaria report 2010. 2010. WHO Global Malaria Programme. Available: http://whqlibdoc.who.int/publications/2010/9789241564106_eng.pdf via internet. Accessed May 5, 2011.
- 2.WHO. Progress report 2000–2009 and strategic plan 2010–2020 of the global programme to eliminate lymphatic filariasis: halfway towards eliminating lymphatic filariasis. 2010. WHO/HTM/NTD/PCT/2010.6.
- 3.WHO. Weekly epidemiological record Relevé 25 march 2011, 86th year/25 mars 2011, No. 13, 86, 113–128. 2011. Available: http://www.who.int/wer via internet. Accessed May 12, 2011.
- 4.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database of Systematic Reviews Issue 2. Art. No. 2004:CD000363. doi: 10.1002/14651858.CD000363.pub2. DOI: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Pluess B, Tanser FC, Lengeler C, Sharp BL. 2010. 49 Indoor residual spraying for preventing malaria (Review) Published by JohnWiley & Sons, Ltd.
- 6.ANVR Quarterly Newsletter. Strengthening the role of local research institutes to improve malaria vector control. 2007. 2. Available: https://apps.who.int/tdr/topics/mol_entomology/files/anvr_2.pdf. Accessed May 12, 2011.
- 7.ANVR Newsletter. Malaria Vector Surveillance in Africa: Building up malaria entomology skills at the level of National Malaria Control Programmes is the way forward.1. 2006. Available: https://apps.who.int/tdr/topics/mol_entomology/files/anvr_1.pdf. Accessed May 12, 2011.
- 8.Katz I, Komatsu R, Low-Beer D, Atun R. Scaling up towards international targets for AIDS, tuberculosis, and malaria: contribution of global fund-supported programs in 2011–2015. PLoS One. 2011;6:e17166. doi: 10.1371/journal.pone.0017166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigeria FY. 2011 Malaria Operational Plan. 2010. Available: http://www.fightingmalaria.gov/countries/mops/fy11/nigeria_mop-fy11.pdf via internet. Accessed May 16, 2011.
- 10.Ranson H, N'guessan R, Lines J, Moiroux N, Nkuni Z, et al. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Kelly-Hope L, Ranson H, Hemingway J. Lessons from the past: managing insecticide resistance in malaria control and eradication programmes. Lancet Infect Dis. 2008;8:387–9. doi: 10.1016/S1473-3099(08)70045-8. DOI: 10.1016/S0140-6736(08)60424-9. [DOI] [PubMed] [Google Scholar]
- 12.IRAC (Insecticide Resistance Action Committee) Prevention and management of insecticide resistance in vectors of public health importance. 2011. 72 A manual produced by: Insecticide Resistance Action Committee (IRAC) Second Edition 2011. Available: http://www.irac-online.org/wp-content/uploads/2009/09/VM-Layout-v2.6_LR.pdf via internet. Accessed 19th June 2011.
- 13.Bockarie MJ, Molyneux DH. The end of lymphatic filariasis? BMJ. 2009;338:b1686. doi: 10.1136/bmj.b1686. doi: 10.1136/bmj.b1686. [DOI] [PubMed] [Google Scholar]
- 14.Lindsay SW, Parson L, Thomas CJ. Mapping the ranges and relative abundance of the two principal African malaria vectors, Anopheles gambiae sensu stricto and An. arabiensis, using climate data. Proc Biol Sci. 1998;265:847–854. doi: 10.1098/rspb.1998.0369. doi: 10.1098/rspb.1998.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coetzee M, Craig M, le Sueur D. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae Complex. Parasitol Today. 2000;16:74–77. doi: 10.1016/s0169-4758(99)01563-x. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay SW, Thomas CJ. Mapping and estimating the population at risk from lymphatic filariasis in Africa. Trans R Soc Trop Med Hyg. 2000;94:37–45. doi: 10.1016/s0035-9203(00)90431-0. [DOI] [PubMed] [Google Scholar]
- 17.Bayoh MN, Thomas CJ, Lindsay SW. Mapping distributions of chromosomal forms of Anopheles gambiae in West Africa using climate data. Med Vet Entomol. 2001;15:267–74. doi: 10.1046/j.0269-283x.2001.00298.x. [DOI] [PubMed] [Google Scholar]
- 18.Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MARA/ARMA (Mapping Malaria Risk in Africa/Atlas du Risque de la Malaria en Afrique) 2005. [ http://www.mara.org.za/]
- 20.de Souza D, Kelly-Hope L, Lawson B, Wilson M, Boakye D. Environmental factors associated with the distribution of Anopheles gambiae s.s in Ghana; an important vector of Lymphatic Filariasis and Malaria. PLoS One. 2010;5(3):e9927. doi: 10.1371/journal.pone.0009927. doi: 10.1371/journal.pone.0009927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okara RM, Sinka ME, Minakawa N, Mbogo CM, Hay SI, et al. Distribution of the main malaria vectors in Kenya. Malar J. 2010;9:69. doi: 10.1186/1475-2875-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sogoba N, Vounatsou P, Bagayoko MM, Doumbia S, Dolo G, et al. The spatial distribution of Anopheles gambiae sensu stricto and An. arabiensis (Diptera: Culicidae) in Mali. Geospat Health. 2007;1:213–222. doi: 10.4081/gh.2007.269. [DOI] [PubMed] [Google Scholar]
- 23.Sogoba N, Vounatsou P, Bagayoko MM, Doumbia S, Dolo G, et al. Spatial distribution of the chromosomal forms of Anopheles gambiae in Mali. Malar J. 2008;7:205. doi: 10.1186/1475-2875-7-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenhart A, Eigege A, Kal A, Pam D, Miri ES, et al. Contributions of different mosquito species to the transmission of lymphatic filariasis in central Nigeria: implications for monitoring infection by PCR in mosquito pools. Filaria J. 2007;6:14. doi: 10.1186/1475-2883-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awolola TS, Okwa O, Hunt RH, Ogunrinade AF, Coetzee M. Dynamics of the malaria-vector populations in coastal Lagos, south–western Nigeria. Ann Trop Med Parasitol. 2002;96:75–82. doi: 10.1179/000349802125000538. [DOI] [PubMed] [Google Scholar]
- 26.Okwa OO, Akinmolayan FI, Carter V, Hurd H. Transmission dynamics of malaria in four selected ecological zones of Nigeria in the rainy season. Ann Afr Med. 2009;8:1–9. doi: 10.4103/1596-3519.55756. [DOI] [PubMed] [Google Scholar]
- 27.Oyewole V, Ibidapo CA, Okwa OO, Oduola AO, Adeoye GO, et al. Species Composition and role of Anopheles mosquitoes in malaria transmission along Badagry axis of Lagos lagoon, Lagos, Nigeria. International Journal of Insect Science. 2010;2:51–57. [Google Scholar]
- 28.Onyabe DY, Conn JE. The distribution of two major malaria vectors, Anopheles gambiae and Anopheles arabiensis, in Nigeria. Mem Inst Oswaldo Cruz. 2001;96:1081–1084. doi: 10.1590/s0074-02762001000800009. [DOI] [PubMed] [Google Scholar]
- 29.Onyabe DY, Conn JE. Population genetic structure of the malaria mosquito Anopheles arabiensis across Nigeria suggests range expansion. Mol Ecol. 2001;10:2577–2591. doi: 10.1046/j.0962-1083.2001.01387.x. [DOI] [PubMed] [Google Scholar]
- 30.Onyabe DY, Vajime CG, Nock IH, Ndams IS, Akpa AU, et al. The distribution of M and S molecular forms of Anopheles gambiae in Nigeria. Trans R Soc Trop Med Hyg. 2003;97:605–608. doi: 10.1016/s0035-9203(03)80045-7. [DOI] [PubMed] [Google Scholar]
- 31.Awolola TS, Oyewole IO, Amajoh CN, Idowu ET, Ajayi MB, et al. Distribution of the molecular forms of Anopheles gambiae and pyrethroid knock down resistance gene in Nigeria. Acta Tropica. 2005;95:204–209. doi: 10.1016/j.actatropica.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Service MW. The anopheline mosquitoes of Nigeria and the Cameroons. West Afr Med J. 1961;10:109–121. [PubMed] [Google Scholar]
- 33.Awolola TS, Bitsindou P, Bagayoko M, Manga L. Malaria Entomological Profile for Nigeria. WHO Document; 2007. 50 [Google Scholar]
- 34.FMEN (Federal Ministry of Environment of Nigeria) National Action Programme to combat desertification. 2001. Available: http://www.unccd.int/actionprogrammes/africa/national/2001/nigeria-eng.pdf via the internet. Accessed May 16, 2011.
- 35.FMH (Federal Ministry of Health) Federal Ministry of Health, National Malaria Control Programme, Abuja, Nigeria. Strategic Plan 2009–2013. 2008. Available: http://nmcpnigeria.org/f/Nigeria%20Annex%201_National%20Malaria%20Control%20Strategic%20Plan%202009-2013.pdf via the internet. Accessed May 16, 2011.
- 36.Iloeje NP. A new geography of Nigeria. 2001. 200 New Revised Edition. Longman Nigeria PLC.
- 37.NGA GEOnet Names Server (GNS) 2008. Available: http://earth-info.nga.mil/gns/html/namefiles.htm via the internet. Accessed May 16, 2011.
- 38.Directory of Cities and Towns in the World, Global Gazetteer Version 2.1. Available: http://www.fallingrain.com/world/NI/index.html via the internet. Copyright 1996–2006 by Falling Rain Genomics, Inc. Accessed May 16, 2011.
- 39.Service MW. Identification of the Anopheles gambiae complex in Nigeria by larval and adult chromosomes. Ann Trop Med Parasitol. 1970;64:131–136. doi: 10.1080/00034983.1970.11686674. [DOI] [PubMed] [Google Scholar]
- 40.Service MW, Davidson GA. High incidence of Dieldrin-resistance in Anopheles gambiae Giles from an unsprayed area in Northern Nigeria. Nature. 1964;203:209–210. doi: 10.1038/203209a0. [DOI] [PubMed] [Google Scholar]
- 41.Pant CP, Self LS. Field trials of Bromophos and Schering 34615 residual sprays and of cheesecloth impregnated with Bayer 39007 for control of Anopheles gambiae and A. funestus in Nigeria. Bull World Health Organ. 1966;35:709–719. [PMC free article] [PubMed] [Google Scholar]
- 42.De Meillon B. New records and species of biting insects from the Ethiopian region. J Entomol Soc South Afr. 1947;10:110–124. [PubMed] [Google Scholar]
- 43.Gillies MT, De Meillon B. The Anophelinae of Africa, south of the sahara (Ethiopian zoogeographical region). Publication of the South African Institute for Medical Research. 1968;54:265–314. [Google Scholar]
- 44.Gillett JD. Common African Mosquitoes and their medical importance (with colour illustrations). 1972. pp. 1–5, 18–26 50, 68, 94, 102. William Heinemann, London.
- 45.Gillies MT, Coetzee M. A supplement to the Anophellinae of Africa south of the sahara Publication of the South African. Institute for Medical Research. 1987;55:143. [Google Scholar]
- 46.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 47.Favia G, della Torre A, Bagayoko M, Lanfrancotti A, Sagnon N, et al. Molecular identification of sympatric chromosomal forms of Anopheles gambiae and further evidence of their reproductive isolation. Insect Mol Biol. 1997;6:377–383. doi: 10.1046/j.1365-2583.1997.00189.x. [DOI] [PubMed] [Google Scholar]
- 48.Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction (PCR) assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66:804–811. doi: 10.4269/ajtmh.2002.66.804. [DOI] [PubMed] [Google Scholar]
- 49.De Meillon B. Illustrated keys to the full-grown larva and adults of South African Anopheline mosquitoes. South Africa Invest Med Res. 1931;28:578. [Google Scholar]
- 50.Edwards FW. Mosquitoes of the Ethiopian region III - culicidae adult and pupae Anopheles mosquitoes. Pub Inst Med Res. 1941;23:28–32. [Google Scholar]
- 51.Hopkins GHE. Mosquitoes of the Ethiopian region. 1952. pp. 1–3 100, 136–139, 194, 250, 305. I. Larval Binomics and Taxonomy of Culicine Larvae. Brit. Nat. Hist. Museum.
- 52.Chandler AC. Introduction to parasitology. XIX edn. New York: John Willey and Sons Inc; 1955. pp. 693–741. [Google Scholar]
- 53.Smart J. A hand book for the Identification of insects of medical importance. 1956. Jarold and Sons Ltd, Norwich.
- 54.Service MW. Mosquito biology, Field sampling methods. 1976. Applied Science Publication Limited, London.
- 55.Service MW. A guide to medical entomology. 1980. The Macmillan Press Ltd. London.
- 56.Bruce-Chwatt LJ. Essential Malariology William. 1985. 425 Heinemann Medical Books. London.
- 57.Gordon RM, Lavoipierre MMJ. Entomology for students of Medicine, 5th Ed. 1978. 330 Blackwell Sc. Publ., London.
- 58.Brunhes J, Le Goff G, Manga L, Geoffroy B. Anophèles afro-tropicaux. IV. Mise au point sur les espèces et sous-espèces du groupe Anopheles (Cellia) I ; réhabilitation d ' An. (C.) multicinctus et d ' An. ( Cellia ) garnhami basilewskyi. Annales de la Société Entomologique de France. 1998;34:397–405. [Google Scholar]
- 59.Fanello C, Santolamazza F, della Torre A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol. 2002;16:461–464. doi: 10.1046/j.1365-2915.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- 60.Hackett BJ, Gimnig J, Guelbeogo W, Costantini C, Koekemoer LL, et al. Ribosomal DNA internal transcribed spacer (ITS2) sequences differentiate Anopheles funestus and An. rivulorum, and uncover a cryptic taxon. Insect Mol Biol. 2000;9:369–374. doi: 10.1046/j.1365-2583.2000.00198.x. [DOI] [PubMed] [Google Scholar]
- 61.Kengne P, Awono-Ambene HP, Antonio-Nkondjio C, Simard F, Fontenille D. Molecular identification of the Anopheles nili group African malaria vectors. Med Vet Entomol. 2003;17:67–74. doi: 10.1046/j.1365-2915.2003.00411.x. [DOI] [PubMed] [Google Scholar]
- 62.Okwa OO, Rasheed A, Adeyemi A, Omoyeni M, Oni L, et al. Anopheles species abundances, composition and vectoral competence in six areas of Lagos: Nigeria. Journal of Cell and Animal Biology. 2007;1:019–023. [Google Scholar]
- 63.Dodge JS. 1965. Outdoor malaria transmission in a DDT-sprayed area of Western Sokoto, northern Nigeria, Unpublished document WHO/MAL/520.65.
- 64.Muirhead Thomson RC. Studies on Anopheles gambiae and Anopheles melas in and around Lagos. Bull Entomol Res. 1948;38:527–558. doi: 10.1017/s0007485300023221. [DOI] [PubMed] [Google Scholar]
- 65.Noutcha MA, Anumudua CI. Variations in species composition and infection rates in Anopheles gambiae s.l. across eco-vegetational zones in Nigeria and Cameroon. J Vector Borne Dis. 2010;47:51–54. [PubMed] [Google Scholar]
- 66.Hanney PW. The mosquitos of Zaria Province. Northern Nigeria Bull Entomol Res. 1960;51:145–171. [Google Scholar]
- 67.Oyewole IO, Awolola TS, Ibidapo CA, Oduola AO, Okwa OO, et al. Behaviour and population dynamics of the major anopheline vectors in a malaria endemic area in southern Nigeria. J Vector Borne Dis. 2007;44:56–64. [PubMed] [Google Scholar]
- 68.Richards FO, Jr, Pam DD, Kal A, Gerlong GY, Onyeka J, et al. Significant decrease in the prevalence of Wuchereria bancrofti infection in Anopheline mosquitoes following the addition of albendazole to annual, ivermectin-based, mass treatments in Nigeria. Ann Trop Med Parasitol. 2005;99:155–164. doi: 10.1179/136485905X19838. [DOI] [PubMed] [Google Scholar]
- 69.Ogunba EO. Observations on Culex pipiens fatigans in Ibadan, Western Nigeria. Ann Trop Med Parasitol. 1971;65:399–402. doi: 10.1080/00034983.1971.11686769. [DOI] [PubMed] [Google Scholar]
- 70.Udonsi JK. Bancroftian filariasis in the Igwun Basin, Nigeria. An epidemiological, parasitological, and clinical study in relation to the transmission dynamics. Acta Trop. 1988;45:171–179. [PubMed] [Google Scholar]
- 71.Agi PI, Ebenezer A. Observations on Filarial Infection in Amassoma Community in the Niger Delta, Nigeria. J Appl Sci Environ Manage. 2009;13:15–19. [Google Scholar]
- 72.Richards FO, Jr, Eigege A, Pam D, Kal A, Lenhart A, et al. Mass ivermectin treatment for onchocerciasis: lack of evidence for collateral impact on transmission of Wuchereria bancrofti in areas of co-endemicity. Filaria J. 2005;4:6. doi: 10.1186/1475-2883-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Annett E, Dutton JE, Elliott JH. Report of Malaria Expedition to Nigeria. Liverpool Sch Trop Med Mem. 1901;IV:69–72. [Google Scholar]
- 74.Johnson WB. Domestic Mosquitos of the Northern Provinces of Nigeria. Bull Entomol Res. 1919;9:325–332. [Google Scholar]
- 75.Taylor AW. The domestic Mosquitos of Gadau, Northern Nigeria, and their relation to malaria and filariasis. Ann Trop Med Parasit. 1930;24:425–435. [Google Scholar]
- 76.Archibald HM. Malaria in south-western and north-western Nigerian communities. Bull World Health Organ. 1956;15:695–709. [PMC free article] [PubMed] [Google Scholar]
- 77.Elliott R, Ramakrishna V. Insecticide resistance in Anopheles gambiae Giles. Nature. 1956;177:532–533. doi: 10.1038/177532a0. [DOI] [PubMed] [Google Scholar]
- 78.Mellanby K. Mosquito populations at Ibadan in Nigeria. Bull Entomol Res. 1956;47:125–136. [Google Scholar]
- 79.Kuhlow F. Field experiments on the behaviour of malaria vectors in an unsprayed hut and in a hut sprayed with DDT in Northern Nigeria. Bull World Health Organ. 1962;26:93–102. [PMC free article] [PubMed] [Google Scholar]
- 80.Service MW. The behaviour of malaria vectors in huts sprayed with DDT and with a mixture of DDT and malathion in northern Nigeria. Trans R Soc Trop Med Hyg. 1964;58:1. [Google Scholar]
- 81.Service MW. An analysis of the numbers of Anopheles gambiae Giles and A. funestus Giles (Diptera, Culicidae) in huts in Northern Nigeria. Bull Entomol Res. 1964;55:29–34. [Google Scholar]
- 82.Barzeev M. Some effects of dichlorvos in huts on the behaviour of mosquitos in southern Nigeria. Bull World Health Organ. 1965;33:583–586. [PMC free article] [PubMed] [Google Scholar]
- 83.Barzeev M, Bracha P. A village-scale trial with the insecticides carbaryl and folithion in Southern Nigeria. Bull World Health Organ. 1965;33:461–470. [PMC free article] [PubMed] [Google Scholar]
- 84.Foll CV, Pant CP, Lietaert PE. A large-scale field trial with dichlorvos as a residual fumigant insecticide in northern Nigeria. Bull World Health Organ. 1965;32:531–550. [PMC free article] [PubMed] [Google Scholar]
- 85.Foll CV, Pant CP. The conditions of malaria transmission in Katsina Province, Northern Nigeria, and a discussion of the effects of dichlorvos application. Bull World Health Organ. 1966;34:395–404. [PMC free article] [PubMed] [Google Scholar]
- 86.Self LS, Pant CP. Insecticide susceptibility and resistance in populations of Anopheles gambiae, Culex fatigans and Aedes aegypti in southern Nigeria. Bull World Health Organ. 1966;34:960–962. [PMC free article] [PubMed] [Google Scholar]
- 87.Pant CP, Rosen P, Ramasamy M, Joshi G, Renaud P, et al. A village scale trial of OMS-597 for the control of An. gambiae and An. funestus in Northern Nigeria. 1968. Unpublished document WHO/VBC/68.104. [PMC free article] [PubMed]
- 88.Pant CP, Rosen P, Joshi GP, Pearson JA, Ramasamy M, et al. A village-scale trial of OMS-708 (Mobam) for the control of Anopheles gambiae and Anopheles funestus in northern Nigeria. Bull World Health Organ. 1969;41:316–319. [PMC free article] [PubMed] [Google Scholar]
- 89.Pant CP, Joshi GP, Rosen P, Pearson JA, Renaud P, et al. A village-scale trial of OMS-214 (Dicapthon) for the control of Anopheles gambiae and Anopheles funestus in northern Nigeria. Bull World Health Organ. 1969;41:311–315. [PMC free article] [PubMed] [Google Scholar]
- 90.Garrett-Jones C, Shidrawi GR. Malaria vectorial capacity of a population of Anopheles gambiae: an exercise in epidemiological entomology. Bull World Health Organ. 1969;40:531–545. [PMC free article] [PubMed] [Google Scholar]
- 91.Fontaine RE, Rosen P, Ramasamy M, Renaud P. A village scale trial of OMS-1211 for the control of Anopheles gambiae and Anopheles funestus in North Central Nigeria. 1971. Unpublished document WHO/VBC/71.284.
- 92.Molineaux L, Shidrawi GR, Clarke JL, Boulzaguet R, Ashkar T, et al. The impact of propoxur on Anopheles gambiae s.1. and some other anopheline populations, and its relationship with some pre-spraying variables. Bull World Health Organ. 1976;54:379–389. [PMC free article] [PubMed] [Google Scholar]
- 93.Molineaux L, Gramiccia G. The Garki project: Research on the epidemiology and control of malaria in the sudan savanna of western Nigeria. World health Organization. 1980;312:53–106. [Google Scholar]
- 94.Awolola TS, Oyewole IO, Koekemoer LL, Coetzee M. Identification of three members of the Anopheles funestus (Diptera: Culicidae) group and their role in malaria transmission in two ecological zones in Nigeria. Trans R Soc Trop Med Hyg. 2005;99:525–531. doi: 10.1016/j.trstmh.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 95.Morgan JC, Irving H, Okedi LM, Steven A, Wondji CS. Pyrethroid resistance in an Anopheles funestus population from Uganda. PLoS One. 2010;5:e11872. doi: 10.1371/journal.pone.0011872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kristan M, Fleischmann H, della Torre A, Stich A, Curtis CF. Pyrethroid resistance/susceptibility and differential urban/rural distribution of Anopheles arabiensis and An. gambiae s.s. malaria vectors in Nigeria and Ghana. Med Vet Entomol. 2003;17:326–332. doi: 10.1046/j.1365-2915.2003.00449.x. [DOI] [PubMed] [Google Scholar]
- 97.Awolola TS, Brooke BD, Koekemoer LL, Coetzee M. Absence of the kdr mutation in the molecular ‘M’ form suggests different pyrethroid resistance mechanisms in the malaria vector mosquito Anopheles gambiae s.s. Trop Med Int Health. 2003;8:420–422. doi: 10.1046/j.1365-3156.2003.01034.x. [DOI] [PubMed] [Google Scholar]
- 98.Awolola TS, Ibrahim K, Hunt RH, Coetzee M. Species composition and biting activities of anthropophilic Anopheles mosquitoes and their role in malaria transmission in a holoendemic area in south-western Nigeria. Afr Entomol. 2003;11:78–82. [Google Scholar]
- 99.Awolola TS, Oduola AO, Oyewole IO, Obansa JB, Amajoh CN, et al. Dynamics of knockdown pyrethroid insecticide resistance alleles in a field population of Anopheles gambiae s.s. in southwestern Nigeria. J Vector Borne Dis. 2007;44:181–188. [PubMed] [Google Scholar]
- 100.Santolamazza F, Calzetta M, Etang J, Barrese E, Dia I, et al. Distribution of knock-down resistance mutations in Anopheles gambiae molecular forms in west and west-central Africa. Malar J. 2008;7:74. doi: 10.1186/1475-2875-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oduola AO, Olojede JB, Ashiegbu CO, Adeogun AO, Otubanjo OA, et al. High level of DDT resistance in the malaria mosquito: Anopheles gambiae s.l. from rural, semi urban and urban communities in Nigeria. Journal of Rural and Tropical Public Health. 2010;9:114–120. [Google Scholar]
- 102.Lehmann T, Licht M, Elissa N, Maega BT, Chimumbwa JM, et al. Population Structure of Anopheles gambiae in Africa. J Hered. 2003;94:133–147. doi: 10.1093/jhered/esg024. [DOI] [PubMed] [Google Scholar]
- 103.Lehmann T, Hume JC, Licht M, Burns CS, Wollenberg K, et al. Molecular evolution of immune genes in the malaria mosquito Anopheles gambiae. PLoS One. 2009;4:e4549. doi: 10.1371/journal.pone.0004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Djouaka RF, Bakare AA, Bankole HS, Doannio JM, Coulibaly ON, et al. Does the spillage of petroleum products in Anopheles breeding sites have an impact on the pyrethroid resistance? Malar J. 2007;6:159. doi: 10.1186/1475-2875-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Djouaka RF, Bakare AA, Coulibaly ON, Akogbeto MC, Ranson H, Hemingway J, Strode C, et al. Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC Genomics. 2008;9:538. doi: 10.1186/1471-2164-9-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kelly-Hope LA, Diggle PJ, Rowlingson BS, Gyapong JO, Kyelem D, et al. Negative spatial association between lymphatic filariasis and malaria in West Africa. Trop Med Int Health. 2006;11:129–135. doi: 10.1111/j.1365-3156.2005.01558.x. [DOI] [PubMed] [Google Scholar]
- 107.RBM Report. Building capacity in monitoring and evaluating roll back malaria in africa a conceptual framework for the roll back malaria partnership. 2005. 65 Roll Back Malaria Monitoring and Evaluation Reference Group.
- 108.Greenwood BM, Bhasin A, Bowler CH, Naylor H, Targett GA. Capacity strengthening in malaria research: the Gates Malaria Partnership. Trends Parasitol. 2006;22:278–284. doi: 10.1016/j.pt.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 109.Sikulu M, Govella NJ, Ogoma SB, Mpangile J, Kambi SH. Comparative evaluation of the Ifakara tent trap-B, the standardized resting boxes and the human landing catch for sampling malaria vectors and other mosquitoes in urban Dar es Salaam, Tanzania. Malar J. 2009;8:197. doi: 10.1186/1475-2875-8-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Govella NJ, Moore JD, Killeen GF. An exposure-free tool for monitoring adult malaria mosquito populations. Am J Trop Med Hyg. 2010;83:596–600. doi: 10.4269/ajtmh.2010.09-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Service MW. A critical review of procedures for sampling populations of adult mosquitoes. Bull of Entomol Res. 1977;67:343–382. [Google Scholar]
- 112.Hay SI, Rogers DJ, Toomer JF, Snow RW. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, Internet access and review. Trans R Soc Trop Med Hyg. 2000;94:113–127. doi: 10.1016/s0035-9203(00)90246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kelly-Hope LA, McKenzie FE. The multiplicity of malaria transmission: a review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malar J. 2009;8:19. doi: 10.1186/1475-2875-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramsdale CD, Leport GH. Studies of the Anopheles gambiae Complex in West Africa. Bull World Health Organ. 1967;36:494–500. [PMC free article] [PubMed] [Google Scholar]
- 115.Anyanwu IN, Agbede RI, Ajanusi OJ, Umoh JU, Ibrahim ND. The incrimination of Aedes (stegomyia) aegypti as the vector of Dirofilaria repens in Nigeria. Vet Parasitol. 2000;92:319–327. doi: 10.1016/s0304-4017(00)00311-3. [DOI] [PubMed] [Google Scholar]
- 116.Michel AP, Ingrasci MJ, Schemerhorn BJ, Kern M, Le Goff G. Rangewide population genetic structure of the African malaria vector Anopheles funestus. Mol Ecol. 2005;14:4235–4248. doi: 10.1111/j.1365-294X.2005.02754.x. [DOI] [PubMed] [Google Scholar]
- 117.Shidrawi GR. The distribution and seasonal prevalence of the Anopheles gambiae species copmlex (Species A and B) in Garki district Northern Nigeria. 1972. Unpublished document WHO/MAL/72.776 WHO/VBC/72.387.
- 118.White GB, Rosen P. Comparative studies in sibling species of the Anopheles gambiae complex (Diptera culicidae). II. Ecology of species A and B in savanna around Kaduna, Nigeria during transition from wet to dry season. Bull Entomol Res. 1973;62:613–625. [Google Scholar]
- 119.Coluzzi M, Sabatini A, Petraca V, Dideco MA. Chromosomal investigation on species A and B of the Anopheles gambiae complex in Garki district (Kano state Nigeria) Results of species identification from 1971–1974. 1975. pp. 16–25. Unpublished WHO Technical note no 24 MPD/TN/75.1.
- 120.Smith DM. Mosquito records from the Republic of Niger, with reference to the construction of the new ‘Trans-Sahara Highway’. J Trop Med Hyg. 1981;84:95–100. [PubMed] [Google Scholar]
- 121.Rishikesh N, Di Deco MA, Petrarca V, Coluzzi M. Seasonal variations in indoor resting Anopheles gambiae and Anopheles arabiensis in Kaduna, Nigeria. Acta Trop. 1985;42:165–170. [PubMed] [Google Scholar]
- 122.Pombi M, Caputo B, Simard F, Di Deco MA, Coluzzi M, et al. Chromosomal plasticity and evolutionary potential in the malaria vector Anopheles gambiae sensu stricto: insights from three decades of rare paracentric inversions. BMC Evol Biol. 2008;8:309. doi: 10.1186/1471-2148-8-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Okogun GR, Anosike JC, Okere AN, Nwoke BE. Ecology of mosquitoes of Midwestern Nigeria. J Vector Borne Dis. 2005;42:1–8. [PubMed] [Google Scholar]
- 124.Kengne P, Antonio-Nkondjio C, Awono-Ambene HP, Simard F, Awolola TS. Molecular differentiation of three closely related members of the mosquito species complex, Anopheles moucheti, by mitochondrial and ribosomal DNA polymorphism. Med Vet Entomol. 2007;21:177–182. doi: 10.1111/j.1365-2915.2007.00681.x. [DOI] [PubMed] [Google Scholar]
- 125.WHO. Atlas of insecticide resistance in malaria vectors of the WHO African region. 2005. Geneva: World Health Organization; Available: http://www.afro.who.int/des/phe/publications/atlas_final_version.pdf via internet. Accessed May 19, 2011. [Google Scholar]
- 126.Penilla RP, Rodriguez AD, Hemingway J, Torres JL, Arredondo-Jimenez JI, et al. Resistance management strategies in malaria vector mosquito control. Baseline data for a largescale field trial against Anopheles albimanus in Mexico. Med Vet Entomol. 1998;12:217–233. doi: 10.1046/j.1365-2915.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- 127.Brooke BD, Hunt RH, Koekemoer LL, Dossouyovo J, Coetzee M. Evaluation of polymerase chain reaction assay for detection of pyrethroid insecticide resistance in a malaria vector species of the Anopheles gambiae complex. J Am Mosq Control Assoc. 1999;15:565–568. [PubMed] [Google Scholar]
- 128.Awolola TS, Oduola OA, Strode C, Koekemoer LL, Brooke B, et al. Evidence of multiple pyrethroid resistance mechanisms in the malaria vector Anopheles gambiae sensu stricto from Nigeria. Trans R Soc Trop Med Hyg. 2009;103:1139–1145. doi: 10.1016/j.trstmh.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 129.Kelly-Hope LA, Yapabandara AM, Wickramasinghe MB, Perera MD, Karunaratne SH, et al. Spatiotemporal distribution of insecticide resistance in Anopheles culicifacies and Anopheles subpictus in Sri Lanka. Trans R Soc Trop Med Hyg. 2005;99:751–761. doi: 10.1016/j.trstmh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 130.Dialynas E, Topalis P, Vontas J, Louis C. MIRO and IRbase: IT tools for the epidemiological monitoring of insecticide resistance in mosquito disease vectors. PLoS Negl Trop Dis. 2009;3:e465. doi: 10.1371/journal.pntd.0000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.IRAC (Insecticide Resistance Action Committee) The increase in the utility and importance of biomolecular techniques in resistance monitoring in insect vectors. 2010. Available: http://www.irac-online.org/wp-content/uploads/2009/09/Biomolecular-monitoring-in-insect-vectorsNov10-v1.pdf via internet. Accessed 19th June 2011.
- 132.IRAC (Insecticide Resistance Action Committee) The importance of Insecticide Resistance Management in the control of the mosquito vectors of malaria. 2010. Available: http://www.irac-online.org/wp-content/uploads/2009/09/IRM-control-mosquito-vectors-of-malariaNov10-v1.pdf via internet. Accessed 19th June 2011.
- 133.Oyewole IO, Awolola TS. Impact of urbanisation on bionomics and distribution of malaria vectors in Lagos, southwestern Nigeria. J Vect Borne Dis. 2006;43:173–178. [PubMed] [Google Scholar]
- 134.Anosike HC, Nwoke BEB, Ajayi EG, Onwuliri CO, Okoro OU, Oku EE, Asor JE, Amajuoyi OU, Ikpeama CA, Ogbusu FI, Meribe CO. Lymphatic filariasis among the Ezza people of Ebonyi state, Eastern Nigeria. Ann Agric Environ Med. 2005;12:181–186. [PubMed] [Google Scholar]
- 135.Manguin S, Bangs MJ, Pothikasikorn J, Chareonviriyaphap T. Review on global co-transmission of human Plasmodium species and Wuchereria bancrofti by Anopheles mosquitoes. Infect Genet Evol. 2009;10:159–177. doi: 10.1016/j.meegid.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 136.Njenga SM, Mwandawiro CS, Wamae CN, Mukoko DA, Omar AA, et al. Sustained reduction in prevalence of lymphatic filariasis infection in spite of missed rounds of mass drug administration in an area under mosquito nets for malaria control. Parasit Vectors. 2011;4:90. doi: 10.1186/1756-3305-4-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.WHO. Assessment of susceptibility to insecticides in anopheline mosquitos. Summary of information received by the Malaria Section of WHO. Bull World Health Organ. 1957;16:874–890. [PMC free article] [PubMed] [Google Scholar]
- 138.Armstrong JA, Ramsdale CD, Ramakrishna V. Insecticide resistance in Anopheles gambiae Giles in Western Sokoto, Northern Nigeria. Ann Trop Med Parasitol. 1957;52:247–256. doi: 10.1080/00034983.1958.11685865. [DOI] [PubMed] [Google Scholar]
- 139.Service MW. The ecology of the mosquitos of the northern guinea savannah of Nigeria. Bull Ento Res. 1963;54:601–632. [Google Scholar]
- 140.Elliott R, Barnes JM. Organophosphorus insecticides for the control of mosquitos in Nigeria: Trials with fenthion and malathion conducted by the WHO Insecticide Testing Unit in 1960–61. Bull World Health Organ. 1963;28:35–54. [PMC free article] [PubMed] [Google Scholar]
- 141.Service MW. Studies on sampling larval populations of the Anopheles gambiae complex. Bull World Health Organ. 1971;45:169–180. [PMC free article] [PubMed] [Google Scholar]
- 142.Gratz NG, Bracha P, Carmichael A. A village-scale trial with dichlorvos as a residual fumigant insecticide in Southern Nigeria. Bull World Health Organ. 1963;29:251–270. [PMC free article] [PubMed] [Google Scholar]
- 143.Service MW. The Behaviour of Anopheles nili Theo. in sprayed huts in Northern Nigeria. J Trop Med Hyg. 1964;67:11–12. [PubMed] [Google Scholar]
- 144.Service MW. Two albinoid females of Anopheles gambiae Giles (Diptera: Culicidae) from Northern Nigeria. Proc R Ent Soc Lond (B) 1964;33:73–116. [Google Scholar]
- 145.Service MW. Dieldrin Resistance in Anopheles funestus Giles from an unsprayed area in Northern Nigeria. J Trop Med Hyg. 1964;67:190. [PubMed] [Google Scholar]
- 146.Wright JW, Fritz RF, Hocking KS, Babione R, Gratz NG, et al. Ortho-Isopropoxyphenyl Methylcarbamate (OMS-33) as a residual spray for control of Anopheline mosquitos. Bull Wld Hlth Org. 1969;40:67–90. [PMC free article] [PubMed] [Google Scholar]
- 147.Rosen P, Fontaine RE, Sundararaman S. A village scale trial of OMS-597 for the control of An. gambiae and An. funestus in Northern Nigeria. 1970. Unpublished document WHO/VBC/70.192.
- 148.Molineaux L, Shidrawi GR, Clarke JL, Boulzaguet JR, Ashkar TS. Assessment of insecticidal impact on the malaria mosquito's vectorial capacity, from data on the man-biting rate and age-composition. Bull World Health Organ. 1979;57:265–274. [PMC free article] [PubMed] [Google Scholar]
- 149.Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- 150.Boreham PF, Lenahan JK, Boulzaguet R, Storey J, Ashkar TS, et al. Studies on multiple feeding by Anopheles gambiae s.l. in a Sudan savanna area of north Nigeria. Trans R Soc Trop Med Hyg. 1979;73:418–423. doi: 10.1016/0035-9203(79)90167-6. [DOI] [PubMed] [Google Scholar]
- 151.Afolabi BM, Amajoh CN, Adewole TA, Salako LA. Seasonal and temporal variations in the population and biting habit of mosquitoes on the Atlantic coast of Lagos, Nigeria. Med Princ Pract. 2006;15:200–208. doi: 10.1159/000092182. [DOI] [PubMed] [Google Scholar]
- 152.Amusan AAS, Mafiana CF, Idowu AB, Olatunde GO. Sampling mosquitoes with CDC trap in rice field and plantation communities in Ogun State. Tanzania Health Res Bull. 2005;7:111–116. doi: 10.4314/thrb.v7i3.14247. [DOI] [PubMed] [Google Scholar]
- 153.Kristan M, Fleischmann H, della Torre A, Stich A, Curtis CF. Pyrethroid resistance/susceptibility and differential urban/rural distribution of Anopheles arabiensis and An. gambiae s.s. malaria vectors in Nigeria and Ghana. Med Vet Entomol. 2003;17:326–332. doi: 10.1046/j.1365-2915.2003.00449.x. [DOI] [PubMed] [Google Scholar]
- 154.Noutcha MA, Anumdu CI. Entomological indices of Anopheles gambiae sensu lato at a rural community in south-west Nigeria. J Vector Borne Dis. 2009;46:43–51. [PubMed] [Google Scholar]
- 155.Ogba OM, Alaribe AA, Ogban GI, Osuchukwu NC, Inyang-Etoh PC. Malaria transmission potential in Adim community of Biase Local Government Area of Cross River State of Nigeria. J Medical Lab Science. 2007;16:1. [Google Scholar]
- 156.Amusan AAS, Mafiana CF, Idowu AB, Oke OA. A survey of adult mosquitoes in the hostels of the University of Agriculture, Abeokuta, Ogun State, Nigeria. Nig J Parasitol. 2003;24:167–172. [Google Scholar]
- 157.Davidson G, Jackson CE. Incipient speciation in Anopheles gambiae Giles. Bull World Health Organ. 1962;27:303–305. [PMC free article] [PubMed] [Google Scholar]
- 158.Anosike HC, Nwoke BEB, Okere AN, Oku EE, Asor AE, Emmy-Egbe IO, Adimike DA. Epidemiology of tree-hole breeding mosquitoes in the tropical rainforest of Imo state, South-east Nigeria. Ann Agric Environ Med. 2007;14:31–38. [PubMed] [Google Scholar]
- 159.Awolola TS, Brooke BD, Hunt RH, Coetze M. Resistance of the malaria vector Anopheles gambiae s.s. to pyrethroid insecticides in Nigeria. Ann Trop Med Parasitol. 2002;96:849–852. doi: 10.1179/000349802125002581. [DOI] [PubMed] [Google Scholar]
- 160.Ayanda OI. Relative abundance of adult female anopheline mosquitoes in Ugah, Nasarawa State, Nigeria. J Parasitol Vector Biol. 2009;1:005–008. [Google Scholar]
- 161.Oyewole IO, Ibidapo A, Oduola AO, Obansa JB, Awolola TS. Molecular identification and population dynamics of the major malaria vectors in a rainforest zone of Nigeria. Biokemistri. 2005;17:171–178. [Google Scholar]
- 162.Adeleke MA, Mafiana CF, Idowu AB, Adekunle MF, Sam-Wobo SO. Mosquito larval habitats and public health implications in Abeokuta, Ogun State, Nigeria. Tanzan J Health Res. 2008;2008 10:103–107. doi: 10.4314/thrb.v10i2.14348. [DOI] [PubMed] [Google Scholar]
- 163.Adeleke MA, Mafiana CF, Idowu AB, Amusan AAS, Adekunle MF. Morphometric studies on Anopheles gambiae complex (Diptera: Culicidae) in Abeokuta, Southwest Nigeria. Acta Entomologica Sinica. 2008;51:1289–1292. [Google Scholar]
- 164.Ndo C, Antonio-Nkondjio C, Cohuet A, Ayala D, Kengne P, et al. Population genetic structure of the malaria vector Anopheles nili in sub-Saharan Africa. Malar J. 2010;9:161. doi: 10.1186/1475-2875-9-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Oguoma VM, Ikpeze OO. Species composition and abundance of mosquitoes of a tropical irrigation ecosystem. Animal Research International. 2008;5:866–871. [Google Scholar]
- 166.Oguoma VM, Nwaorgu OC, Mbanefo EC, Ikpeze OO, Umeh JM, et al. Species composition of Anopheles mosquito in three villages of Uratta Owerri north local government area of Imo state Nigeria. Reviews in Infection. 2010;1:192–196. [Google Scholar]
- 167.Ndams IS, Laila KM, Tukur Z. Susceptibility of some species of mosquitoes to permethrin pyrethroid in Zaria Nigeria. Science World Journal. 2006;1:15–19. [Google Scholar]
- 168.Sow GJ, Ndams IS, Kogi E, Tukur Z, Adamu H. Efficacy of permethrin insecticide tested against populations of Anopheles and Aedes from different larval habitats in southern guinea savanna, Nigeria. Science World Journal. 2007;2:5–8. [Google Scholar]
- 169.Ivoke N, Okafor FC, Owoicho LO. Evaluation of ovicidal and larvicidal effects of leave extracts of Hyptis suaveolens (L) POIT (Lamiaceae) against Anopheles gambiae (Diptera: Anophelidae) Complex. Animal Research International. 2009;6:1072–1076. [Google Scholar]
- 170.Obomanu FG1, Ogbalu OK, Gabriel UU, Fekarurhobo GK, Adediran BI. Larvicidal properties of Lepidagathis alopecuroides and Azadirachta indica on Anopheles gambiae and Culex quinquefasciatus Afr. J Biotechnol. 2006;5:761–765. [Google Scholar]
- 171.Olayemi IK, Ande AT. Life table analysis of Anopheles gambiae (diptera: culicidae) in relation to malaria transmission. J Vector Borne Dis. 2009;46:295–298. [PubMed] [Google Scholar]
- 172.Oparaocha ET, Iwu I, Ahanakuc JE. Preliminary study on mosquito repellent and mosquitocidal activities of Ocimum gratissimum (L.) grown in eastern Nigeria. J Vector Borne Dis. 2010;47:45–50. [PubMed] [Google Scholar]
- 173.Pinto J, Lynd A, Vicente JL, Santolamazza F, Randle NP, et al. Multiple origins of knockdown resistance mutations in the Afrotropical mosquito vector Anopheles gambiae. PLoS One. 2007;2:e1243. doi: 10.1371/journal.pone.0001243. doi: 10.1371/journal.pone.0001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ramkrishna V, Elliot R. Normal resistance level of Anopheles funestus Giles to insecticide. Nature. 1957;179:1140–1141. doi: 10.1038/1791140b0. [DOI] [PubMed] [Google Scholar]
- 175.Adeleke MA, Mafiana CF, Idowu AB, Sam-Wobo SO, Idowu OA. Population dynamics of indoor sampled mosquitoes and their implication in disease transmission in Abeokuta, south-western Nigeria. J Vector Borne Dis. 2010;47:33–38. [PubMed] [Google Scholar]
- 176.Olayemi IK, Ande AT. Survivorship of Anopheles gambiae in relation to malaria transmission in Ilorin, Nigeria. Online J Health Allied Scs. 2008;7:1–5. [Google Scholar]
- 177.Manga SB, Ameh IG, Galadima M, Dangogo SM, Ukatu VE. Larvicidal efficacy of stock Bacillus sphaericus on local species of Anopheles mosquito in Sokoto, Nigeria. Nig J Parasitol. 2008;29:103–105. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nigeria Anopheles vector database.
(XLSX)
The number of data points by each species and each year from 1900–1999.
(XLSX)
The number of data points by each species and each year from 2000–2010.
(XLSX)
Number of specimen of Anopeles gambiae s.s. M form and S form from 1900–1999.
(XLSX)
Number of specimen of Anopeles gambiae s.s. M form and S form from 2000–2010.
(XLSX)
Localities and species recording resistance and the frequency.
(XLSX)