Abstract
Oxidative stress is a well-known etiologic factor in the development of cardiovascular disease. Oxidation of lipoproteins, and in particular of low density lipoprotein, is a necessary if not obligatory mechanism for the generation of macrophage-derived foam cells, the first major initiating factor in the development of an atherosclerotic plaque. Oxidation of lipoproteins does not result in the generation of a single, defined molecular species, but of a variety of oxidation-specific epitopes, such as oxidized phospholipids and malondialdehyde-lysine epitopes. Unique monoclonal antibodies have been developed to bind these well-defined epitopes, and have been used in in vitro assays to detect them on circulating lipoproteins present in plasma. This article will summarize the accumulating clinical data of one oxidation-specific biomarker, oxidized phospholipids (OxPL) on apoB-100 lipoproteins. Elevated levels of OxPL/apoB predict the presence and progression of coronary, femoral and carotid artery disease, are increased following acute coronary syndromes and percutaneous coronary intervention, and predict the development of death, myocardial infarction, stroke and need for revascularization in unselected populations. OxPL/apoB levels are independent of traditional risk factors and the metabolic syndrome, and enhance the risk prediction of the Framingham Risk Score. The OxPLs measured in this assay reflect the biological activity of the most atherogenic lipoprotein(a) (Lp(a)) particles, reflected in patients with high plasma Lp(a) levels with small apo(a) isoforms. The predictive value of OxPL/apoB is amplified by Lp(a) and phospholipases such as lipoprotein-associated phospholipase A2 and secretory phospholipase A2, which are targets of therapy in clinical trials. This assay has now been validated in over 10,000 patients and efforts are underway to make it available to the research and clinical communities.
Keywords: atherosclerosis, biomarker, lipoproteins, outcome, oxidation, oxidative stress, oxidized phospholipid
Oxidative stress is considered to be a key mechanism for the initiation and progression of atherosclerosis and the development of cardiovascular disease (CVD) [1,2]. Excessive oxidative stress occurs in response to many underlying cardiovascular risk factors, such as hypercholesterolemia, hypertension, diabetes mellitus and underlying genetic predisposition. Oxidative stress and subsequent oxidation of low-density lipoprotein (LDL) to produce oxidized (Ox)LDL is created through the reaction of reactive oxygen species, such as superoxide anion, and hydrogen peroxide and peroxynitrite. These oxidative species are generated during cellular metabolic pathways by lipoxygenases, myeloperoxidase, nitric oxide synthase, NADPH oxidase, xanthine oxidase and other oxidases, with polyunsaturated fatty acids, lipoproteins and amino acids, causing their modification to proinflammatory and atherogenic particles. One LDL particle is composed of approximately 600 molecules of free cholesterol, 1600 molecules of cholesteryl esters, 700 molecules of phospholipids and 185 molecules of triglycerides [3]. The polyunsaturated acyl chains of cholesteryl esters, polyunsaturated acyl and triglycerides are vulnerable to oxidation, as is the sterol of free cholesterol and cholesteryl esters. LDL contains one molecule of apoB-100, made up of 4536 amino acid residues with many exposed lysines, which can be directly oxidized or modified by lipid oxidation products [4]. Owing to its molecular composition, LDL is particularly susceptible to oxidation and OxLDL is considered among the strongest proinflammatory components of vulnerable plaques [5].
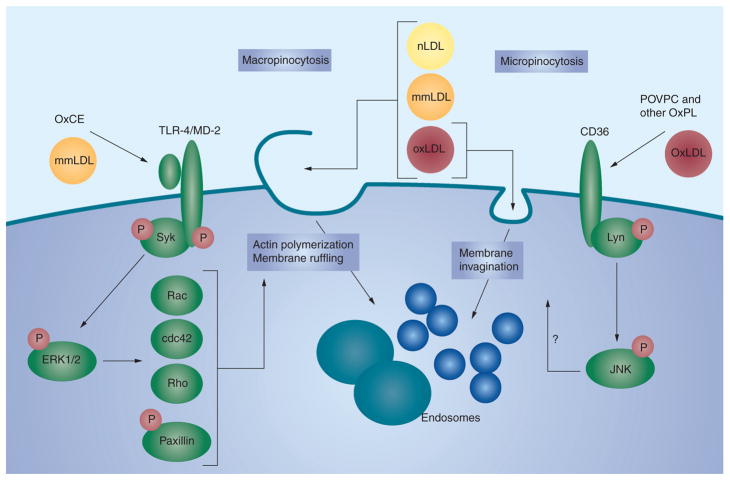
Oxidation of LDL leads to the generation of a variety of oxidation-specific epitopes (OSE), such as the oxidized phospholipid (OxPL) and malondialdehyde epitopes on LDL. Oxidation of LDL is thought to occur primarily in the vessel wall rather than in plasma, which is strongly enriched in antioxidants. These OSE are biologically active and upregulate adhesion molecules to attract monocytes into the vessel wall, mediate proinflammatory responses in cytokines and upregulatie proinflammatory genes, promote macrophage retention and apoptosis [6,7], and are cytotoxic. These OSE are proatherogenic owing to their ability to enhance the unregulated uptake of OxLDL in macrophages through specific pathways generating activated macrophage foam cells (Figure 1). The accumulation of foam cells leads to fatty streak formation. Foam cell necrosis and/or apoptosis and continued accumulation of oxidized lipids in the extracellular space eventually lead to atheroma formation.
Figure 1. Oxidized lipid moieties induce lipoprotein accumulation in macrophages.
Macrophage lipoprotein uptake mechanisms can be separated into: macropinocytosis, when actin polymerization and extensive membrane ruffling result in the ruffles closing into large endosomes and capture of large volumes of extracellular material, including all classes of native and OxLDL present in the vicinity of the cell; and micropinocytosis, when ligand–receptor binding leads to membrane invagination and nearly stoichiometric internalization of the ligand or the lipoprotein carrying this ligand. mmLDL and polyoxygenated OxCEs induce Syk recruitment to TLR-4, Syk and TLR-4 phosphorylation and subsequent ERK1/2-dependent activation of small GTPases Rac, cdc42 and Rho and phosphorylation of paxillin, leading to actin reorganization and membrane ruffling. Resulting macropinocytosis promotes foam cell formation [74]. Binding of OxLDL or OxPL to CD36 initiates Lyn-dependent phosphorylation of JNK, which is essential for CD36-mediated OxLDL uptake, although the mechanism linking JNK with the membrane dynamics is unclear [75]. The TLR-4- and CD36-mediated uptake mechanisms are only examples; there are numerous other pattern recognition receptors involved in oxidation-specific epitope-stimulated lipoprotein internalization by macrophages.
mmLDL: Minimally modified low-density lipoprotein; nLDL: Native low-density lipoprotein; OxCE: Oxidized cholesterol ester; OxLDL: Oxidized low-density lipoprotein; OxPL: Oxidized phospholipid; POVPC: 1-palmitoyl-2-oxovaleroyl-sn-glycero-3-phosphorylcholine; TLR: Toll-like receptor.
Adapted with permission from [26].
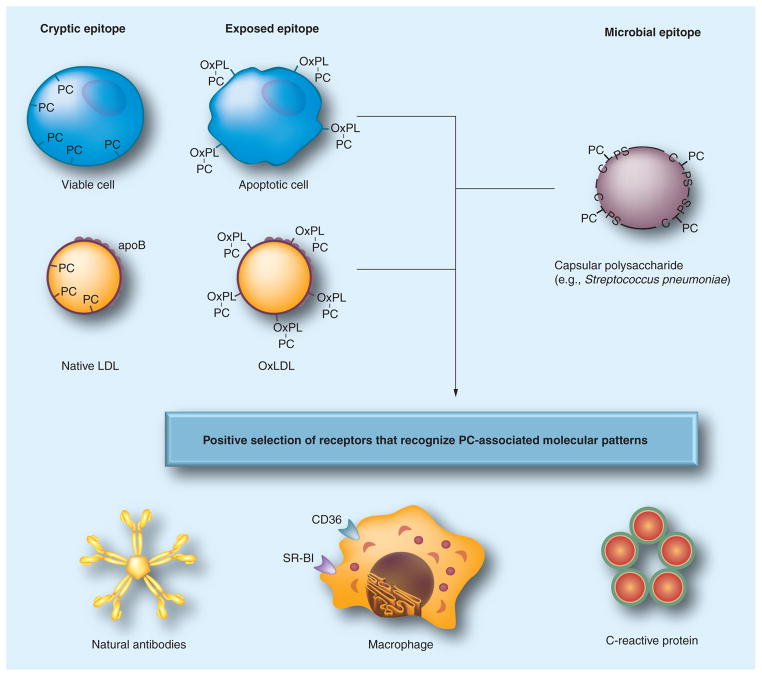
Oxidation-specific epitopes are also potent immunogens and lead to activation of T cells and B cells, resulting in the generation of autoantibodies to specific epitopes, which have been described in both humans and animals [8–10]. In mice, natural antibodies, secreted from OxLDL-specific B-1 cell lines, bind to OSE, block uptake of OxLDL by macrophages, recognize apoptotic cells, and are deposited in atherosclerotic lesions, suggesting a role of the innate immunity system in protecting hosts against these proinflammatory antigens (Figure 2).
Figure 2. Pattern recognition of oxidation-specific danger-associated molecular patterns and microbial pathogen-associated molecular patterns.
Using the example of the PC epitope, in this illustration, we demonstrate our hypothesis of the emergence and positive selection of multiple pattern recognition receptors (PRRs) that recognize common epitopes, shared by modified self- and microbial pathogens. According to this hypothesis, oxidation of plasma membrane phospholipids in apoptotic cells alters the conformation of the PC head group, yielding an exposed epitope, accessible to recognition by macrophage scavenger receptors, natural antibodies and pentraxins, such as C-reactive protein. These PRRs were selected to clear apoptotic cells from developing or regenerating tissues. Recognition by the same receptors of the PC epitope of capsular polysaccharide in Gram-positive bacteria (e.g., Streptococcus pneumoniae) strengthened positive selection of these PRRs and probably helped to select additional strong proinflammatory components to PRR-dependent responses. (Note that the PC on the bacteria is not part of a phospholipid.) Finally, oxidized lipoproteins, prevalent in experimental animals and humans as a result of enhanced oxidative stress, dyslipidemia and impact of environmental factors, bear OxPLs with the PC epitope exposed in an analogous manner to that of apoptotic cells. This leads to OxLDL recognition by PRRs and initiation of innate immune responses. The balance between proinflammatory responses of cellular PRRs and atheroprotective roles of natural antibodies plays an important role in the development of atherosclerosis. There are likely many more oxidation-specific epitopes that represent such danger-associated molecular patterns and corresponding PRRs that represent respective innate responses.
C-PS: Cell wall polysaccharide; OxPL: Oxidized phospholipid; PC: Phosphocholine.
Adapted with permission from [26].
Oxidized phospholipids play an important role in atherosclerosis and accumulate in human and mouse lesions. Specific OxPLs have been identified as major regulators of many cell types present in the vessel wall, including endothelial cells, smooth muscle cells, macrophages, dendritic cells and platelets [11,12]. Furthermore, several receptors and signaling pathways associated with OxPL action have been identified and demonstrated to be upregulated in human lesions [12,13]. OxPLs mediate plaque destabilization, being present in higher quantities (70-fold) in plaque than plasma [5,14]. OxPL are key components of OxLDL, apoptotic cells and atherosclerotic lesions, and are important contributors to early events in atherogenesis by activating proinflammatory genes, leading to inflammatory cascades in the vessel wall [11,12], which reflect features of chronic inflammatory disease [1,15].
We have cloned a series of immuno-dominant IgM antibodies binding to OxLDL from apoE−/− mice [10,16,17]. E06 is a well-characterized murine monoclonal antibody that binds to the phosphocholine (PC) head group of oxidized but not native phospholipids [18]. E06 is encoded by non-mutated germline genes and is 100% identical in the variable region of the heavy-chain and κ-light-chains sequences of the T15 natural antibody, which provides the optimal protection to mice against lethal infection with Streptococcus pneumoniae [19]. E06/T15 binds to PC exposed on OxPLs on Cu-OxLDL, as well as OxPL present on apoptotic cells, but also to PC coupled to techoic/lipotechoic acid on the cell wall of bacteria such as S. pneumoniae. Indeed, E06 recognizes OxPL on an equimolar basis when simply present as a PC salt or as PC on OxPL such as 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-PC attached to a variety of different peptides, as well as PC on OxPL covalently linked (via its sn-2 oxidized side chain) to a variety of synthetic peptides irrespective of amino acid sequence [18]. E06 inhibits OxLDL uptake by macrophages, preventing recognition by scavenger receptors, and inhibits a number of other proinflammatory properties of OxPL generated via acute lung injury and infections [20,21]. E06 also exhibits other important biological functions, such as inhibition of uptake of apoptotic cells by macrophages in vitro [17,22], but may promote complement-mediated enhanced clearance of apoptotic cells in vivo, as has been demonstrated for IgM antibodies [23]. Interestingly, pneumococcal vaccination of cholesterol-fed LDLR−/− mice increased the T15/E06 titers and, most strikingly, reduced the progression of atherosclerosis [24,25]. Overall, these data suggest that PC-based OxPL represents a danger-associated molecular pattern and that effector molecules, such as IgM natural antibodies (e.g., antibody E06/T15), scavenger receptors (e.g., CD36 and SR-B1) and C-reactive protein of the innate immune system evolved to bind and potentially neutralize them [26].
In the next section we provide a detailed summary of the OxPL/apoB assay in various applications in CVD. Other assays to measure the presence of OSEs on lipoprotein particles exist, and the interested reader is referred to recent articles on this topic that review the advantages and limitations of each assay [27–29].
OxPL/apoB methodology
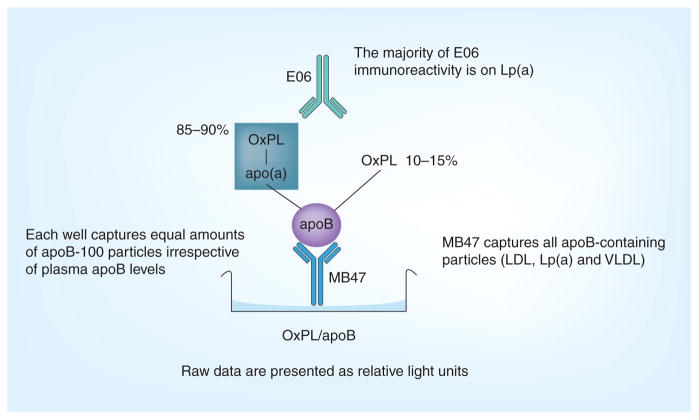
Using E06, we have developed a chemiluminescent ELISA to detect OxPL on human apoB-100 (OxPL/apoB)-containing lipoprotein particles in plasma. This well-validated assay has been previously described in detail [27,28,30,31]. The assay is performed by plating overnight murine monoclonal antibody MB47 (50 μl at 5 μg/ml) [29,32], which under the conditions used, captures a saturating amount of apoB-100 on all apoB-containing lipoproteins from plasma. After washing, the plates are coated with 1% bovine serum albumin in tris-(hydroxymethyl) aminomethane-buffered saline. Plasma (50 μl at 1:50 dilution) is added and allowed to incubate for 75 min. This initial step is designed so that each well captures a constant, saturating amount of apoB-100 from plasma and therefore normalizes the OxPL measure to an equal amount of apoB in the well for each patient. Thus, by definition, the OxPL/apoB measurement is independent of apoB and LDL cholesterol levels. Biotinylated E06 (1 μg/ml) is added and allowed to incubate for 1 h. Alkaline phosphatase neutravidin (1:40,000 dilution) is added for 1 h. Lumi-Phos® 530 (Lumigen, Inc., Southfield, MI, USA; 25 μl) is added for 75 min to detect OxPL per unit of apoB captured (e.g., OxPL in relative light units [RLU]/apoB). Since an equal amount of apoB-100 is captured in each well from each subject, the denominator is the same in all wells (e.g., 1) and, thus, the actual read out is the amount of OxPL as detected by E06. This is detected by chemiluminescent technique and reported in RLU in 100 ms (Figure 3). The OxPL/apoB assay is highly specific to the number of OxPL epitopes on individual apoB-100 particles, but does not measure the total amount of OxPL in plasma.
Figure 3. The oxidized phospholipid/apoB assay.
Microtiter well plates are coated with the murine antibody MB47 and plasma added to bind apoB-100 particles. OxPL on apoB-100 are then detected with biotinylated murine monoclonal antibody E06.
LDL: Low-density lipoprotein; Lp(a): Lipoprotein(a); OxPL: Oxidized phospholipid; VLDL: Very low-density lipoprotein.
In an early version of this assay prior to 2006, parallel plates were used to document that the wells captured equal amounts of apoB by first capturing apoB with MB47 and then adding the biotinylated murine monoclonal antibody MB24, which detects a separate apoB epitope, to quantitate the amount of captured apoB. The numerator represented OxPL on apoB detected by E06 (in RLU) and the denominator represented the amount of apoB (in RLU) detected on a parallel plate (i.e., E06 in RLU/apoB in RLU = OxPL:apoB ratio, without units). Subsequently, we demonstrated that the correlation between OxPL/apoB RLU vs OxPL/apoB ratio was r = 0.99 in more than 1500 samples [28]. Therefore, this additional step is not performed routinely in current human studies and the data are presented as OxPL/apoB in RLU, measured as the E06 binding only. In the near future, we will report the values as moles of oxidized PC, as we have generated a standard curve using a small peptide with a known amount of OxPL to equate the amount of OxPL detected by E06 as moles PC. For the data reported thus far, one can compare relative changes or values within assays, but not absolute values among different studies. To minimize variability we have generally performed all the samples in one batch with high and low OxPL standards on each plate. Our coefficient of variability has been 5–10%, which should improve further with the standard curve.
Table 1 displays the various studies performed with this assay prior to 2006. The OxPL/apoB assay is reported as OxPL/apoB ratio prior to 2006 and as OxPL/apoB RLU following 2006.
Table 1.
Clinical studies examining the role of oxidized phospholipids/apoB in cardiovascular disease.
| Study | Year | Study name | Patient population | Number of patients/samples | Outcome: change in OxPL/apoB levels | Ref. |
|---|---|---|---|---|---|---|
| Wu et al. | 1999 | – | Borderline hypertension | 146/146 | Increased in borderline hypertension and may reflect early vascular changes | [68] |
| Penny et al. | 2001 | UCSD regression study | Hypercholesterolemic patients undergoing quantitative angiography before/after lipid-lowering therapy | 29/54 | Related significantly to the severity of endothelial dysfunction and was the single most powerful independent risk factor | [69] |
| Tsimikas et al. | 2003 | ACS | Acute coronary syndromes Acute MI, unstable angina, stable CAD |
66/272 | Increased after acute MI | [27] |
| Tsimikas et al. | 2004 | Toronto PCI | Patients with stable angina pectoris undergoing PCI | 141/1269 | Increased immediately after PCI and return to baseline after 6 h | [33] |
| Segev et al. | 2005 | No relationship to restenosis | [70] | |||
| Tsimikas et al. | 2004 | MIRACL | Impact of atorvastatin in ACS | 2341/4682 | Increased 9.6% with atorvastatin 80 mg/day | [71] |
| Fraley et al. | 2009 | Baseline levels varied according to specific CVD risk factors and were largely independent of inflammatory biomarkers | [72] | |||
| Silaste et al. | 2004 | – | Healthy young women | 37/74 | Increased 19–27% with low-fat, high-vegetable diet | [62] |
| Tsimikas et al. | 2005 | Mayo | Coronary angiography | 504/504 | Strong and graded association with presence and extent of CAD | [48] |
| Tsimikas et al. | 2006 | Bruneck | Random sample of population (40–79 year old males and females) | 765/1436 | Predict presence and progression of carotid and femoral atherosclerosis | [28] |
| Kiechl et al. | 2007 | Predict 10-year CVD event rates independently of traditional risk factors, hsCRP and FRS | [44] | |||
| Rodenburg et al. | 2006 | – | Children with familial hypercholesterolemia and unaffected siblings | 256/512 | Increase 29% with Step II AHA diet Increase 49% with pravastatin 40 mg/day |
[61] |
| Bossola et al. | 2007 | – | End-stage renal failure patients undergoing chronic hemodialysis | 52/104 | Reduced in end-stage renal failure patients following hemodialysis | [73] |
| Ky et al. | 2008 | PROXI | Hypercholesterolemic patients were randomized to different types and doses of statin | 120/240 | Increased 26% with pravastatin 40 mg/day and 20% with atorvastatin 80 mg/day | [60] |
| Choi et al. | 2008 | REVERSAL | Patients with CAD who underwent coronary IVUS and were assigned to statin therapy | 214/428 | Increased 48% with atorvastatin 80 mg/day and 39% with pravastatin 40 mg/day | [65] |
| Tsimikas et al. | 2009 | Dallas Heart Study | Multiethnic, probability-based sample of the Dallas County population | 3481/3481 | Varied according to race/ethnicity, independent of cardiovascular risk factors and were inversely associated with apo(a) isoform size | [49] |
| Tsimikas et al. | 2010 | EPIC-Norfolk Study | 45–79-years-old healthy males and females followed for 6 years | 2160/2160 | The highest tertiles are associated with higher risk of CAD events | [47] |
| Budoff et al. | 2009 | Garlic study | Asymptomatic patients with CAD treated with aged garlic extract plus supplement followed with coronary artery calcium scan | 60/120 | Increase with aged garlic extract predicts lack of coronary artery calcium progression | [63] |
| Ahmadi et al. | 2010 | Increase with aged garlic extract correlates with improvement in vascular function | [58] | |||
| Arai et al. | 2010 | I4399M SNP | Carriers and noncarriers of I4399M single nucleotide LPA polymorphism | 174/174 | Elevated in carriers compared with noncarriers, while patients with small apo(a) isoforms had the highest OxPL/apoB levels | [57] |
| Faghihnia et al. | 2010 | CHORI | Healthy subjects consuming a high-fat low-carbohydrate diet and a low-fat high-carbohydrate diet | 63/126 | OxPL/apoB and OxPL/apo(a) are increased by a low-fat high-carbohydrate diet | [64] |
| Total: 21 | – | – | – | 10609/15782 | – | – |
ACS: Acute coronary syndrome; AHA: American Heart Association; CAD: Coronary artery disease; CHORI: Children’s Hospital Oakland Research Institute; CVD: Cardiovascular disease; EPIC: European Prospective Investigation of Cancer; FRS: Framingham Risk Score; hsCRP: High-sensitivity C-reactive protein; IVUS: Intravascular ultrasound; MI: Myocardial infarction; MIRACL: Myocardial Ischemia Reduction With Aggressive Cholesterol Lowering; OxPL: Oxidized phospholipid; PCI: Percutaneous coronary intervention; PROXI: Pravastatin and Atorvastatin on Markers of Oxidative Stress in Hypercholesterolemic Humans; REVERSAL: Reversal of Atherosclerosis with Aggressive Lipid Lowering; SNP: Single nucleotide polymorphism; UCSD: University of California at San Diego.
Relationship of OxPL/apoB & Lp(a): Lp(a) as a preferential carrier of E06-detectable OxPL
Several early clinical studies documented, unexpectedly, that OxPL/apoB correlated strongly with Lp(a) [27,33], and not with LDL as expected [31]. Lp(a), which is secreted from the liver, is an independent, causal, genetic risk factor for cardiovascular death and myocardial infarction, and this risk is continuous and linear with increasing Lp(a) levels [34–41]. A physiological role of Lp(a) and the underlying mechanisms through which it contributes to CVD are still unknown. However, we have shown that Lp(a) preferentially binds OxPL, compared with other lipoproteins [42,43], and have proposed that a unique physiological role of Lp(a) may be to bind and transport proinflammatory OxPL in plasma. This would suggest that a sufficient and low level of Lp(a) is beneficial. Indeed, a J-shaped curve relates Lp(a) levels to CVD [44,45], suggesting that a small amount of Lp(a) (2–7 mg/dl) is associated with reduced CVD risk, but that higher levels are associated with increased risk (>25 mg/dl). The OxPL content may also explain this pathophysiological role [31]. When present at high plasma concentrations, Lp(a) would be more atherogenic than native LDL, as it binds with increased affinity to arterial intimal proteoglycans [46] resulting in increased intimal concentration of LDL along with associated proinflammatory OxPL. This hypothesis is now supported by several levels of evidence, including the correlation between OxPL/apoB and Lp(a) in multiple clinical studies [30,44,47]; the presence of OxPL on apo(a) and Lp(a), as detected by a variety of biochemical, immuno-precipitation and ultracentrifugation experiments, which demonstrate that approximately 85% of E06 reactivity (i.e., OxPL) coimmunoprecipitated with Lp(a) [42,43]; in vitro transfer studies demonstrating that OxPL from OxLDL are preferentially transferred to Lp(a) rather than LDL in a time-/temperature-dependent fashion [42]; extraction of purified human Lp(a) with organic solvents followed by liquid chromatogrphy tandem mass spectrometry studies showing that 30–70% of OxPL, both E06-detectable and E06-nondetectable, are extractable; lack of evidence of oxidation of Lp(a) itself (e.g., the lack of malondialdehyde epitopes) [42]; large clinical studies showing CVD event prediction by elevated baseline levels of OxPL/apoB, particularly those with small isoforms [28,42,44,47–49]; and accentuation of CVD risk and event prediction by OxPL/apoB with either lipoprotein-associated phospholipase A2 (Lp-PLA2) or secretory-PLA2 (sPLA2), suggesting an additive effect of substrate (OxPL) and enzyme activity of phospholipases [44,47].
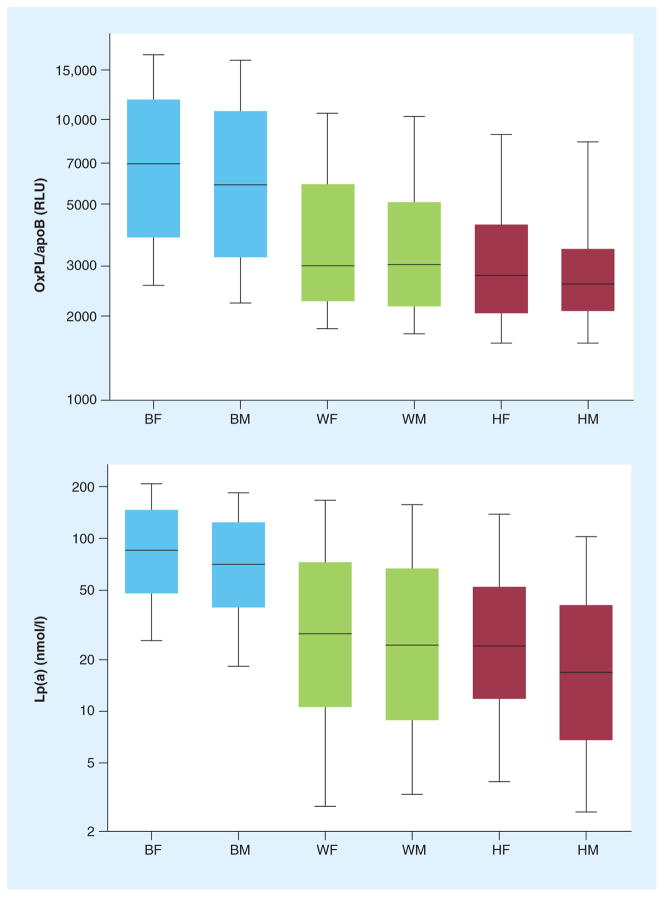
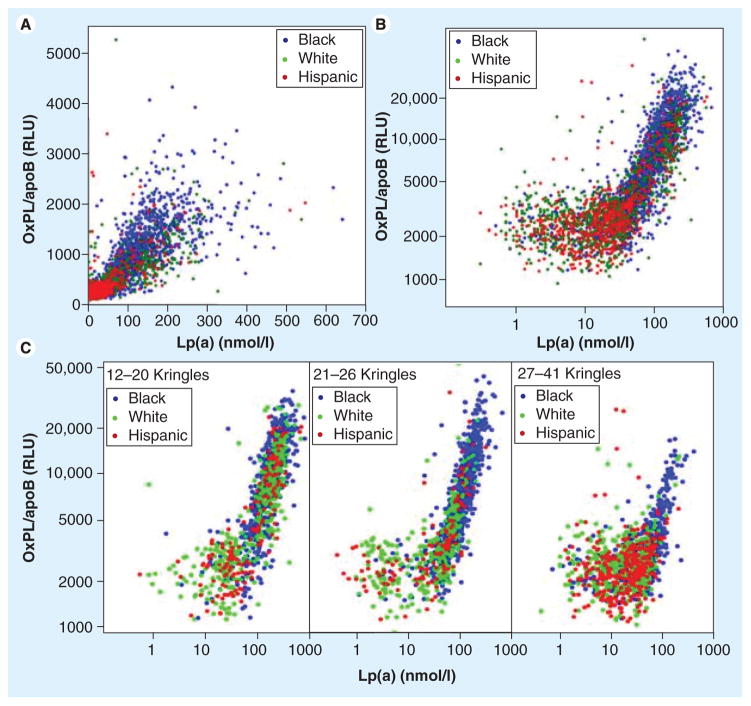
Oxidized phospholipid/apoB levels were measured in 3481 subjects (1831 black, 1047 white and 603 Hispanic) in the Dallas Heart Study, where it was demonstrated that they were highest in black people followed by white and Hispanic people (p < 0.001 for each comparison) (Figure 4). OxPL/apoB levels did not correlate significantly with cardiovascular risk factors, age or gender. However, OxPL/apoB levels strongly correlated with Lp(a) (r = 0.85, p < 0.001), with the correlation showing a ‘reverse L’ shape when values were log-transformed (Figure 5). In this relationship, there was no correlation between OxPL/apoB and Lp(a) at Lp(a) levels <30 nmol/l (~10 mg/dl), but a very strong correlation above this threshold. Within racial groups, the highest r-values were highest in black individuals, then white and the Hispanic individuals. The OxPL-Lp(a) correlation was highly dependent on underlying apo(a) isoform size, with strong correlations in subjects with small apo(a) isoforms (number of kringle type-IV repeats) that became progressively weaker or absent with larger apo(a) isoforms. Interestingly, there was a negative association between the size of the major apo(a) isoform and OxPL/apoB (r = −0.50, p < 0.001) (Table 2), irrespective of racial group. The relationship between OxPL/apoB and Lp(a) remained significant (r = 0.67, p < 0.001) after adjusting for apo(a) isoform size. In summary, this suggests that the OxPL/apoB levels reflect the most atherogenic Lp(a) particles, irrespective of race, and may allow clinical selection of risk profiles above and beyond measuring Lp(a) levels, as recently shown in the European Prospective Investigation of Cancer (EPIC)-Norfolk study [47]. Although basal Lp(a) levels are mainly genetically determined, modest changes above this pre-set baseline do occur in a number of situations, such as in acute phase responses [34], low fat diet [50,51] and statin therapy [52]. As Lp(a) is a lipoprotein carrier of OxPL, it is likely that the OxPL/apoB levels are also genetically determined to some extent, but in certain situations these values do change from baseline. Studies in twins are underway to examine the strength of the heritability of this relationship.
Figure 4. Levels of oxidized phospholipid/apoB and lipoprotein(a) categorized by racial group.
Boxes indicate medians, 25th and 75th percentile, whiskers indicate 10th and 90th percentile. Differences among racial groups are all significant (p < 0.001).
BF: Black females; BM: Black males; HF: Hispanic females; HM: Hispanic males; Lp(a): Lipoprotein(a); OxPL: Oxidized phospholipid; RLU: Relative light units; WF: White females; WM: White males.
Adapted with permission from [49].
Figure 5. Correlation between oxidized phospholipid/apoB and Lipoprotein(a) in the Dallas Heart Study.
(A) Relationship plotted on a geometric scale. (B) Relationship plotted on a logarithmic scale. (C) Relationship in the entire cohort according to apo(a) isoform sizes.
Lp(a): Lipoprotein(a); OxPL: Oxidized phospholipid; RLU: Relative light units.
Adapted with permission from [49].
Table 2.
Spearman correlation (r-values) between lipoprotein(a) and oxidized phospholipid/apoB by race and sex.
| Correlation | All | BF | BM | WF | WM | HF | HM |
|---|---|---|---|---|---|---|---|
| Correlation between Lp(a) and OxPL/apoB by race and sex | |||||||
| Lp(a) vs OxPL/apoB | 0.84* | 0.87* | 0.87* | 0.72* | 0.68* | 0.69* | 0.53* |
| Correlation between major apo(a) allele and OxPL/apoB by race and sex | |||||||
| apo(a) vs OxPL/apoB | −0.50* | −0.47* | −0.48* | −0.46* | −0.46* | −0.50* | −0.32* |
| Correlation between Lp(a) and OxPL/apoB by race and sex stratified by number of apo(a) isoforms in the major allele | |||||||
| 12–20 | 0.85* | 0.81* | 0.84* | 0.84* | 0.85* | 0.85* | 0.80* |
| 21–26 | 0.88* | 0.86* | 0.85* | 0.74* | 0.62* | 0.80* | 0.69* |
| 27–41 | 0.47* | 0.67* | 0.71* | 0.16** | 0.13** | 0.38* | 0.25* |
p < 0.001.
p < 0.05.
BF: Black females; BM: Black males; HF: Hispanic females; HM: Hispanic males; Lp(a): Lipoprotein(a); OxPL: Oxidized phospholipid; WF: White females; WM: White males.
Data taken from [49].
Relationship of OxPL/apoB & CVD
Association with acute coronary syndromes & percutaneous coronary intervention
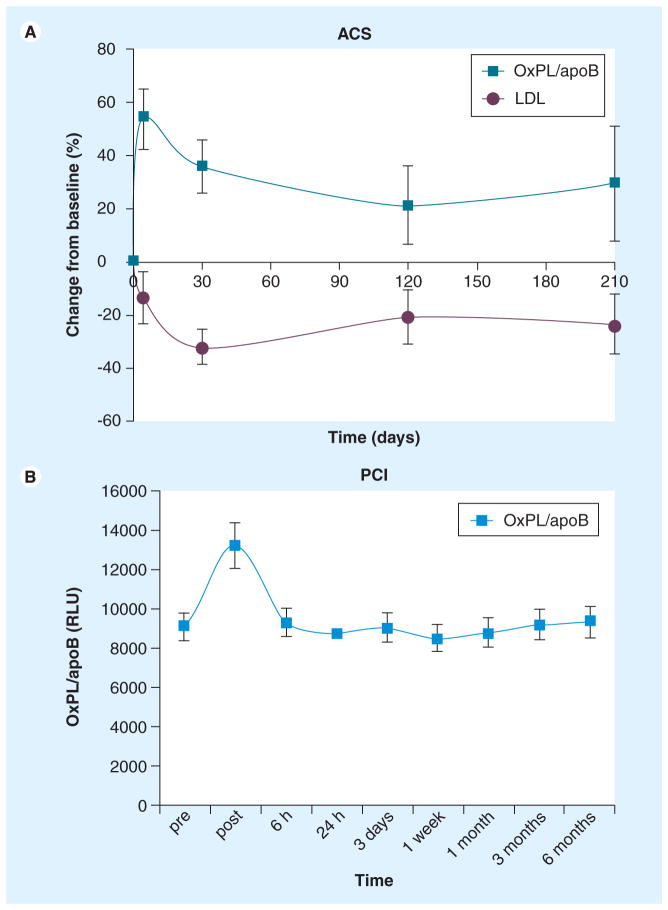
Acute coronary syndromes are associated with increased oxidative stress [53–55]. In two studies, it was demonstrated that acute increases occur in OxPL/apoB in patients following acute coronary syndrome [27] or during uncomplicated percutaneous coronary intervention [33], suggesting generation and/or release of oxidized lipids into the circulation from atherosclerotic lesions. In a prospective study in patients with acute coronary syndrome [27], it was demonstrated that OxPL/apoB levels rise rapidly by approximately 54% after an acute myocardial infarction and then tend to decrease toward baseline levels over the next 7 months (Figure 6a). By contrast, no significant changes were noted in patients with stable coronary artery disease (CAD), patients with normal coronary angiograms and a control group of healthy subjects followed for the same period of time. Further supporting this finding, a follow-up study demonstrated that OxPL/apoB and Lp(a) levels also acutely increased (by 36%; p < 0.0001) (Figure 6b) and 64%; p < 0.0001, respectively) immediately following percutaneous coronary intervention and then returned to baseline by 6 h [33]. Immunoprecipitation experiments showed that approximately 50% of OxPLs were present on Lp(a) immediately after percutaneous coronary intervention, whereas the rest were present on non-Lp(a) apoB-100 particles. However, by 6 h more than 90% of OxPL were again present on Lp(a). This supports the role of Lp(a) as a preferential binder and transporter of OxPL [42].
Figure 6. Change in oxidized phospholipid/apoB following acute coronary syndromes and uncomplicated percutaneous coronary intervention.
(A) Percent change from baseline in OxPL/apoB measured by antibody E06 in patients with ACS. The p-values at the 30-, 120- and 210-day labels represent differences between groups at each time point. Changes in LDL cholesterol are given for comparison. (B) Absolute changes in RLU in oxidized phospholipid/apoB after PCI. p < 0.001 compared with other time points.
ACS: Acute coronary syndrome; LDL: Low-density lipoprotein; OxPL: Oxidized phospholipid; PCI: Percutaneous coronary intervention; RLU: Relative light units.
Association with CAD
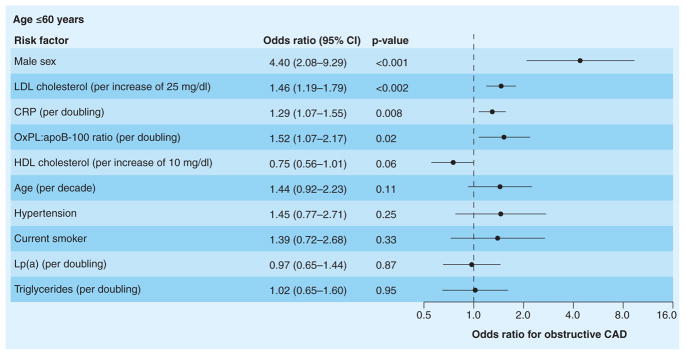
It was demonstrated in 504 patients undergoing clinically indicated coronary angiography that elevated levels of OxPL/apoB were strongly and independently correlated with the presence and extent of angiographically documented CAD, defined by >50% diameter stenosis and measured as one-, two- or three-vessel CAD, particularly in patients 60 years of age or younger [48]. In patients <60 years old, the highest quartile of OxPL/apoB was associated with an odds ratio (OR) for CAD of 3.12 (p = 0.004), compared with the lowest quartile. This relationship was markedly accentuated in the setting of hyper-lipidemia (OR: 16.8) (Table 3). Importantly, in patients <60 years old, OxPL/apoB remained an independent predictor of CAD, even with Lp(a) in the model (Figure 7). In the entire cohort, OxPL/apoB levels were independently associated with obstructive CAD for all clinical and lipid measures except for Lp(a), suggesting a common biologic influence on CAD risk. These observations support the hypothesis that although much of the risk attributable to Lp(a) can be explained by its binding of OxPL, additional risk associated with OxPL may be present in younger patients, perhaps through proinflammatory pathways independent of Lp(a). Furthermore, in this specific population selected for clinically indicated coronary angiography, the most atherogenic Lp(a) particles may be better reflected by OxPL/apoB, as suggested by the significantly higher OR for CAD. It is likely that smaller Lp(a) particles have the most OxPL on them, and this leads to some dissociation of OxPL/apoB and Lp(a) levels per se. In fact, in all studies published to date OxPL/apoB was equivalent or superior to risk compared with Lp(a) in risk prediction.
Table 3.
Odds ratios for obstructive coronary artery disease according to quartiles of the ratio of oxidized phospholipids to apoB-100 and levels of lipoprotein(a) in patients with and without hypercholesterolemia.
| Patient group | Oxidized phospholipid:apoB-100 ratio
|
|||
|---|---|---|---|---|
| No hypercholesterolemia
|
Hypercholesterolemia
|
|||
| Number with CAD (%) | OR (95% CI) | Number with CAD (%) | OR (95% CI) | |
|
All patients
| ||||
| Quartile I | 29 | 1.00 | 67 | 4.93 (2.31–10.5) |
|
| ||||
| Quartile II | 44 | 1.92 (0.91–4.06) | 56 | 3.10 (1.54–6.25) |
|
| ||||
| Quartile III | 38 | 1.47 (0.68–3.19) | 64 | 4.36 (2.16–8.79) |
|
| ||||
| Quartile IV | 39 | 1.54 (0.70–3.40) | 77 | 8.13 (3.88–17.1) |
|
| ||||
| Total | 150 | – | 264 | – |
|
| ||||
|
Age ≤ 60 years
| ||||
| Quartile I | 14 | 1.00 | 61 | 9.33 (2.64–33.0) |
|
| ||||
| Quartile II | 27 | 2.21 (0.61–7.97) | 37 | 3.53 (1.03–12.0) |
|
| ||||
| Quartile III | 28 | 2.33 (0.64–8.45) | 57 | 8.00 (2.51–25.5) |
|
| ||||
| Quartile IV | 43 | 4.59 (1.39–15.1) | 74 | 16.8 (5.11–55.2) |
|
| ||||
| Total | 112 | – | 229 | – |
|
| ||||
|
Age >60 years
| ||||
| Quartile I | 45 | 1.00 | 71 | 3.00 (1.10–8.18) |
|
| ||||
| Quartile II | 61 | 1.85 (0.67–5.15) | 68 | 2.57 (1.01–6.54) |
|
| ||||
| Quartile III | 48 | 1.11 (0.39–3.14) | 71 | 2.90 (1.11–7.58) |
|
| ||||
| Quartile IV | 31 | 0.55 (0.15–1.92) | 80 | 4.95 (1.76–13.9) |
|
| ||||
| Total | 185 | – | 290 | – |
For the oxidized phospholipid:apo B-100 ratio, quartiles I–IV correspond to the following values: <0.047, 0.047–0.089, 0.089–0.294 and >0.294, respectively. For lipoprotein(a), quartiles I–IV correspond to the following values: <8.8, 8.8–21.1, 21.1–39.7 and >39.7 mg per deciliter, respectively. The p-values indicate whether any two of the eight groups (defined by quartile and hypercholesterolemia status) have significantly different proportions of subjects with CAD.
CAD: Coronary artery disease; OR: Odds ratio.
Data taken from [48].
Figure 7. Odds ratios for obstructive coronary artery disease associated with selected risk factors among patients 60 years of age or younger from the multivariable analysis.
Risk factors are shown in descending order of significance. In this analysis, Lp(a) was forced into the model with the OxPL:apoB-100 ratio.
CAD: Coronary artery disease; CRP: C-reactive protein; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; Lp(a): Lipoprotein(a); OxPL:apoB-100 ratio: Ratio of oxidized phospholipid content per particle of apoB-100.
Adapted with permission from [48].
Relationship to peripheral artery disease
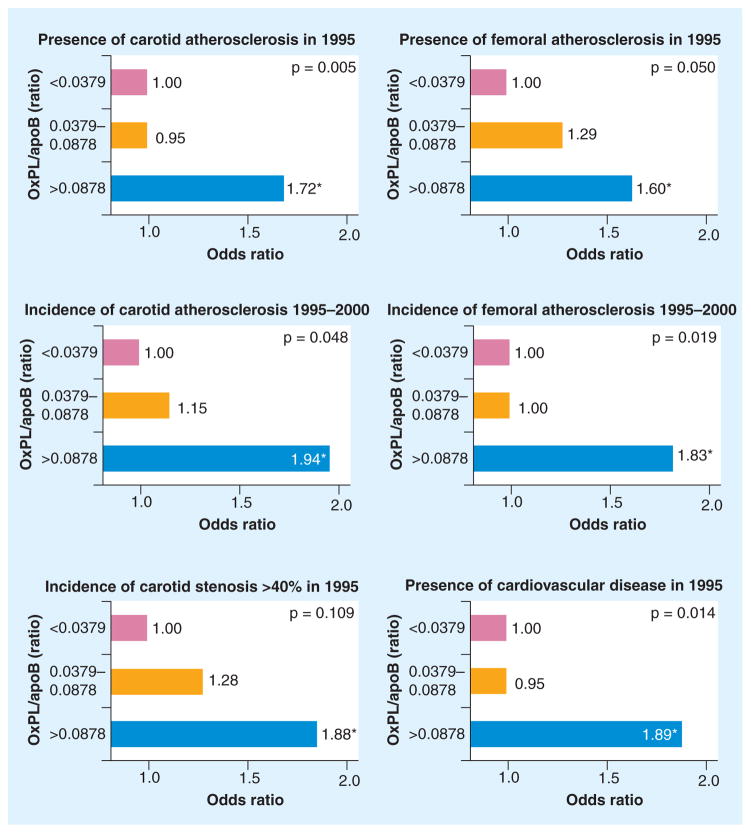
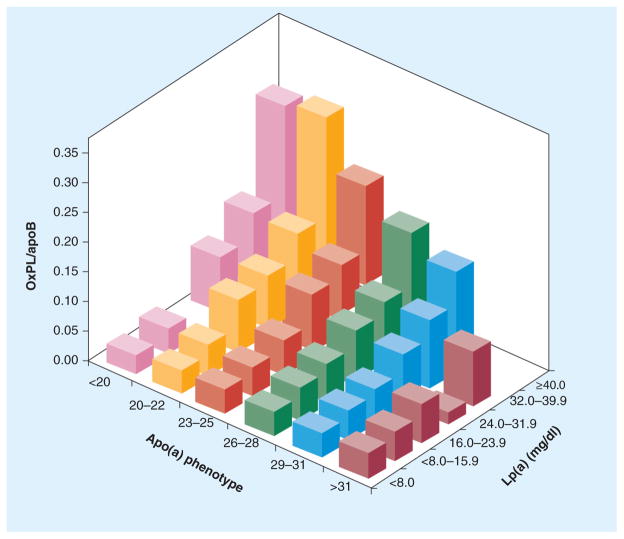
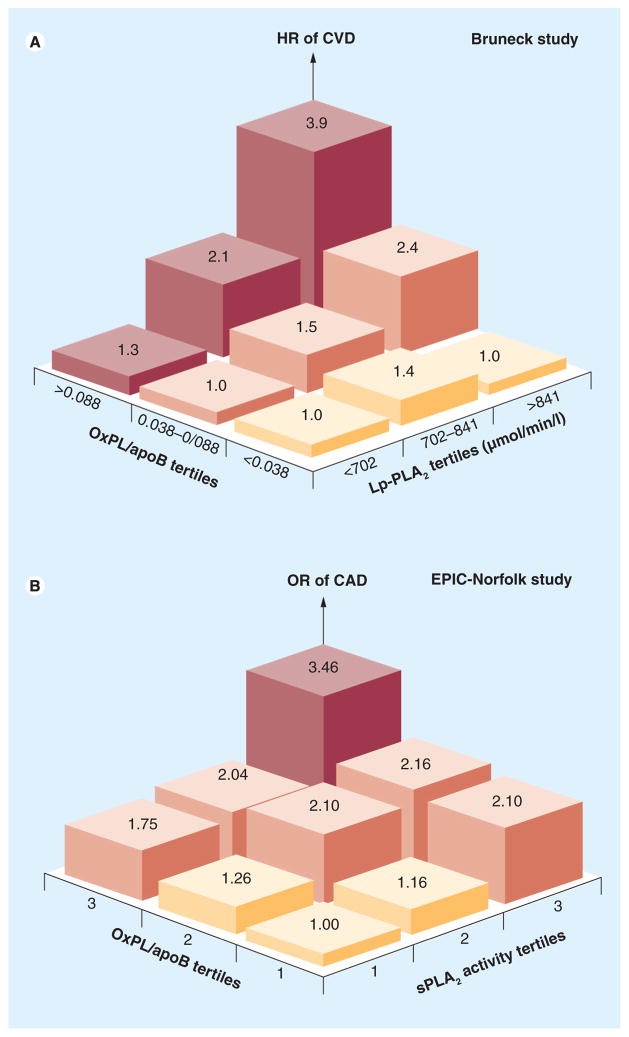
In the Bruneck study, a large prospective population-based survey of 40–79-year-old men and women initiated in 1990 where serial plasma levels of OxPL/apoB were measured in 765 and 671 subjects in 1995 and 2000, OxPL/apoB levels predicted the presence of symptomatic CVD and were significantly associated with the presence, extent and development (1995–2000) of carotid and femoral atherosclerosis (Figure 8) [28]. The association of OxPL/apoB and Lp(a) was strongest in subjects with small apo(a) isoforms and the highest Lp(a) concentration, suggesting that OxPL/apoB levels may be influenced by the number of K-IV2 repeats (Figure 9).
Figure 8. Multivariate analysis showing the association of oxidized phospholipids/apoB-100 particle tertile groups with the presence and progression of carotid and femoral artery atherosclerosis and with cardiovascular disease.
*p < 0.05 for the comparison between the first tertile group (reference category) and the third tertile group. The p-values presented in the figures are the overall p-values for the three tertiles (test for trend).
Adapted with permission from [28].
Figure 9. 3D plot of oxidized phospolipid/apoB levels according to lipoprotein(a) mass and apo(a) phenotypes expressed as the number of kringle IV type 2 repeats.
The OxPL/apoB levels presented are geometric means (taken as the anti-log of the mean of log-transformed OxPL/apoB values).
Lp(a): Lipoprotein(a); OxPL: Oxidized phospolipid.
Adapted with permission from [28].
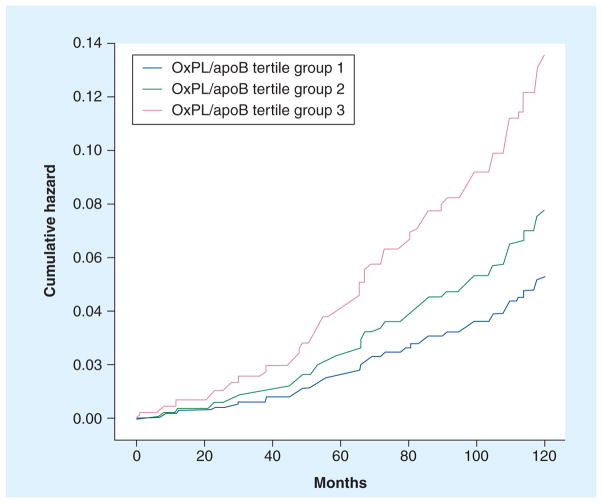
Prediction of cardiovascular events
The Bruneck study [28,44] was the first prospective epidemiological study in an unselected population derived from the general community to demonstrate the prognostic utility of OxPL/apoB levels in predicting future death, myocardial infarction, stroke, and transient ischemic attack and revascularization. OxPL/apoB levels predicted the development of cardiovascular events over a 10-year prospective follow-up period. For example, subjects in the highest tertile of OxPL/apoB had a significantly higher risk of cardiovascular events than those in the lowest tertile (hazard ratio: 2.4, 95% CI: 1.3–4.3, p = 0.004) (Figure 10) following multivariable adjustment for traditional risk factors, high-sensitivity C-reactive protein and Lp-PLA2 activity. These findings were confirmed and extended in the prospective case-controlled EPIC-Norfolk study, consisting of 763 cases and 1397 matched controls composed of 45–79-year-old apparently healthy men and women followed for 6 years [47]. This study showed the highest tertiles of OxPL/apoB were associated with a significantly higher risk of CAD events (OR: 1.67; p < 0.001) compared with the lowest tertiles (Table 4), after adjusting for age, smoking, diabetes, LDL and high-density lipoprotein cholesterol, and systolic blood pressure.
Figure 10. Cumulative hazard curves of incident cardiovascular disease from 1995 to 2005 for tertiles of oxidized phospolipid/apoB in the Bruneck study.
OxPL: Oxidized phospholipid.
Adapted with permission from [44].
Table 4.
Odds ratio (95% CI) of coronary artery disease by tertiles of oxidized phospolipid/apoB.
| OxPL/apoB | Tertile 1 <1150 RLU; OR | Tertile 2 1150–2249 RLU; OR (95% CI) | Tertile 3 >2249 RLU; OR (95% CI) | p-value trend linearity |
|---|---|---|---|---|
| Entire cohort | ||||
| Unadjusted | 1.00 | 1.21 (0.97–1.52) | 1.61 (1.29–2.01) | <0.001 |
| Adjusted model 1 | 1.00 | 1.32 (1.04–1.68) | 1.67 (1.32–2.12) | <0.001 |
| Adjusted model 2 | 1.00 | 1.27 (1.01–1.60) | 1.66 (1.32–2.09) | <0.001 |
| Men | ||||
| Unadjusted | 1.00 | 1.31 (0.99–1.75) | 1.59 (1.20–2.11) | 0.001 |
| Adjusted model 1 | 1.00 | 1.43 (1.05–1.94) | 1.65 (1.21–2.24) | 0.001 |
| Adjusted model 2 | 1.00 | 1.42 (1.06–1.92) | 1.70 (1.26–2.29) | <0.001 |
| Women | ||||
| Unadjusted | 1.00 | 0.96 (0.67–1.39) | 1.60 (1.12–2.28) | 0.009 |
| Adjusted model 1 | 1.00 | 1.05 (0.70–1.56) | 1.67 (1.14–2.44) | 0.007 |
| Adjusted model 2 | 1.00 | 0.97 (0.67–1.41) | 1.58 (1.10–2.26) | 0.01 |
Model 1 matched for sex, age and enrollment time, and adjusted for diabetes, smoking, systolic blood pressure, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol. Model 2 matched for sex, age and enrollment time, and adjusted for Framingham Risk Score.
OR: Odds ratio; OxPL: Oxidized phospolipid; RLU: Relative light units.
Data taken from [47].
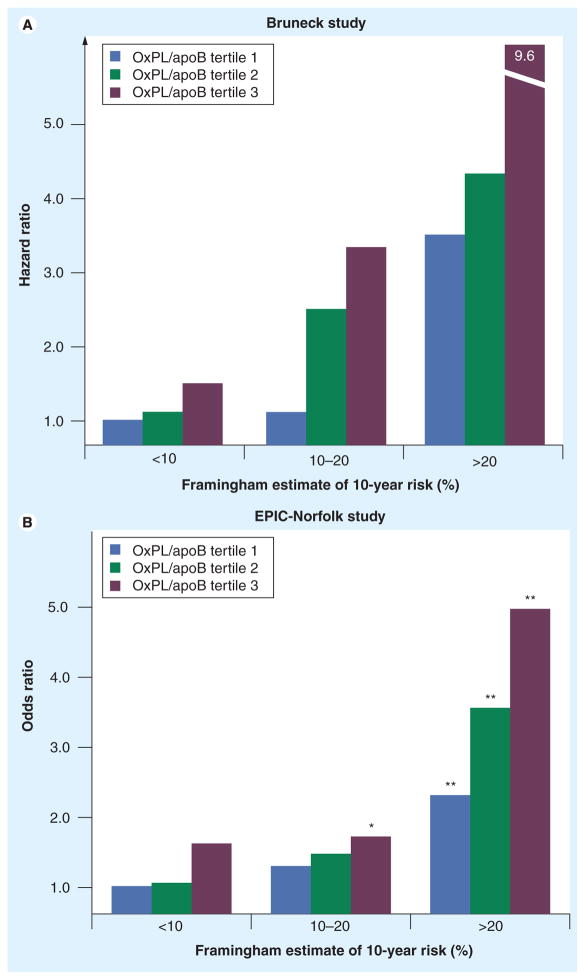
Furthermore, OxPL/apoB levels provided additional predictive value to the Framingham Risk Score (FRS). By measuring OxPL/apoB in each tertile of FRS, the predicted risk can be either increased or decreased depending on the tertiles of OxPL/apoB. This would allow fine tuning of risk prediction and more accurate assessment of treatment options. For example, in the Bruneck Study, the graded increase in CVD risk across OxPL/apoB tertile groups was evident in the low-, moderate- and high-risk groups as defined by the FRS (Figure 11a) [44]. This result was validated in the EPIC-Norfolk study (Figure 11b) [47]. Finally, OxPL/apoB values are independent of metabolic syndrome parameters [47].
Figure 11. Oxidized phospholipid/apoB ratio within each Framingham Risk Score group.
(A) Relationship between tertile groups and cardiovascular disease risk (tertile 1: <0.0379 relative light units (RLU), tertile 2: 0.0379–0.0878 RLU and tertile 3: >0.0878 RLU). (B) Relationship between tertile groups and future coronary artery disease risk (tertile 1: <1150 RLU, tertile 2: 1151–2249 RLU and tertile 3: >2249 RLU). Framingham Risk Score was calculated as low risk (<10% risk of events over 10 years), moderate risk (10–20%) and high risk (>20%).
*p< 0.05 and **p< 0.001 for comparison of each tertile of the respective biomarkers with the lowest tertile in the low Framingham Risk Score category of each biomarker.
EPIC: European Prospective Investigation of Cancer; Lp(a): Lipoprotein(a); OxPL: Oxidized phospholipid.
(A) Adapted with permission from [44].
(B) Adapted with permission from [47].
Receiver-operator characteristic c-index values
To assess the predictive value of the utility of these biomarkers above the FRS, receiver-operator characteristic unconditional c-indexes were generated [47]. The c-index discriminates between individuals at different risk levels and measures the probability that a randomly chosen individual who experienced an event has a higher risk score than a randomly chosen individual who did not experience an event during the same, specific follow-up interval. The c-index is a standard measure of the effect of a new marker in risk prediction and helps to quantify its predictive discrimination, but does have limitations [56].
The c-index for the FRS was 0.584 (95% CI: 0.558–0.609), a relatively low value that reflects the fact that age and sex were already accounted for as part of the matching design. Adding individual biomarkers to the FRS shows that the c-index increased from 0.584 (95% CI: 0.558–0.609) to 0.618 (95% CI: 0.593–0.642), in progressing order of myeloperoxidase mass, Lp-PLA2 activity, OxPL/apoB, high-sensitivity C-reactive protein, Lp(a), sPLA2 mass and sPLA2 activity. Adding biomarkers to FRS until all biomarkers were present in the model progressively increased the c-index from 0.584 (95% CI: 0.558–0.609) to 0.651 (95% CI: 0.627–0.675) (Table 5).
Table 5.
Statistical values for area under receiver operating characteristic curves.
| Biomarkers | c-index (95% CI) |
|---|---|
| FRS | 0.584 (0.558–0.609) |
| FRS, MPO | 0.586 (0.561–0.612) |
| FRS, Lp-PLA2 | 0.587 (0.562–0.613) |
| FRS, OxPL/apoB | 0.597 (0.572–0.623) |
| FRS, hsCRP | 0.605 (0.580–0.630) |
| FRS, Lp(a) | 0.607 (0.582–0.632) |
| FRS, sPLA2 mass | 0.609 (0.584–0.634) |
| FRS, sPLA2 activity | 0.618 (0.593–0.642) |
| FRS, MPO, Lp-PLA2, | 0.590 (0.565–0.616) |
| FRS, MPO, Lp-PLA2, OxPL/apoB | 0.603 (0.578–0.628) |
| FRS, MPO, Lp-PLA2, OxPL/apoB, hsCRP | 0.614 (0.590–0.639) |
| FRS, MPO, Lp-PLA2, OxPL/apoB, hsCRP, Lp(a) | 0.625 (0.600–0.649) |
| FRS, MPO, Lp-PLA2, OxPL/apoB, hsCRP, Lp(a), sPLA2 mass | 0.635 (0.610–0.659) |
| FRS, MPO, Lp-PLA2, OxPL/apoB, hsCRP, Lp(a), sPLA2 mass, sPLA2 activity | 0.651 (0.627–0.675) |
FRS: Framingham Risk Score; hsCRP: High-sensitivity C-reactive protein; Lp(a): Lipoprotein(a); Lp-PLA2: Lipoprotein-associated phospholipase A2 activity; MPO: Myeloperoxidase; OxPL: Oxidized phospolipid; sPLA2: Soluble phospholipase A2.
Data taken from [47].
Relationship of OxPL/apoB to Lp(a), Lp-PLA2 and sPLA2
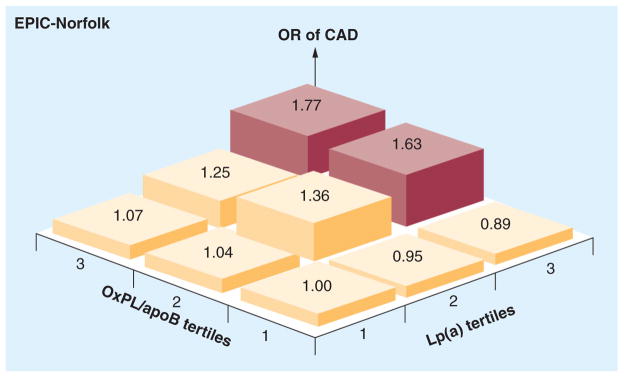
In prior studies, we have demonstrated that OxPL/apoB reflect the biological activity of small apo(a) isoforms associated with high Lp(a) levels [44,49]. Furthermore, the OxPL/apoB assay represents OxPL bound by Lp(a) (~85–90%) and non-Lp(a) apoB (10–15%), on average [42]. This relationship is not constant and depends on the underlying LPA genetics, where small isoforms are associated with high OxPL/apoB and high correlations with Lp(a) [49,57], but large isoforms are associated with low Lp(a) levels (i.e., <25 mg/dl) and weak-to-absent correlations [49]. To assess whether adding Lp(a) levels to OxPL/apoB enhances the predictive value for CVD events, we performed a 3 × 3 tertile analysis evaluating relationship to CAD events in EPIC-Norfolk. This demonstrated that the relationship of OxPL/apoB and Lp(a) to fatal and nonfatal CAD was accentuated in the highest tertiles of both biomarkers (OR: 1.77, 95% CI: 1.31–2.37), suggesting that they can provide independent and additive information for risk prediction (Figure 12) [47].
Figure 12. Odds ratios for fatal and nonfatal coronary artery disease based on tertiles of oxidized phospholipid/apoB and lipoprotein.
(a). The tertile cutoffs for OxPL/apoB are tertile 1: <1150 relative light units (RLU), tertile 2: 1151–2249 RLU and tertile 3: >2249 RLU, and for Lp(a) they are tertile 1: <7.25 mg/dl, tertile 2: 7.25–11.69 mg/dl and tertile 3: >11.69 mg/dl.
CAD: Coronary artery disease; Lp(a): Lipoprotein(a); OR: Odds ratio; OxPL: Oxidized phospolipid.
Adapted with permission from [47].
Lipoprotein-associated phospholipase A2 and sPLA2 are enzymes that react with OxPLs and cleave the oxidized fatty acid side chain at the sn-2 position of OxPL to generate lyso-phosphatidylcholine and an oxidized free fatty acid. Both of these biomarkers have been associated with the prediction of cardiovascular events when elevated and are targets of ongoing therapeutic trials to inhibit their activities [50]. Since OxPL and phospholipases may share similar pathophysiology, we evaluated whether the combination of these biomarkers provides enhanced predictive value. In the Bruneck Study, the strength of the association between OxPL/apoB and CVD risk significantly increased with increasing Lp-PLA2 activity (p = 0.018 for interaction) (Figure 13a). Similarly, the OR of CAD events associated with the highest tertiles of OxPL/apoB was significantly potentiated (approximately doubled) by the highest tertiles of sPLA2 activity and mass (Figure 13b). In clinical risk prediction, using combinations of these biomarkers may allow stronger predictive value for ascertaining CVD risk. Although these phospholipases are clearly risk predictors, their role as causal risk factors has not been proven yet [58]. In fact, Phase III clinical trials are currently underway to assess whether inhibition of their mass and/or activity will lead to improvement in cardiovascular outcomes [51,52].
Figure 13. Relationship of oxidized phospolipid/apoB to lipoprotein-associated phospholipase A2 and soluble phospholipase A2 activity.
(A) Relationship between OxPL/apoB tertile groups and CVD risk according to tertiles of Lp-PLA2 activity. (B) ORs for CAD based on tertiles of OxPL/apoB and sPLA2 activity. The tertile cutoffs for OxPL/apoB are tertile 1: <1150 relative light units (RLU), tertile 2: 1151–2249 RLU and tertile 3: >2249 RLU, and for sPLA2 activity levels, tertile 1: <4.05 nmol/min/ml, tertile 2: 4.05–4.83 nmol/min/ml and tertile 3: >4.83 nmol/min/ml.
CAD: Coronary artery disease; CVD: Cardiovascular disease; EPIC: European Prospective Investigation of Cancer; HR: Hazard ratio; Lp-PLA2: Lipoprotein-associated phospholipase A2 activity; OR: Odds ratio; OxPL: Oxidized phospolipid; sPLA2: Soluble phospholipase A2.
(A) Adapted with permission from [44].
(B) Adapted with permission from [47].
Change in OxPL/apoB & therapeutic interventions
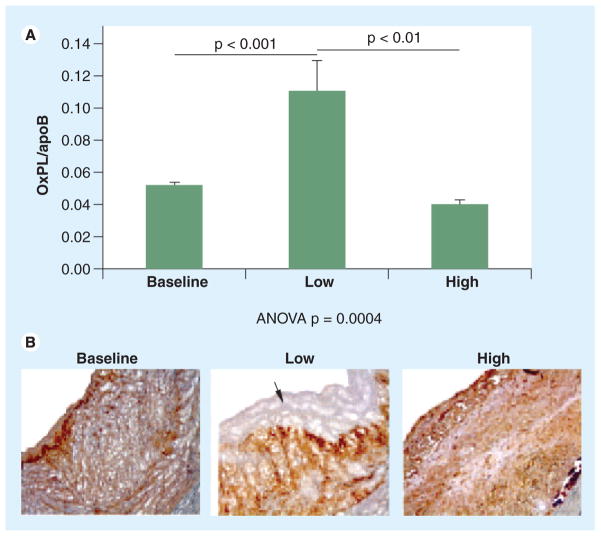
The OxPL/apoB assay was originally developed as an indicator of minimally oxidized LDL in plasma that might reflect the overall content of circulating OxLDL. We initially postulated that OxPL/apoB levels would increase during hypercholesterolemia and/or atherosclerosis progression and decrease during atherosclerosis regression. Counterintuitively, we have found the opposite, in both animals [59] and humans [33,58,60–65], suggesting that increases in OxPL/apoB in plasma may reflect reduction of the OxPL content of arterial lesions. For example, in New Zealand white rabbits and cynomolgus monkey models of atherosclerosis, which do not have Lp(a) or their Lp(a) does not bind OxPL and/or has E06 immunoreactivity, respectively, OxPL/apoB levels increased 50–100% in plasma in the setting of lesion regression, concomitant with reduced immunostaining of OxPL in atherosclerotic lesions (Figure 14) [59]. Note that the OxPL staining in the middle panel of Figure 14 has disappeared from the luminal surface, while the OxPL/apoB level goes up in plasma, suggesting a flux of OxPL from the vessel wall to the circulation during dietary induced regression. This suggests that efflux of OxPL occurs preferentially early during atherosclerosis regression from arterial lesions into plasma, even more extensively than depletion of apoB-100 or physical plaque regression. These data suggest that changes in the OxPL/apoB ratio may reflect early atherosclerosis regression. Similarly, in human studies with low fat diets [60,62], aged garlic supplementation [58,63] and statin therapy (Table 1) [33,60,61,65], significant increases in OxPL/apoB have been noted shortly after the initiation of the intervention. Although the etiology of these changes is not fully defined, the data are consistent across studies and are associated with treatments considered to be of clinical benefit. For example, in the Myocardial Ischemia Reduction With Aggressive Cholesterol Lowering (MIRACL) trial, an increase in OxPL/apoB and Lp(a) was observed at 16 weeks after initiation of atorvastatin therapy, consistent with a decreased reoccurrence of clinical events, while no such change was observed in the placebo arm. Similarly, an increase in OxPL/apoB, and Lp(a) in response to aged garlic supplements was associated with less progression of coronary artery calcium and improvement in vascular function [58,63]. Based on the animal data, one may postulate a flux of OxPL from sites of vessel injury or inflammation to the circulation, where they are bound to apoB lipoproteins. In humans, the OxPL preferentially bind to Lp(a), but we have also noted an increase in the Lp(a) levels as well, suggesting that perhaps there is some signaling mechanism leading to increased Lp(a) levels in these settings. These findings are consistent with our suggestion noted above that a ‘physiological’ role of Lp(a) may be to bind and transport cellular sources of OxPL, such as from apoptotic cells or during normal cellular metabolism where OxPL may be generated. Whether these increases in OxPL/apoB found in the settings of low fat diets, statin therapy, or other potentially beneficial interventions are biomarkers of enhanced efflux from the artery of OxPL, and hence a surrogate of regression, remains to be established.
Figure 14. Oxidized phospholipid:apoB ratio and immunohistochemistry in a New Zealand white rabbit study.
(A) OxPL:apoB ratio in the New Zealand white rabbit study in the baseline (n = 15), low cholesterol (n = 10) and high cholesterol (n = 5) groups. (B) Immunochemistry of New Zealand white aortas with antibody E06 staining (brown color pattern) for OxPL in the baseline, low cholesterol and high cholesterol diet groups. The arrow represents lack of OxPL at the luminal surface in a representative rabbit with pre-existing atherosclerosis that was subsequently switched to a low-cholesterol diet.
OxPL: Oxidized phospholipid.
Adapted with permission from [59].
The apparent paradox is that OxPL/apoB remain strongly independent predictors of CVD in prospective studies, yet here we find they are increased, at least in the short term, in settings of effective therapy. We suggest that a resolution of this paradox may be that with continued therapy, the initial efflux of OxPL from artery into plasma will return to baseline, and the elevated OxPL/apoB will revert to basal levels, or even lower, consistent with reduced risk caused by the intervention. These ideas are currently being tested by extended analyses of adequately powered prospective intervention trials. If confirmed, an early rise in OxPL/apoB might then be used as a useful surrogate biomarker of a beneficial intervention. Such biomarkers are urgently needed.
Conclusion & future perspective
Oxidized phospholipids are strongly implicated in several aspects of CVD. The OxPL/apoB assay provides diagnostic information by strongly reflecting the presence and progression of CVD and prognostic information for predicting future cardiovascular events. It independently complements established risk factors in risk prediction, is independent of the metabolic syndrome and optimizes the predictive value of the FRS. Importantly, from a pathophysiological perspective, OxPL/apoB appears to most closely reflect the biological activity of the most atherogenic Lp(a) particles that are associated with both high Lp(a) levels and small apo(a) isoforms. Since apo(a) isoforms are laborious and expensive to measure, OxPL/apoB levels may accurately reflect the CVD risk of these atherogenic lipoproteins. With Lp(a) now being established as an independent, genetic risk factor for CVD, it may become a target of therapy in future studies. In fact, the antisense oligonucleotide mipomersen and a more specific antisense oligonucleotide targeted to KIV-2 repeats of apo(a) have recently been shown to reduce plasma levels of Lp(a)/apo(a), and their associated OxPL, by 75–86% in Lp(a)-transgenic mice [66,67], setting the stage for future clinical development.
Currently, the OxPL/apoB assay is a research tool, but the large clinical database already established suggests that with standardization of the methodology, it will be a useful biomarker that can be used clinically for risk stratification. Future research will focus on the early (and possibly late) changes in OxPL/apoB that occur with therapeutic interventions and whether therapeutic decisions can be guided by assessing these changes in response to the intervention. Finally, since OxPL are implicated in a variety of disorders that have oxidative stress as a key component, such as Alzheimer’s disease, multiple sclerosis, nonalcoholic steatohepatitis, rheumatologic disease such as lupus and rheumatoid arthritis, infectious disease and cancer, it would be of interest to assess whether OxPL/apoB and related oxidative biomarkers are associated with and predict noncardiovascular outcomes in patients with these disorders.
Executive summary.
Oxidation of lipoproteins, especially low-density lipoprotein (LDL), is a necessary mechanism for foam cell generation as well as initiation and destabilization of atherosclerotic lesions.
A variety of oxidation-specific epitopes, such as oxidized phospholipids (OxPL) and malondialdehyde-lysine epitopes, are generated during oxidation.
EO6 is a murine monoclonal antibody that binds to the phosphocholine head group of oxidized but not native phospholipids.
The OxPL/apoB-lipoprotein(a) (Lp(a)) correlation is highly dependent on apo(a) isoform size, with strong correlations with small apo(a) isoforms and weak or absent with larger apo(a) isoforms.
OxPL/apoB levels correlate with Lp(a) and not with LDL, which could propose a unique physiological role of Lp(a) to bind and transport pro-inflammatory OxPL in plasma. OxPL/apoB reflects the most atherogenic Lp(a) particles associated with small isoforms and high Lp(a) levels.
Relationship of OxPL/apoB & cardiovascular disease
OxPL/apoB increases acutely in patients following acute coronary syndromes or during uncomplicated percutaneous coronary intervention.
OxPL/apoB levels are independently correlated with the presence and extent of angiographically documented coronary artery disease and the presence and progression of carotid and femoral atherosclerosis.
Elevated baseline levels of OxPL/apoB independently predict the development of cardiovascular events over a 10-year prospective follow-up in a population derived from general community and provide additional predictive value to the Framingham Risk Score, are independent of the metabolic syndrome and increase the c-index for predicting events in a model with Framingham Risk Score, traditional risk factors, and other oxidative and inflammatory biomarkers, including high-sensitivity C-reactive protein.
The strength of the association between OxPL/apoB and cardiovascular disease risk significantly increases with increasing Lp-PLA2 activity, secretory phospholipase A2 activity and mass.
In both animals and humans, efflux of OxPL from arterial lesions into plasma was observed to occur preferentially early during atherosclerosis regression (low fat diets, statin therapy and aged garlic supplements).
Conclusion
OxPL/apoB levels provide diagnostic and prognostic information in strongly reflecting the presence and progression of cardiovascular disease and predicting future cardiovascular events, and may be a useful assay in research and clinical applications.
Acknowledgments
The authors would like to thank the large number of collaborating investigators and collaborating institutions that have worked closely with them and supplied many of the blood samples to help validate many of the concepts described in this article.
Footnotes
Financial & competing interests disclosure
This study was supported by the Fondation Leducq. JL Witztum and S Tsimikas are named as co-inventors of patents and patent applications owned by the University of California for the clinical use of oxidation-specific antibodies. JL Witztum is a consultant to ISIS, Inc, Regulus and Lpath. S Tsimikas is a consultant to Quest, ISIS, Genzyme and Sanofi, Inc. and has received investigator-initiated grant research funding from Merck and Pfizer, Inc. JL Witztum and S Tsimikas are directors and have equity in Atherotope, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
For reprint orders, please contact: reprints@futuremedicine.com
Bibliography
Papers of special note have been highlighted as: ▪ of interest
- 1.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104(4):503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 2.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8(4):222–332. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 3.Esterbauer H, Gebicki J, Puhl H, Jürgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Rad Biol Med. 1992;13(4):341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 5.Nishi K, Itabe H, Uno M, et al. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol. 2002;22(10):1649–1654. doi: 10.1161/01.atv.0000033829.14012.18. [DOI] [PubMed] [Google Scholar]
- 6▪.Seimon TA, Nadolski MJ, Liao X, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12(5):467–482. doi: 10.1016/j.cmet.2010.09.010. Demonstrates that oxidized phospholipids (OxPL) and lipoprotein(a) (Lp(a)) induce macrophage apoptosis in a CD36-TLR2-dependent mechanism and link the atherogenicity of OxPL/Lp(a) with a late stage of atherosclerosis that leads to clinical events. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13(3):184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salonen JT, Yla-Herttuala S, Yamamoto R, et al. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992;339(8798):883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 9.Palinski W, Rosenfeld ME, Yla-Herttuala S, et al. Low density lipoprotein undergoes oxidative modificationin vivo. Proc Natl Acad Sci USA. 1989;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, Witztum JL. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14(4):605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 11▪.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. J Lipid Res. 2009;50(Suppl):S207–S212. doi: 10.1194/jlr.R800074-JLR200. Recent updated review on the role of OxPL in atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12(8):1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg ME, Li XM, Gugiu BG, et al. The lipid whisker model of the structure of oxidized cell membranes. J Biol Chem. 2008;283(4):2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 14.Ravandi A, Harkewicz R, Leibundgut G, et al. Identification of oxidized phospholipids and cholesteryl esters in embolic protection devices post percutaneous coronary, carotid and peripheral interventions in humans. Arterioscler Thromb Vasc Biol. 2011:P385. [Google Scholar]
- 15.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 16.Horkko S, Bird DA, Miller E, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103(1):117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw PX, Horkko S, Chang MK, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105(12):1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪.Friedman P, Horkko S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J Biol Chem. 2002;277(9):7010–7020. doi: 10.1074/jbc.M108860200. Describes in great detail the characteristics of the epitope to which E06 binds, and shows it to be a conformational epitope of phosphocholine on OxPL, but not native phospholipids. [DOI] [PubMed] [Google Scholar]
- 19.Briles DE, Forman C, Hudak S, Claflin JL. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective againstStreptococcus pneumoniae. J Exp Med. 1982;156(4):1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz D, Watson AD, Miller CS, et al. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest. 2008;118(8):2917–2928. doi: 10.1172/JCI34189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang MK, Bergmark C, Laurila A, et al. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA. 1999;96(11):6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IGM is required for efficient complement mediated phagocytosis of apoptotic cellsin vivo. Autoimmunity. 2005;38(4):259–264. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 24.Binder CJ, Horkko S, Dewan A, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9(6):736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 25.Hartvigsen K, Chou MY, Hansen LF, et al. The role of innate immunity in atherogenesis. J Lipid Res. 2009;50(Suppl):S388–S393. doi: 10.1194/jlr.R800100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪.Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108(2):235–248. doi: 10.1161/CIRCRESAHA.110.223875. Comprehenive review that develops the idea that oxidation-specific epitopes are danger associated molecular patterns that are targets of pattern recognition receptors of innate immunity. It provides a framework to understand the role of innate immunity in inflammation and atherogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27▪.Tsimikas S, Bergmark C, Beyer RW, et al. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41(3):360–370. doi: 10.1016/s0735-1097(02)02769-9. First paper to describe the relationship between OxPL/apoB and Lp(a) [DOI] [PubMed] [Google Scholar]
- 28.Tsimikas S, Kiechl S, Willeit J, et al. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J Am Coll Cardiol. 2006;47(11):2219–2228. doi: 10.1016/j.jacc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Young SG, Smith RS, Hogle DM, Curtiss LK, Witztum JL. Two new monoclonal antibody-based enzyme-linked assays of apolipoprotein B. Clin Chem. 1986;32(8):1484–1490. [PubMed] [Google Scholar]
- 30.Fraley AE, Tsimikas S. Clinical applications of circulating oxidized low-density lipoprotein biomarkers in cardiovascular disease. Curr Opin Lipidol. 2006;17(5):502–509. doi: 10.1097/01.mol.0000245255.40634.b5. [DOI] [PubMed] [Google Scholar]
- 31.Tsimikas S, Witztum JL. The role of oxidized phospholipids in mediating lipoprotein(a) atherogenicity. Curr Opin Lipidol. 2008;19(4):369–377. doi: 10.1097/MOL.0b013e328308b622. [DOI] [PubMed] [Google Scholar]
- 32.Young SG, Witztum JL, Casal DC, Curtiss LK, Bernstein S. Conservation of the low density lipoprotein receptor-binding domain of apoprotein B. Demonstration by a new monoclonal antibody, MB47. Arteriosclerosis. 1986;6(2):178–188. doi: 10.1161/01.atv.6.2.178. [DOI] [PubMed] [Google Scholar]
- 33.Tsimikas S, Lau HK, Han KR, et al. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109(25):3164–3170. doi: 10.1161/01.CIR.0000130844.01174.55. [DOI] [PubMed] [Google Scholar]
- 34.Rhoads GG, Dahlen G, Berg K, Morton NE, Dannenberg AL. Lp(a) lipoprotein as a risk factor for myocardial infarction. JAMA. 1986;256(18):2540–2544. [PubMed] [Google Scholar]
- 35.Seed M, Hoppichler F, Reaveley D, et al. Relation of serum lipoprotein(a) concentration and apolipoprotein(a) phenotype to coronary heart disease in patients with familial hypercholesterolemia. N Engl J Med. 1990;322(21):1494–1499. doi: 10.1056/NEJM199005243222104. [DOI] [PubMed] [Google Scholar]
- 36.Dangas G, Ambrose JA, D’agate DJ, et al. Correlation of serum lipoprotein(a) with the angiographic and clinical presentation of coronary artery disease. J Am Coll Cardiol. 1999;83(4):583–585. doi: 10.1016/s0002-9149(98)00917-5. [DOI] [PubMed] [Google Scholar]
- 37.Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000;102(10):1082–1085. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 38.Marcovina SM, Koschinsky ML, Albers JJ, Skarlatos S. Report of the National Heart, Lung, and Blood Institute Workshop on Lipoprotein(a) and Cardiovascular Disease: recent advances and future directions. Clin Chem. 2003;49(11):1785–1796. doi: 10.1373/clinchem.2003.023689. [DOI] [PubMed] [Google Scholar]
- 39.Bennet A, Di Angelantonio E, Erqou S, et al. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch Int Med. 2008;168(6):598–608. doi: 10.1001/archinte.168.6.598. [DOI] [PubMed] [Google Scholar]
- 40.Berglund L, Anuurad E. Role of lipoprotein(a) in cardiovascular disease: current and future perspectives. J Am Coll Cardiol. 2008;52(2):132–134. doi: 10.1016/j.jacc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117(2):176–184. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 42▪.Bergmark C, Dewan A, Orsoni A, et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49(10):2230–2239. doi: 10.1194/jlr.M800174-JLR200. Demonstrates the presence of OxPL on Lp(a) with a variety of biochemical and analytical techniques. [DOI] [PubMed] [Google Scholar]
- 43.Edelstein C, Pfaffinger D, Hinman J, et al. Lysine-phosphatidylcholine adducts in kringle V impart unique immunological and potential proinflammatory properties to human apolipoprotein(a) J Biol Chem. 2003;278(52):52841–52847. doi: 10.1074/jbc.M310425200. [DOI] [PubMed] [Google Scholar]
- 44▪.Kiechl S, Willeit J, Mayr M, et al. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: prospective results from the Bruneck study. Arterioscler Thromb Vasc Biol. 2007;27(8):1788–1795. doi: 10.1161/ATVBAHA.107.145805. First study to demonstrate the predictive value of OxPL/apoB for death, myocardial infarction, stroke and revascularization over a 10-year follow-up period in an unselected population and to also link the atherogenicity of OxPL and Lp(a) with lipoprotein-associated phospholipase A2 activity. [DOI] [PubMed] [Google Scholar]
- 45.Berg K, Dahlen G, Christophersen B, Cook T, Kjekshus J, Pedersen T. Lp(a) lipoprotein level predicts survival and major coronary events in the Scandinavian Simvastatin Survival Study. Clin Genet. 1997;52(5):254–261. doi: 10.1111/j.1399-0004.1997.tb04342.x. [DOI] [PubMed] [Google Scholar]
- 46.Pillarisetti S, Paka L, Obunike JC, Berglund L, Goldberg IJ. Subendothelial retention of lipoprotein(a). Evidence that reduced heparan sulfate promotes lipoprotein binding to subendothelial matrix. J Clin Invest. 1997;100(4):867–874. doi: 10.1172/JCI119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪.Tsimikas S, Mallat Z, Talmud PJ, et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol. 2010;56(12):946–955. doi: 10.1016/j.jacc.2010.04.048. Demonstrates the predictive value of OxPL/apoB alone and in combination with a variety of other oxidative and inflammatory biomarkers, including high sensitivity C-reactive protein, and also shows increased c-index values above and beyond Framingham Risk Score estimates. [DOI] [PubMed] [Google Scholar]
- 48▪.Tsimikas S, Brilakis ES, Miller ER, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353(1):46–57. doi: 10.1056/NEJMoa043175. Describes the strong predictive value of OxPL/apoB in angiographically determined obstructive (>50% diameter stenosis) coronary artery disease. [DOI] [PubMed] [Google Scholar]
- 49.Tsimikas S, Clopton P, Brilakis ES, et al. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: results from the Dallas Heart Study. Circulation. 2009;119(13):1711–1719. doi: 10.1161/CIRCULATIONAHA.108.836940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mallat Z, Lambeau G, Tedgui A. Lipoprotein-associated and secreted phospholipases A in cardiovascular disease: roles as biological effectors and biomarkers. Circulation. 2010;122(21):2183–2200. doi: 10.1161/CIRCULATIONAHA.110.936393. [DOI] [PubMed] [Google Scholar]
- 51.Serruys PW, Garcia-Garcia HM, Buszman P, et al. Effects of the direct lipoprotein-associated phospholipase A2 inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118(11):1172–1182. doi: 10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- 52.Rosenson RS, Hislop C, Elliott M, Stasiv Y, Goulder M, Waters D. Effects of varespladib methyl on biomarkers and major cardiovascular events in acute coronary syndrome patients. J Am Coll Cardiol. 2010;56(14):1079–1088. doi: 10.1016/j.jacc.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 53.Ehara S, Ueda M, Naruko T, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103(15):1955–1960. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 54.Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR., Jr Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299(19):2287–2293. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holvoet P, Vanhaecke J, Janssens S, Van De Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–1494. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 56.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119(17):2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arai K, Luke MM, Koschinsky ML, et al. The I4399M variant of apolipoprotein(a) is associated with increased oxidized phospholipids on apolipoprotein B-100 particles. Atherosclerosis. 2010;209(2):498–503. doi: 10.1016/j.atherosclerosis.2009.09.077. [DOI] [PubMed] [Google Scholar]
- 58.Ahmadi N, Tsimikas S, Hajsadeghi F, et al. Relation of oxidative biomarkers, vascular dysfunction, and progression of coronary artery calcium. Am J Cardiol. 2010;105(4):459–466. doi: 10.1016/j.amjcard.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 59▪.Tsimikas S, Aikawa M, Miller FJ, Jr, et al. Increased plasma oxidized phospholipid:apolipoprotein B-100 ratio with concomitant depletion of oxidized phospholipids from atherosclerotic lesions after dietary lipid-lowering: a potential biomarker of early atherosclerosis regression. Arterioscler Thromb Vasc Biol. 2007;27(1):175–181. doi: 10.1161/01.ATV.0000251501.86410.03. Describes the increased of OxPL/apoB ratio in rabbit and monkey models in the setting of dietary regression showing a loss of OxPL epitopes in the vessel wall with a concomitant increase in OxPL/apoB in plasma. This suggests a “reverse OxPL tranport” mechanism that needs to be confirmed but may explain some of the findings in patients treated with low fat diet and statins, who also show an increase in OxPL/apoB. [DOI] [PubMed] [Google Scholar]
- 60.Ky B, Burke A, Tsimikas S, et al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J Am Coll Cardiol. 2008;51(17):1653–1662. doi: 10.1016/j.jacc.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 61.Rodenburg J, Vissers MN, Wiegman A, et al. Oxidized low-density lipoprotein in children with familial hypercholesterolemia and unaffected siblings: effect of pravastatin. J Am Coll Cardiol. 2006;47(9):1803–1810. doi: 10.1016/j.jacc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 62.Silaste ML, Rantala M, Alfthan G, et al. Changes in dietary fat intake alter plasma levels of oxidized low-density lipoprotein and lipoprotein(a) Arterioscler Thromb Vasc Biol. 2004;24(3):498–503. doi: 10.1161/01.ATV.0000118012.64932.f4. [DOI] [PubMed] [Google Scholar]
- 63.Budoff MJ, Ahmadi N, Gul KM, et al. Aged garlic extract supplemented with B vitamins, folic acid and L-arginine retards the progression of subclinical atherosclerosis: a randomized clinical trial. Prev Med. 2009;49(2–3):101–107. doi: 10.1016/j.ypmed.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 64.Faghihnia N, Tsimikas S, Miller ER, Witztum JL, Krauss RM. Changes in lipoprotein(a), oxidized phospholipids, and LDL subclasses with a low-fat high-carbohydrate diet. J Lipid Res. 2010;51(11):3324–3330. doi: 10.1194/jlr.M005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi SH, Chae A, Miller E, et al. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J Am Coll Cardiol. 2008;52(1):24–32. doi: 10.1016/j.jacc.2008.02.066. [DOI] [PubMed] [Google Scholar]
- 66.Merki E, Graham MJ, Mullick AE, et al. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice. Circulation. 2008;118(7):743–753. doi: 10.1161/CIRCULATIONAHA.108.786822. [DOI] [PubMed] [Google Scholar]
- 67.Merki E, Graham M, Taleb A, et al. Antisense oligonucleotide lowers plasma levels of apolipoprotein (a) and lipoprotein(a) in transgenic mice. J Am Coll Cardiol. 2011;57(15):1611–1621. doi: 10.1016/j.jacc.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 68.Wu R, De Faire U, Lemne C, Witztum JL, Frostegard J. Autoantibodies to OxLDL are decreased in individuals with borderline hypertension. Hypertension. 1999;33(1):53–59. doi: 10.1161/01.hyp.33.1.53. [DOI] [PubMed] [Google Scholar]
- 69.Penny WF, Ben-Yehuda O, Kuroe K, et al. Improvement of coronary artery endothelial dysfunction with lipid-lowering therapy: heterogeneity of segmental response and correlation with plasma-oxidized low density lipoprotein. J Am Coll Cardiol. 2001;37(3):766–774. doi: 10.1016/s0735-1097(00)01180-3. [DOI] [PubMed] [Google Scholar]
- 70.Segev A, Strauss BH, Witztum JL, Lau HK, Tsimikas S. Relationship of a comprehensive panel of plasma oxidized low-density lipoprotein markers to angiographic restenosis in patients undergoing percutaneous coronary intervention for stable angina. Am Heart J. 2005;150(5):1007–1014. doi: 10.1016/j.ahj.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Tsimikas S, Witztum JL, Miller ER, et al. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation. 2004;110(11):1406–1412. doi: 10.1161/01.CIR.0000141728.23033.B5. [DOI] [PubMed] [Google Scholar]
- 72.Fraley AE, Schwartz GG, Olsson AG, et al. Relationship of oxidized phospholipids and biomarkers of oxidized low-density lipoprotein with cardiovascular risk factors, inflammatory biomarkers, and effect of statin therapy in patients with acute coronary syndromes: results from the MIRACL (Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering) trial. J Am Coll Cardiol. 2009;53(23):2186–2196. doi: 10.1016/j.jacc.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 73.Bossola M, Tazza L, Merki E, et al. Oxidized low-density lipoprotein biomarkers in patients with end-stage renal failure: acute effects of hemodialysis. Blood Purif. 2007;25(5–6):457–465. doi: 10.1159/000112465. [DOI] [PubMed] [Google Scholar]
- 74.Choi SH, Harkewicz R, Lee JH, et al. Lipoprotein accumulation in macrophages via Toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104(12):1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4(3):211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]