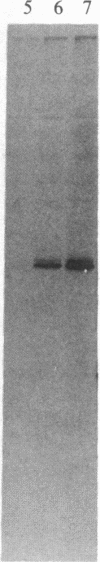
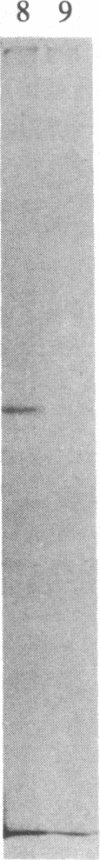
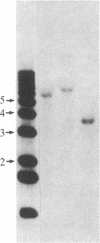
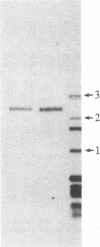
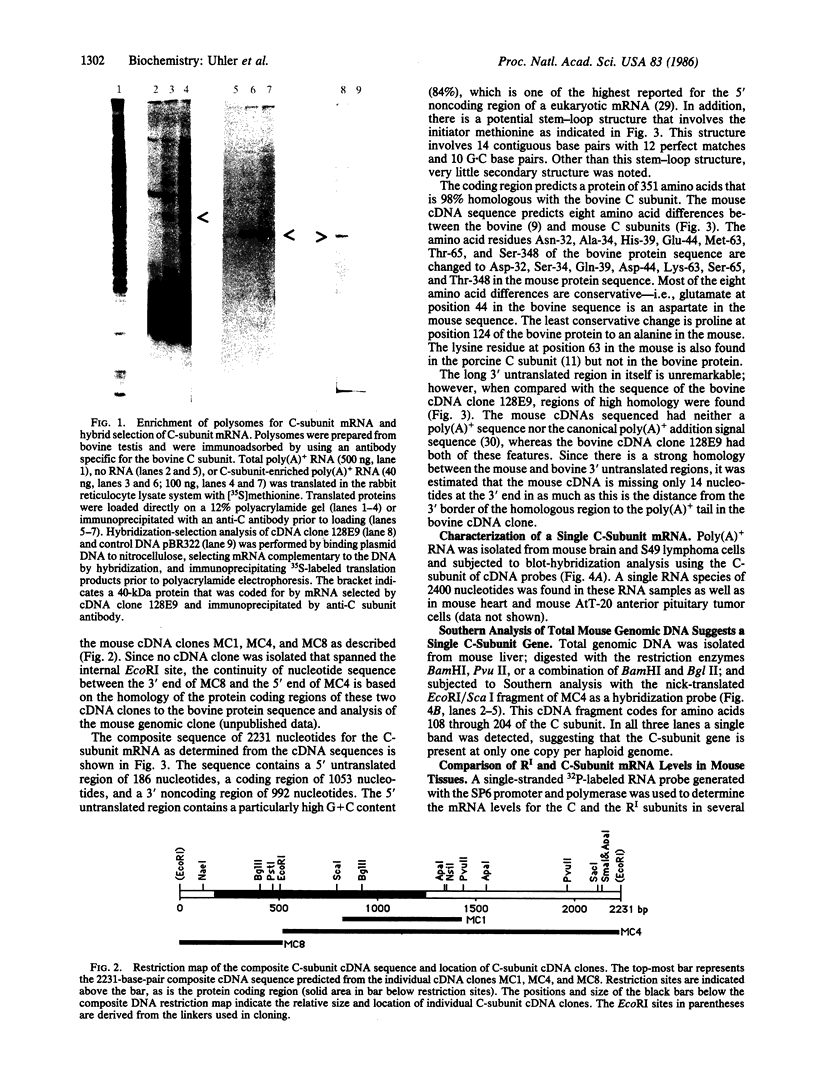
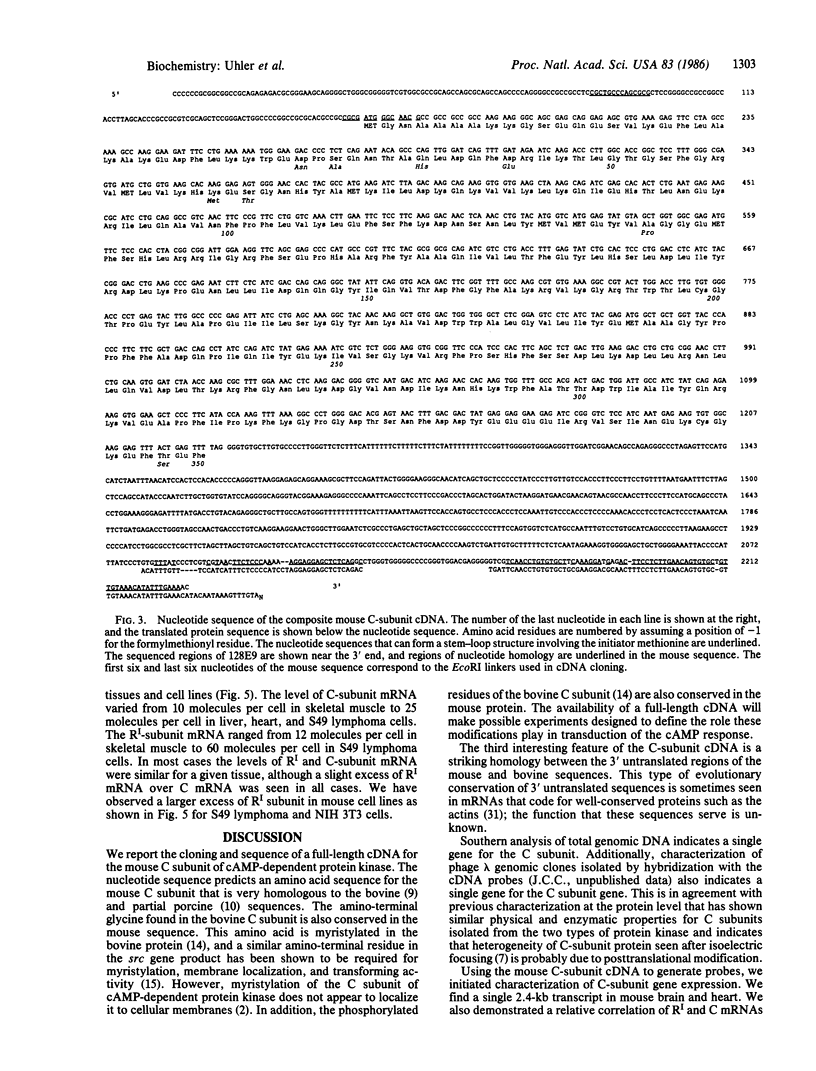
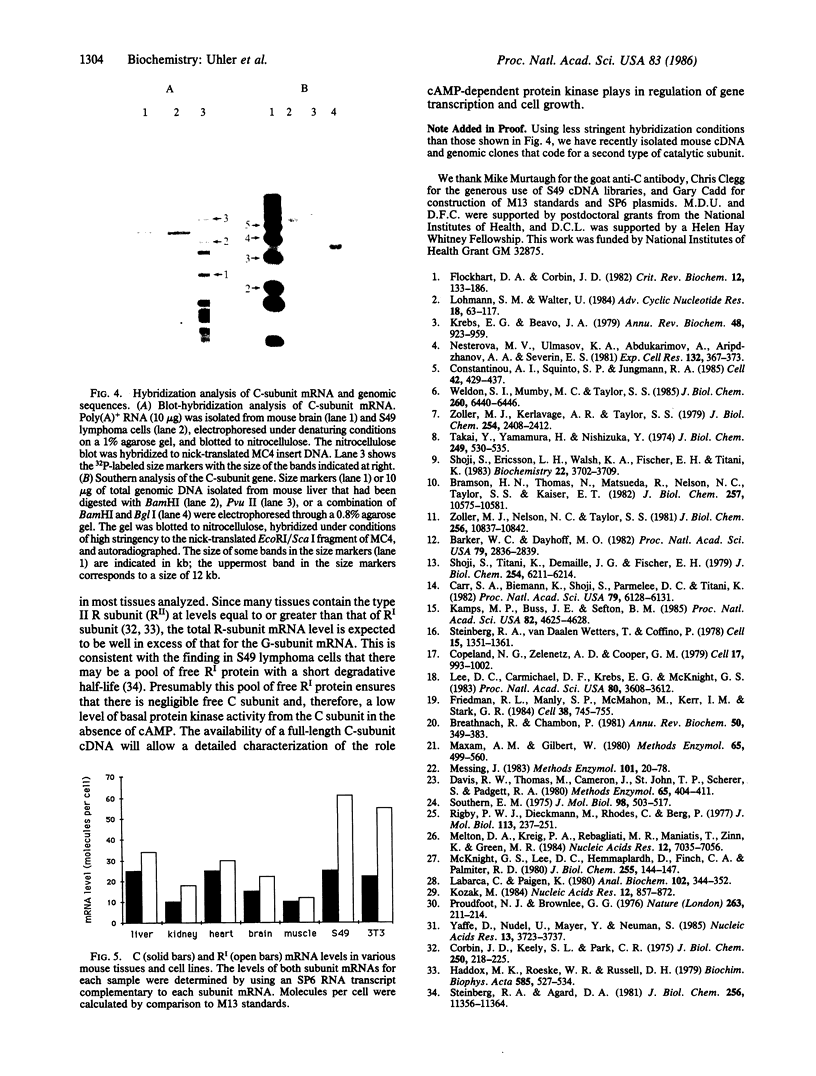
Abstract
mRNA coding for the catalytic (C) subunit of cAMP-dependent protein kinase (ATP: protein phosphotransferase, EC 2.7.1.37) was partially purified from bovine testis by polysome immunoadsorption and oligo(dT)-chromatography. This enriched mRNA preparation was used to prepare and differentially screen a cDNA library. One of the selected cDNA clones was shown to hybrid-select mRNA coding for a 40-kDa protein that was specifically precipitated with antibodies to the C subunit. This bovine cDNA clone was then used to isolate a series of mouse cDNA clones that are complementary to the entire mouse C subunit mRNA. The mouse clones code for a protein of 351 amino acids that shows 98% homology to the bovine C subunit and hybridize to a single mRNA of 2.4 kilobases in mouse heart and brain. Southern blot analysis of total genomic DNA suggests that there is a single mouse gene coding for the C subunit. mRNA levels for both the C subunit and the type I regulatory subunit in various mouse tissues and cell lines were quantitated and compared by using single-stranded RNA probes prepared with SP6 polymerase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker W. C., Dayhoff M. O. Viral src gene products are related to the catalytic chain of mammalian cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 May;79(9):2836–2839. doi: 10.1073/pnas.79.9.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramson H. N., Thomas N., Matsueda R., Nelson N. C., Taylor S. S., Kaiser E. T. Modification of the catalytic subunit of bovine heart cAMP-dependent protein kinase with affinity labels related to peptide substrates. J Biol Chem. 1982 Sep 25;257(18):10575–10581. [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou A. I., Squinto S. P., Jungmann R. A. The phosphoform of the regulatory subunit RII of cyclic AMP-dependent protein kinase possesses intrinsic topoisomerase activity. Cell. 1985 Sep;42(2):429–437. doi: 10.1016/0092-8674(85)90100-x. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Zelenetz A. D., Cooper G. M. Transformation of NIH/3T3 mouse cells by DNA of Rous sarcoma virus. Cell. 1979 Aug;17(4):993–1002. doi: 10.1016/0092-8674(79)90338-6. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Flockhart D. A., Corbin J. D. Regulatory mechanisms in the control of protein kinases. CRC Crit Rev Biochem. 1982 Feb;12(2):133–186. doi: 10.3109/10409238209108705. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Haddox M. K., Roeske W. R., Russell D. H. Independent expression of cardiac type I and II cyclic AMP-dependent protein kinase during murine embryogenesis and postnatal development. Biochim Biophys Acta. 1979 Jul 18;585(4):527–534. doi: 10.1016/0304-4165(79)90185-5. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lee D. C., Carmichael D. F., Krebs E. G., McKnight G. S. Isolation of a cDNA clone for the type I regulatory subunit of bovine cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3608–3612. doi: 10.1073/pnas.80.12.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann S. M., Walter U. Regulation of the cellular and subcellular concentrations and distribution of cyclic nucleotide-dependent protein kinases. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:63–117. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Lee D. C., Hemmaplardh D., Finch C. A., Palmiter R. D. Transferrin gene expression. Effects of nutritional iron deficiency. J Biol Chem. 1980 Jan 10;255(1):144–147. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nesterova M. V., Ulmasov K. A., Abdukarimov A., Aripdzhanov A. A., Severin E. S. Nuclear translocation of cAMP-dependent protein kinase. Exp Cell Res. 1981 Apr;132(2):367–373. doi: 10.1016/0014-4827(81)90112-9. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shoji S., Ericsson L. H., Walsh K. A., Fischer E. H., Titani K. Amino acid sequence of the catalytic subunit of bovine type II adenosine cyclic 3',5'-phosphate dependent protein kinase. Biochemistry. 1983 Jul 19;22(15):3702–3709. doi: 10.1021/bi00284a025. [DOI] [PubMed] [Google Scholar]
- Shoji S., Titani K., Demaille J. G., Fischer E. H. Sequence of two phosphorylated sites in the catalytic subunit of bovine cardiac muscle adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1979 Jul 25;254(14):6211–6214. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Agard D. A. Studies on the phosphorylation and synthesis of type I regulatory subunit of cyclic AMP-dependent protein kinase in intact S49 mouse lymphoma cells. J Biol Chem. 1981 Nov 10;256(21):11356–11364. [PubMed] [Google Scholar]
- Steinberg R. A., van Daalen Wetters T., Coffino P. Kinase-negative mutants of S49 mouse lymphoma cells carry a trans-dominant mutation affecting expression of cAMP-dependent protein kinase. Cell. 1978 Dec;15(4):1351–1361. doi: 10.1016/0092-8674(78)90060-0. [DOI] [PubMed] [Google Scholar]
- Takai Y., Yamamura H., Nishizuka Y. Adenosine 3':5'-monophosphate-dependent protein kinase from yeast. J Biol Chem. 1974 Jan 25;249(2):530–535. [PubMed] [Google Scholar]
- Weldon S. L., Mumby M. C., Taylor S. S. The regulatory subunit of neural cAMP-dependent protein kinase II represents a unique gene product. J Biol Chem. 1985 May 25;260(10):6440–6448. [PubMed] [Google Scholar]
- Yaffe D., Nudel U., Mayer Y., Neuman S. Highly conserved sequences in the 3' untranslated region of mRNAs coding for homologous proteins in distantly related species. Nucleic Acids Res. 1985 May 24;13(10):3723–3737. doi: 10.1093/nar/13.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Kerlavage A. R., Taylor S. S. Structural comparisons of cAMP-dependent protein kinases I and II from porcine skeletal muscle. J Biol Chem. 1979 Apr 10;254(7):2408–2412. [PubMed] [Google Scholar]
- Zoller M. J., Nelson N. C., Taylor S. S. Affinity labeling of cAMP-dependent protein kinase with p-fluorosulfonylbenzoyl adenosine. Covalent modification of lysine 71. J Biol Chem. 1981 Nov 10;256(21):10837–10842. [PubMed] [Google Scholar]