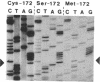
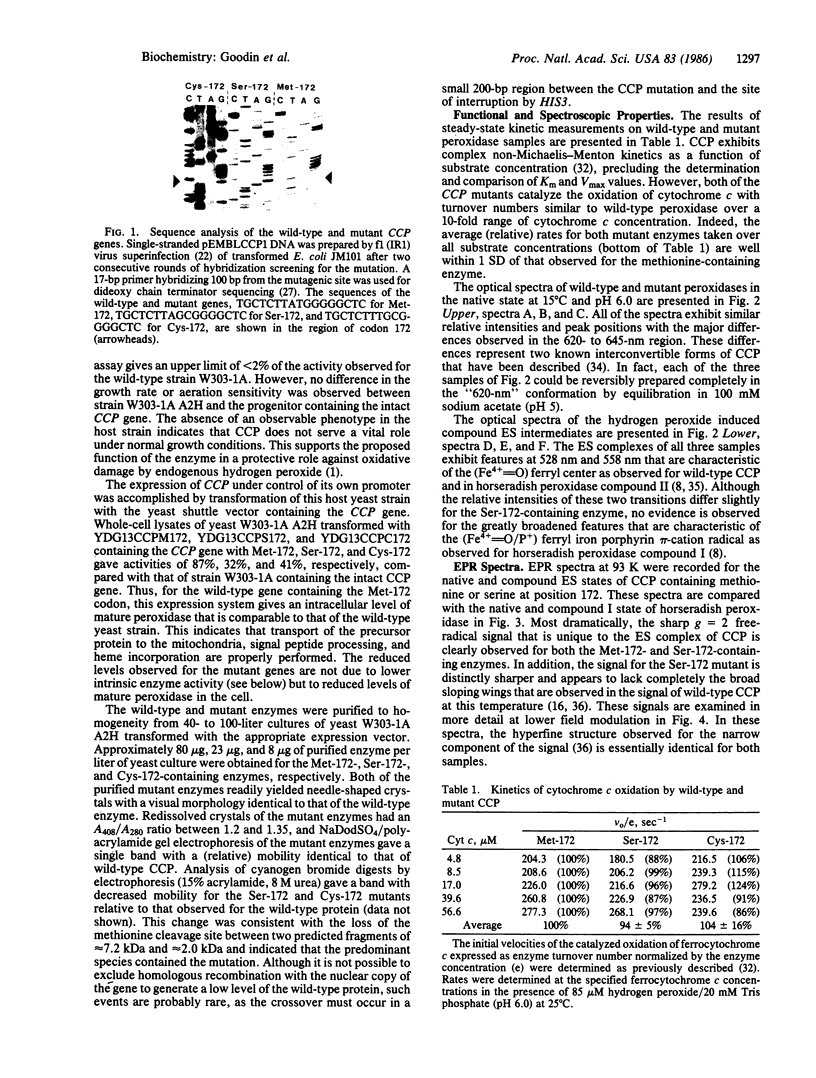
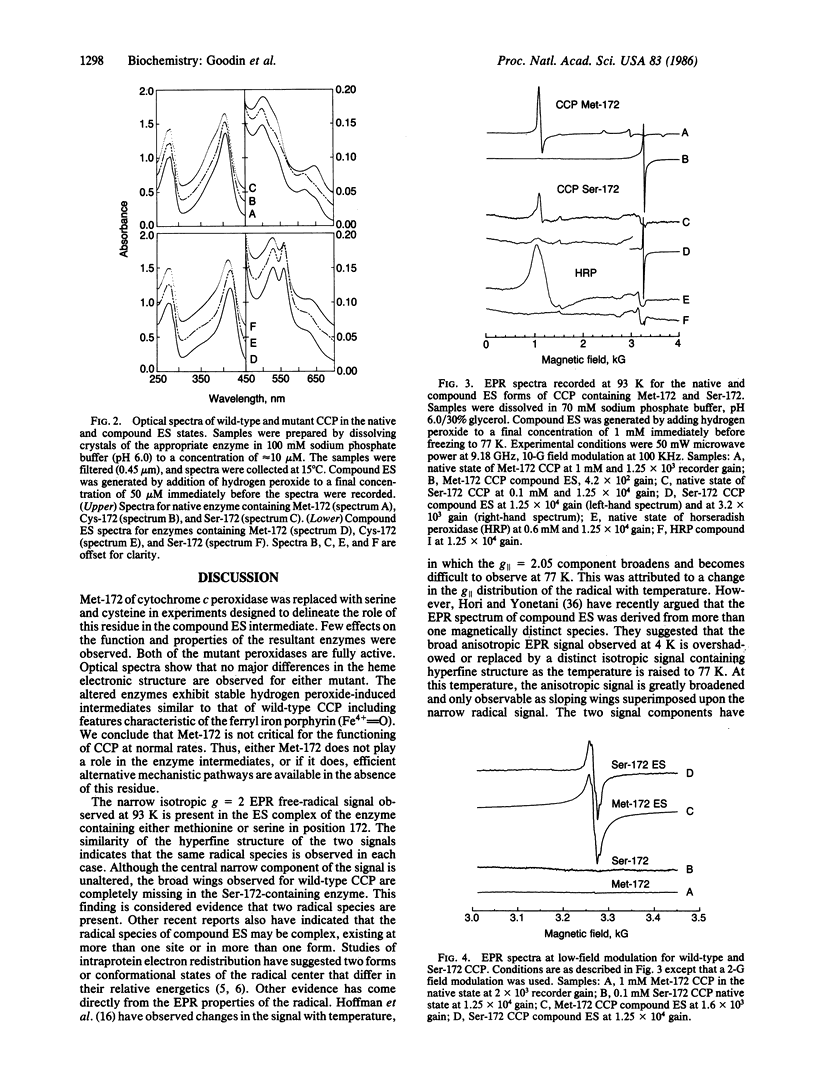
Abstract
Yeast cytochrome c peroxidase reacts with hydrogen peroxide to form an intermediate, compound ES, in which the heme iron atom is converted to a ferryl function (Fe4+ = O) and a radical center is generated on a reversibly oxidizable amino acid residue of uncertain identity. As methionine-172 is a possible site of this radical, we have constructed specific variants of cytochrome c peroxidase in which methionine-172 is replaced by serine or cysteine. These mutants and the wild-type enzyme have been expressed in Saccharomyces cerevisiae, purified, and crystallized. Both mutant enzymes are fully active. A stable, reversible, peroxide-induced intermediate with optical properties characteristic of compound ES is observed for the three forms of the enzyme. The electron paramagnetic resonance spectrum of this intermediate at 93 K for the serine mutant exhibits the narrow free-radical signal and hyperfine structure observed for the wild-type enzyme. However, a broader component of the signal that is observed for the wild-type enzyme at this temperature is absent from the spectrum observed with the serine mutant. These results demonstrate that the narrow component of the free-radical signal observed at 93 K cannot reside at methionine-172. The absence of the broader component of the signal for the serine mutant may reflect the loss of spin density on methionine or, alternatively, could arise from conformationally induced changes in the properties of the radical. The results are consistent with a heterogeneity of radical species in the ES complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Bill K., Broger C. Affinity chromatography purification of cytochrome c binding enzymes. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2447–2450. doi: 10.1073/pnas.79.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Coulson A. F., Yonetani T. Oxidation of cytochrome c peroxidase with hydrogen peroxide: identification of the "endogenous donor". Biochem Biophys Res Commun. 1972 Oct 17;49(2):391–398. doi: 10.1016/0006-291x(72)90423-8. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin D., Forman A., Borg D. C., Fajer J., Felton R. H. Compounds I of catalase and horse radish peroxidase: pi-cation radicals. Proc Natl Acad Sci U S A. 1971 Mar;68(3):614–618. doi: 10.1073/pnas.68.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzel B. C., Poulos T. L., Kraut J. Crystal structure of yeast cytochrome c peroxidase refined at 1.7-A resolution. J Biol Chem. 1984 Nov 10;259(21):13027–13036. [PubMed] [Google Scholar]
- Goltz S., Kaput J., Blobel G. Isolation of the yeast nuclear gene encoding the mitochondrial protein, cytochrome c peroxidase. J Biol Chem. 1982 Sep 25;257(18):11186–11190. [PubMed] [Google Scholar]
- Ho P. S., Hoffman B. M., Kang C. H., Margoliash E. Control of the transfer of oxidizing equivalents between heme iron and free radical site in yeast cytochrome c peroxidase. J Biol Chem. 1983 Apr 10;258(7):4356–4363. [PubMed] [Google Scholar]
- Ho P. S., Hoffman B. M., Solomon N., Kang C. H., Margoliash E. Kinetics and energetics of intramolecular electron transfer in yeast cytochrome c peroxidase. Biochemistry. 1984 Aug 28;23(18):4122–4128. doi: 10.1021/bi00313a017. [DOI] [PubMed] [Google Scholar]
- Hoffman B. M., Roberts J. E., Brown T. G., Kang C. H., Margoliash E. Electron-nuclear double resonance of the hydrogen peroxide compound of cytochrome c peroxidase: identification of the free radical site with a methionyl cluster. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6132–6136. doi: 10.1073/pnas.76.12.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B. M., Roberts J. E., Kang C. H., Margoliash E. Electron paramagnetic and electron nuclear double resonance of the hydrogen peroxide compound of cytochrome c peroxidase. J Biol Chem. 1981 Jul 10;256(13):6556–6564. [PubMed] [Google Scholar]
- Hori H., Yonetani T. Powder and single-crystal electron paramagnetic resonance studies of yeast cytochrome c peroxidase and its peroxide and its peroxide compound, Compound ES. J Biol Chem. 1985 Jan 10;260(1):349–355. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. S., Erman J. E. The cytochrome c peroxidase-catalyzed oxidation of ferrocytochrome c by hydrogen peroxide. Steady state kinetic mechanism. J Biol Chem. 1982 Nov 10;257(21):12775–12779. [PubMed] [Google Scholar]
- Kaput J., Goltz S., Blobel G. Nucleotide sequence of the yeast nuclear gene for cytochrome c peroxidase precursor. Functional implications of the pre sequence for protein transport into mitochondria. J Biol Chem. 1982 Dec 25;257(24):15054–15058. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch K., Mims W. B., Peisach J. Pulsed EPR studies of peroxide-activated cytochrome c peroxidase and of the mercaptoethanol derivative of Neurospora tyrosinase. J Biol Chem. 1981 Oct 10;256(19):10088–10091. [PubMed] [Google Scholar]
- Mathews R. A., Wittenberg J. B. Cytochrome c peroxidase. Interconversion of chemically and enzymatically reactive and unreactive forms of the ferric protein. J Biol Chem. 1979 Jul 10;254(13):5991–5996. [PubMed] [Google Scholar]
- Myers D., Palmer G. Magnetic circular dichroism studies on the heme and tryptophan components of cytochrome c peroxidase. J Biol Chem. 1985 Apr 10;260(7):3887–3890. [PubMed] [Google Scholar]
- Nelson C. E., Sitzman E. V., Kang C. H., Margoliash E. Preparation of cytochrome c peroxidase from baker's yeast. Anal Biochem. 1977 Dec;83(2):622–631. doi: 10.1016/0003-2697(77)90066-5. [DOI] [PubMed] [Google Scholar]
- Pielak G. J., Mauk A. G., Smith M. Site-directed mutagenesis of cytochrome c shows that an invariant Phe is not essential for function. Nature. 1985 Jan 10;313(5998):152–154. doi: 10.1038/313152a0. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Kraut J. A hypothetical model of the cytochrome c peroxidase . cytochrome c electron transfer complex. J Biol Chem. 1980 Nov 10;255(21):10322–10330. [PubMed] [Google Scholar]
- Poulos T. L., Kraut J. The stereochemistry of peroxidase catalysis. J Biol Chem. 1980 Sep 10;255(17):8199–8205. [PubMed] [Google Scholar]
- Roberts J. E., Hoffman B. M., Rutter R., Hager L. P. Electron-nuclear double resonance of horseradish peroxidase compound I. Detection of the porphyrin pi-cation radical. J Biol Chem. 1981 Mar 10;256(5):2118–2121. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg B. A., Kampa L., Wittenberg J. B., Blumberg W. E., Peisach J. The electronic structure of protoheme proteins. II. An electron paramagnetic resonance and optical study of cytochrome c peroxidase and its derivatives. J Biol Chem. 1968 Apr 25;243(8):1863–1870. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Ray G. S. Studies on cytochrome c peroxidase. I. Purification and some properties. J Biol Chem. 1965 Nov;240(11):4503–4508. [PubMed] [Google Scholar]
- Yonetani T., Schleyer H. Studies on cytochrome c peroxidase. VII. Electron paramagnetic resonance absorptions of the enzyme and complex ES in dissolved and crystalline forms. J Biol Chem. 1966 Jul 10;241(13):3240–3243. [PubMed] [Google Scholar]
- Yonetani T. Studies on cytochrome c peroxidase. II. Stoichiometry between enzyme, H2O2, and ferrocytochrome c and enzymic determination of extinction coefficients of cytochrome c. J Biol Chem. 1965 Nov;240(11):4509–4514. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]