Abstract
Objectives
We sought to assess the in vivo importance of scavenger receptor (SR)-mediated uptake of oxidized low density lipoprotein (OxLDL) in atherogenesis and test the efficacy of human antibody IK17-Fab or IK17 single chain Fv fragment (IK17-scFv), which lack immunological properties of intact antibodies other than the ability to inhibit uptake of OxLDL by macrophages, to inhibit atherosclerosis.
Background
The unregulated uptake of OxLDL by macrophage SR contributes to foam cell formation but the importance of this pathway in vivo is uncertain.
Methods
Cholesterol-fed LDLR−/− mice were treated with intraperitoneal infusion of human IK17-Fab (2.5mg/kg) 3 times/week for 14 weeks. Because these mice developed anti-human antibodies, LDLR−/−/Rag−/− mice (lacking ability to make immunoglobulins due to loss of T and B cell function) were treated with an adenoviral vector encoding Adv-IK17-scFv or control adenoviral-enhanced green fluorescent protein (adv-EGFP) vector intravenously every 2 weeks for 16 weeks.
Results
In LDLR−/− mice, infusion of IK17-Fab was able to sustain IK17 plasma levels for the first 8 weeks, but these diminished afterwards due to increasing murine anti-IK17 antibody titers. Despite this, after 14 weeks a 29% decrease in en face atherosclerosis was noted compared to PBS treated mice. In LDLR−/−/Rag−/− mice, sustained levels of plasma IK17-scFv was achieved by Adv-IK17-scFv mediated hepatic expression, which led to a 46% reduction (P<0.001) in en face atherosclerosis compared to adv-EGFP. Importantly, peritoneal macrophages isolated from Adv-IK17-scFv treated mice had decreased lipid accumulation compared to Adv-EGFP treated mice.
Conclusion
These data support an important role for SR-mediated uptake of OxLDL in the pathogenesis of atherosclerosis and demonstrate that oxidation-specific antibodies reduce the progression of atherosclerosis suggesting their potential in treating cardiovascular disease in humans.
Keywords: oxidation, atherosclerosis, gene therapy, antibodies, scavenger receptors
INTRODUCTION
The pathogenesis of atherosclerosis is complex and involves the impact of many well documented traditional risk factors. Among those, hypercholesterolemia plays a dominant role in the initiation of the fatty streak, the earliest morphological change in the artery. After penetration and binding to the matrix of the intima, it is generally thought that modification(s) of LDL lead to its recognition and unregulated uptake by macrophage scavenger receptors (SR), resulting in cholesteryl ester accumulation. Oxidation of LDL (OxLDL) is generally thought to be one of these important modifications, and a variety of macrophage scavenger receptors redundantly bind OxLDL, including SR-A (I, II, III), CD36, SR-B1 MARCO, LOX-1 and others (1–3). Although there is controversy about their quantitative role in foam cell formation, considerable evidence supports important roles for these receptors in atherogenesis (4–6). We have shown that certain antibodies recognizing oxidation-specific epitopes can block the ability of OxLDL to be taken up by macrophages. For example, the natural IgM antibody, E06/T15, which binds to the phosphocholine (PC) group of oxidized but not native phospholipids(7), blocks the binding and uptake of OxLDL mediated by CD36 and SR-B1 on macrophages(8–10). Indeed, marked in vivo elevation of E06/T15 IgM titers in cholesterol-fed LDLR−/− mice, achieved by immunization with S. pneumoniae, which contains the same PC epitope, ameliorated the progression of atherosclerosis(11). Similarly, infusion of IgM T15 decreased lesion formation in a vein graft model(12) and immunization with PC-keyhole limpet hemocyanin (KLH), which also increased PC-specific antibodies that bound to OxLDL, also retarded lesion progression(13). These and other data (reviewed in Hartvigsen et al(14)) suggest the hypothesis that enhanced titers of antibodies that block OxLDL binding to macrophage SR should decrease foam cell formation, and decrease atherosclerosis.
Immunization with antigens to increase titers of oxidation-specific antibodies initiates a cascade of immunological responses that could impact lesion formation aside from the direct impact of the humoral antibody responses. Furthermore, antibodies of different isotypes have different effector functions, such as the ability to opsonize antigens and fix complement, and also to bind to different Fc receptors, which in turn differ in their biological responses. Therefore, even though an oxidation-specific antibody has the capacity to block OxLDL uptake in vitro, it does not rule out the possibility that its ability to inhibit lesion formation in vivo is due to other immunological properties.
We previously reported the cloning of the first human antibody to OxLDL from a Fab antibody phage display library(15). The Fab antibody IK17 was shown to bind to both OxLDL as well as malondialdehyde modified LDL (MDA-LDL) but not to native LDL or to unrelated antigens, including tetanus toxoid, chicken ovalbumin, type VI collagen, and calf thymus single-stranded DNA. The dissociation constant (Kd) for IK17 was 3.7 × 10−8 mol/L calculated according to Klotz plots. MDA-LDL and Cu-OxLDL were effective competitors, whereas native LDL, native HDL, MDA-modified bovine serum albumin (BSA), 4-hydroxynonenal-modified LDL, (another prominent epitope of OxLDL), MDA-polylysine, and MDA-murine IgG did not compete. On Western blots after SDS-PAGE under reduced conditions, IK17 bound extensively to the protein moiety (apoB) of Cu-OxLDL and MDA-LDL, but not to native LDL or native HDL. IK17 inhibited the uptake of OxLDL by macrophages and also bound to apoptotic cells and inhibited their phagocytosis by macrophages. Intravenously injected IK17 also was targeted to and effectively imaged atherosclerotic lesions in vivo (15–18).
Because neither IK17-Fab nor IK17-scFv have immunological properties of intact antibodies other than their ability to inhibit uptake of OxLDL and apoptotic by macrophages, we hypothesized that if mice treated with these IK17 antibody fragments had reduced atherosclerosis, this would support an important role for SR-mediated uptake of OxLDL in the pathogenesis of atherosclerosis.
METHODS (a detailed description of selected methods is in the attached Supplement)
Preparation of IK17-Fab and IK17-scFv
Preparation of IK17-Fab
Recombinant IK17-Fab was produced in BL21 (DE3) E. coli cells (Invitrogen) and purified using a goat anti-human Fab affinity column (Pierce) to >99% purity as shown by western blotting(15). Residual endotoxin was removed (to >99%) using TritonX 114 (Sigma) as previously described(16). The purified IK17-Fab fully retained its immunoreactivity to MDA-LDL and copper oxidized LDL (Cu-OxLDL), using chemiluminescent immunoassays described below, similar to the starting material.
Generation of IK17 single chain antibody fragment (IK17-scFv) and its adenoviral vector
A full description of these procedures can be found in Supplement. In brief, to convert IK17-Fab into an scFv fragment, two rounds of PCR were used to introduce a seven amino acid linker connecting the VL and VH regions and restriction sites for cloning. The coding region of IK17-scFv was amplified by PCR and then subcloned into HindIII and NotI sites of the eukaryotic expression vector pSecTag2A (Invitrogen), which contains a mouse kappa signal sequence for expression and secretion, and a c-myc tag, allowing detection with an anti-myc antibody. The expression and secretion cassette of IK17-scFv gene was isolated from the pSecTag-IK17-scFv plasmid by a PCR reaction and then inserted into the EcoRV (blunt) site of the adenovirus shuttle plasmid pDelatE1Z, which expresses the transgene from the CMV promoter. The resulting adeno-shuttle plasmid pDeltaE1Z-IK17 was co-transfected with E1-deleted adenovirus backbone genome JM17 DNA into HEK293 cells. Two to three weeks after co-transfection, plaques were isolated and amplified to examine the IK17-scFv expression. Adv-vectors were then purified through two-rounds of CsCl centrifugation and titers were measured in two ways: plaque formation on HEK293 cells (pfu/ml) and OD260 reading (particles/ml). The enhanced green florescent protein (EGFP) gene was inserted into the same adenovirus vector to generate adenovirus expressing GFP (Adv-EGFP), which was used as a control.
Murine Models and Atherosclerosis Studies
Animal protocols were approved by the UCSD and VA Institutional Animal Care and Use Committee (IACUC). All mice used were on the C57BL/6 background and included wild type C57BL/6 (The Jackson Laboratories), LDL receptor knockout (LDLR−/−), and LDL receptor-Rag 1 double knockout mice (LDLR−/−/Rag1−/−)(17). The mice were bred and maintained under specific-pathogen-free conditions unless otherwise noted.
Study 1: Infusion of IK17-Fab in LDLR−/− mice
Twenty-two male LDLR−/− mice (6 weeks old) were placed on a 1.25% cholesterol/21% milk fat diet for 2 weeks to rapidly increase total cholesterol levels and to initiate atherosclerosis and then switched to a normal mouse chow diet enriched with 0.5% cholesterol for another 2 weeks as previously described(18). This diet was then continued for the remainder of the study and the mice (n=11 in each group) were randomized to receive either IK-17 Fab (2.5 mg/kg in sterile PBS) or similar volume PBS, given intraperitoneally 3 times per week for 14 weeks. Blood samples were obtained prior to the 1.25% cholesterol/21% milk fat diet, prior to the 0.5% cholesterol diet, and then at 4 week intervals until the end of the study (total of 6 time points). The animals were then sacrificed by CO2 inhalation, at which time the extent of atherosclerosis was determined.
Study 2: Adenoviral expression of IK17-scFv in C57BL/6 and in LDLR−/−/Rag1−/− mice
In initial studies to validate the ability of Adv-IK17-scFv to express biologically active IK17 into plasma, we injected C57BL/6 mice with varying doses of virus and then measured IK17-scFv titers in plasma as described below. Biologically active IK17-scFv was secreted into plasma, but sustained expression was not possible because of rapidly extinguished hepatic expression of IK17-scFv and because of murine humoral responses to the human IK17. To overcome this, we expressed the Adv-IK17-scFv in LDLR−/−/Rag1−/− mice. For gene transfer, 10-week old male mice (20–25 grams) were anesthetized with 2% isoflurane inhalation and adenovirus vectors were diluted into 100 µl of PBS and injected through the retro-orbital plexus with a 27-gauge needle.
The male LDLR−/−/Rag1−/− mice were divided into 2 groups of 15 animals each. Group A was injected with 1011 viral particles (vp) of Adv-IK17-scFv; group B with same amount of Adv-EGFP. One week after the first injection, the mice were started on a high cholesterol (HC) diet (Harlan-Teklad 8604 standard rodent diet containing 1.25% cholesterol) for 16 weeks. These mice were subsequently treated by repeat injections every 2 weeks. Blood (~100 µl) was collected 2 weeks prior to the study and at 2, 4, 8 and 16 weeks after initiation of the HC diet. After 16 weeks of HC diet, the mice were sacrificed by CO2 inhalation and the entire aorta and heart were removed for atherosclerosis measurements. Two of the mice injected with Adv-IK17-scFv died before the end of the study and their data are not included.
Macrophage binding and competition assay
Binding of biotinylated Cu-OxLDL and MDA-LDL ligands to J774 murine macrophages plated in microtiter wells, and the ability of IK17-Fab or IK17-scFv in culture supernatants or plasma to inhibit binding was assessed by a chemiluminescent binding assay as previously described(11,19), with modifications. A detailed protocol can be found in Supplement.
Peritoneal macrophage isolation and lipid content assessment
Foam cell formation in elicited peritoneal macrophages of experimental mice was determined as previously described(20). To derive mechanistic information for Study 2, in a separate experiment, LDLR−/−/Rag1−/− mice were treated with regular mouse chow, high cholesterol diet, or high cholesterol diet and Adv-IK17-scFv or high cholesterol diet and Adv-EGFP every 2 weeks for 8 weeks. Peritoneal macrophages were isolated from mice 4 days after intraperitoneal injection of thioglycollate. 1.0 × 105 cells were resuspended into 0.5 ml of DME media containing 20 % FCS and added to each well of a 24 well culture plate laid with a round cover slip. After the macrophages were attached to the cover slip (about 3 hours), the cells were washed with PBS and fixed with formaldehyde/sucrose solution. The cells were then stained with heated Oil red O/propylene glycol solution and mounted. The lipid loaded macrophages were counted using a microscope with a visual grid, and expressed as percent foam cells per total cells counted.
Chemiluminescent immunoassays, immunohistochemistry and atherosclerosis quantification
Methodologies for these assays are described in the Supplement.
Statistical analysis
Differences between groups in atherosclerosis measures and immunohistochemistry parameters were analyzed by Student’s t-test. Analysis of quantitative parameters of oxidation biomarkers within groups of mice over time (4 timepoints, Fig 1B) and of % Oil Red O staining (4 groups, Fig 6B) was performed with repeated measures ANOVA with post hoc Bonferroni correction. P values <0.05 are considered significant. Data are given as mean ± SEM unless otherwise noted.
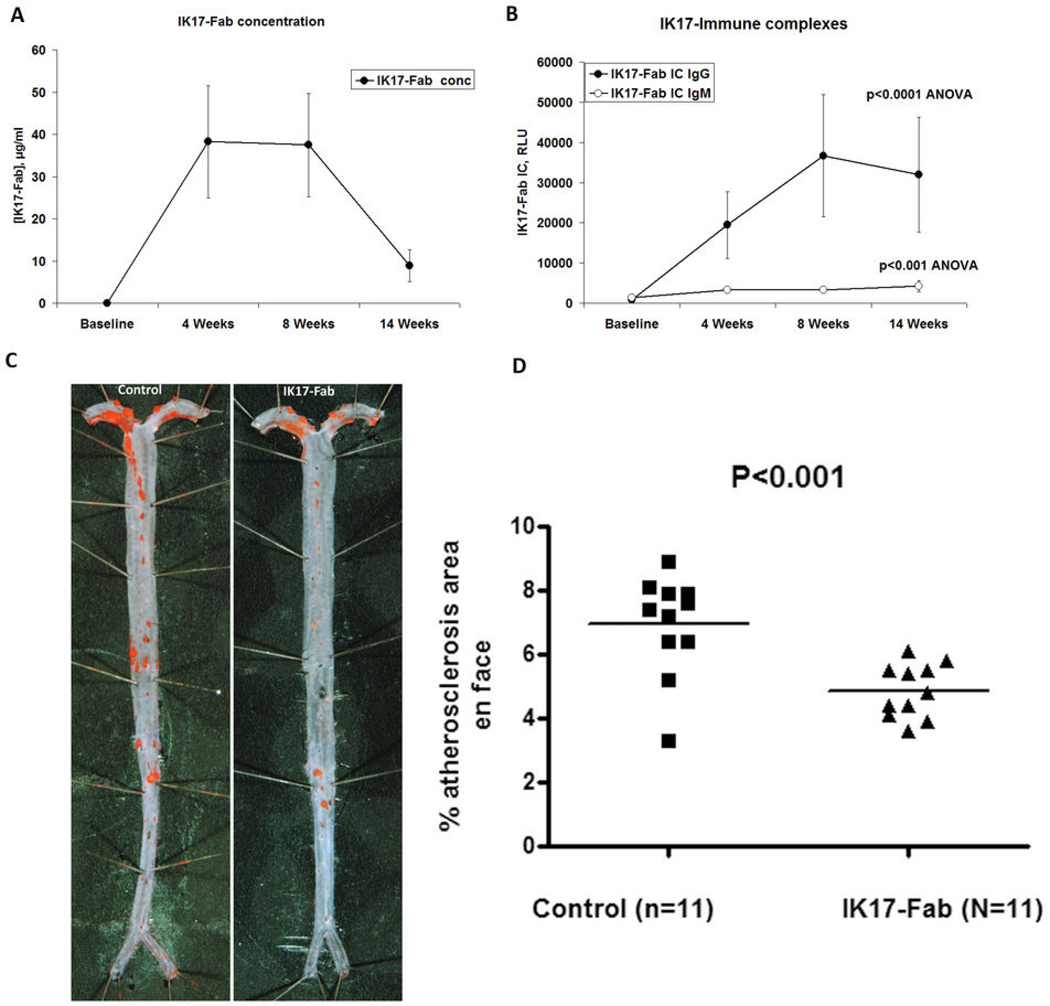
Figure 1. Impact of injection of IK17-Fab on atherosclerosis in LDLR−/− mice in Study 1.
(A) Plasma IK17-Fab concentrations and (B) IK17-Fab mouse IgG or IgM immune complexes in Study 1. Increases in both IgM and IgG titers are significant (P< 0.001 ANOVA with Bonferroni correction). (C) Representative aortic images from a PBS Control or IK17-Fab injected LDLR−/− mouse stained with Sudan IV. (D) Quantitative analysis of en face atherosclerotic area in the entire aorta. Data are expressed as percentage of Sudan IV stained area of entire aorta examined.
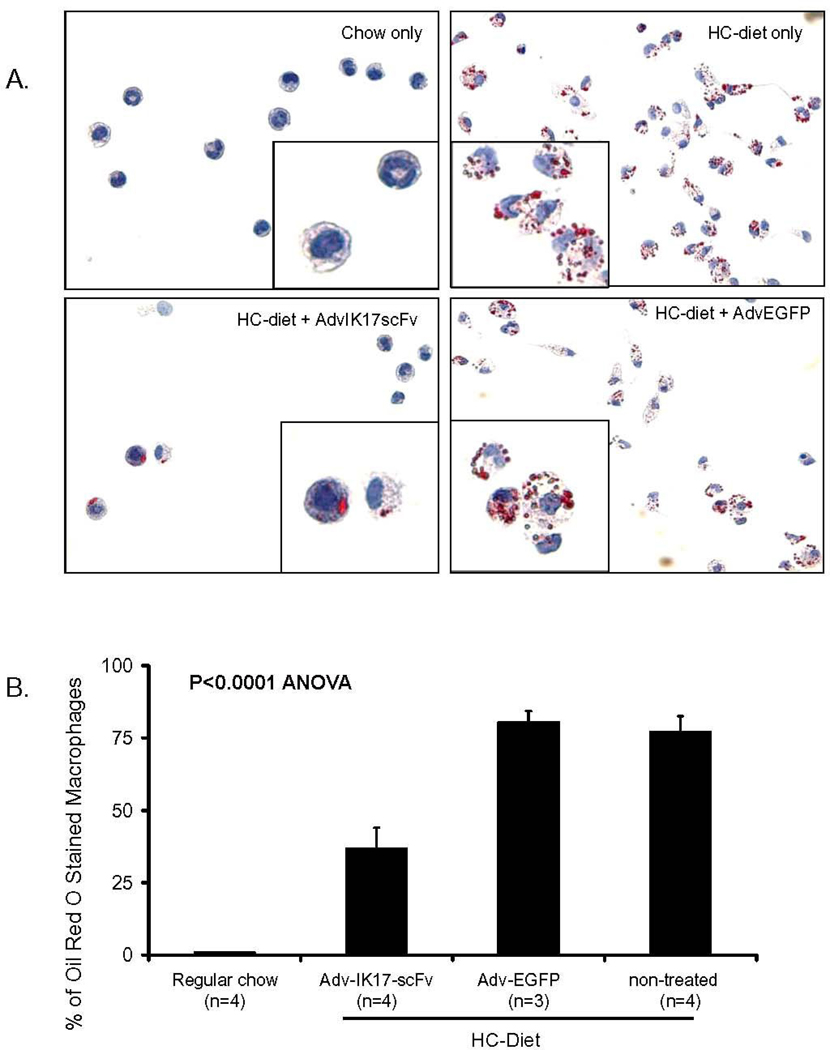
Figure 6. Adv-IK17-scFv reduces foam cell formation in elicited peritoneal macrophages.
(A) Oil red O staining of macrophages isolated from LDLR−/−/Rag1−/− mice fed regular chow, or HC-diet only (non-treated), or HC-diet + Adv-IK17-scFv or HC-diet + Adv-EGFP treatment for 16 weeks as indicated. (B) Quantification of macrophages that contained lipid droplets as a percentage of total cells counted.
RESULTS
Study 1: IK17-Fab infusion inhibits atherosclerosis progression in LDLR−/− mice
There were no significant differences in weight gain, plasma cholesterol or triglyceride levels between groups over time (Table 1). Significant increases in autoantibodies to MDA-LDL, apoB-immune complexes and IgM “T15/E06” levels occurred in both groups in response to the diet, but no significant differences were present between groups (Supplemental Fig. 1).
Table 1.
Effects of high cholesterol diet and IK17-Fab or IK17-scFv on weight and lipid profiles.
| Study Time Course |
− 4 week (21% fat/1.25% chol diet) |
− 2 week (0.5 % HC- diet) |
0 week (Intervention start) |
4 week | 8 week | 14 week (Endpoint) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental Groups (n=11) |
IK17- Fab |
Control | IK17- Fab |
Control | IK17- Fab |
Control | IK17- Fab |
Control | IK17- Fab |
Control | IK17- Fab |
Control |
| Weight (gram) ± SEM | 17.7 ± 0.6 | 17.9 ± 0.4 | 24.2 ± 0.5 | 23.5 ± 0.4 | 24.9 ± 0.6 | 24.1 ± 0.4 | 27.0 ± 0.6 | 25.0 ± 0.5 | 28.0 ± 0.7 | 26.5 ± 0.5 | 30.3 ± 0.8 | 28.0 ± 0.5 |
| Total Cholesterol (mg/dl) ±SEM | 327 ± 9.6 | 337 ± 8.3 | 1420 ± 75.7 | 1532 ± 50.3 | 734 ± 24.3 | 752 ± 24.6 | 612 ± 25.3 | 676 ± 33.8 | 640 ± 30.2 | 654 ± 40.3 | 629 ± 43.9 | 536 ± 23.1 |
| Triglyceride (mg/dl) ±SEM | 201 ± 11.3 | 173 ± 7.3 | 410 ± 51.7 | 372 ±45.2 | 126 ± 6.8 | 109 ± 7.8 | 117 ± 9.5 | 130 ± 13.4 | 98 ± 7.9 | 104 ± 11.1 | 190 ± 30.3 | 145 ± 9.0 |
| Study 2 Time Course |
−2 week (Baseline) |
8 week (Midpoint) |
16 week (Endpoint) |
|||
|---|---|---|---|---|---|---|
| Experimental Groups (n) |
Adv-IK17-scFv (13) |
Adv-EGFP (15) |
Adv-IK17-scFv (13) |
Adv-EGFP (15) |
Adv-IK17-scFv (13) |
Adv-EGFP (15) |
| Weight (gram) ± SEM | 23.9 ± 0.4 | 24.1 ± 0.6 | 23.6 ± 0.5 | 24.1 ± 0.5 | 23.0 ± 0.7 | 23.7 ± 0.6 |
| Total Cholesterol (mg/dl) ± SEM | 187 ± 3.3 | 200 ± 6.5 | 984 ± 41.6 | 1010±46.8 | 741 ± 44.8 | 831 ± 35.9 |
| Triglyceride (mg/dl) ± SEM | 87 ± 5.0 | 89 ± 3.4 | 71 ± 6.3 | 85 ± 6.8 | 104 ± 15.5 | 91 ± 7.8 |
Weight, total cholesterol (TC) and triglyceride (TG) depicted are at the beginning of the diet period. In Study 1, 21% milkfat/1.25% cholesterol diet started at −4 week and switched to 0.5 % HC-diet at −2 week before the infusion of IK17-Fab (0 week); In study 2, adenovirus gene therapy started one week before 1.25 % HC-diet feeding (0 week). Data are expressed as mean ± SEM. No statistically significant differences were noted in total cholesterol levels between groups at each timepoint in both studies.
IK17-Fab plasma concentrations and IK17-Fab Immune complexes
As expected, substantial elevations in plasma levels of IK17-Fab concentrations were documented at 4–8 weeks during the ongoing IK17-Fab infusions. However, IK17-Fab plasma levels diminished significantly by the end of the study (Fig. 1A), corresponding to a rise in titers of murine IgG (31140±8198 vs. 335±105 RLU, p<0.001) and IgM (17140±3397 vs. 2009±262 RLU, p<0.001) anti-IK17 antibodies in the IK17-Fab injected group (data at 1:500 plasma dilution). This was also associated with increased plasma levels of murine IgM and IgG IK17-Fab/immune complexes. (Fig. 1B).
Impact of IK17-Fab on atherosclerosis progression
Despite the immune response to IK17-Fab, there was a 29% decrease in en face atherosclerosis in the IK17 treated LDLR−/− mice compared to mice treated with PBS (4.9±0.25% versus 6.9±0.47%, P<0.001) (Figs. 1C and D). In fact, the mice with the lowest anti-IK17 immune response also had the least atherosclerosis, and for the group as a whole the plasma titers of IgG anti-IK17 antibodies correlated positively with the extent of en face lesion formation in the IK17-Fab treated mice (Spearman correlation r=0.87, P <0.001) Analysis of cross sections at the level of the aortic valve did not reveal differences between the two groups (1.93 ± 0.20 mm2 versus 2.12 ± 0.13 mm2 total lesion area, P=0.42). Immunohistochemistry of the aortic root for macrophages, SMC, collagen and MDA epitopes, revealed a strong trend (p=0.08) for reduced MDA staining but no differences in other measures (Supplemental Fig. 2).
Study 2: Adenoviral expression of IK17-scFv inhibits progression of atherosclerosis in LDLR−/− /Rag1−/− mice
To overcome the likely neutralization of the infused IK17 Fab by mouse anti-IK17 antibodies, we generated IK17 as a functional scFv and expressed it via an adenoviral vector in LDLR−/−/Rag1−/− mice, which lack T and B cells.
Generation of functional IK17-scFv
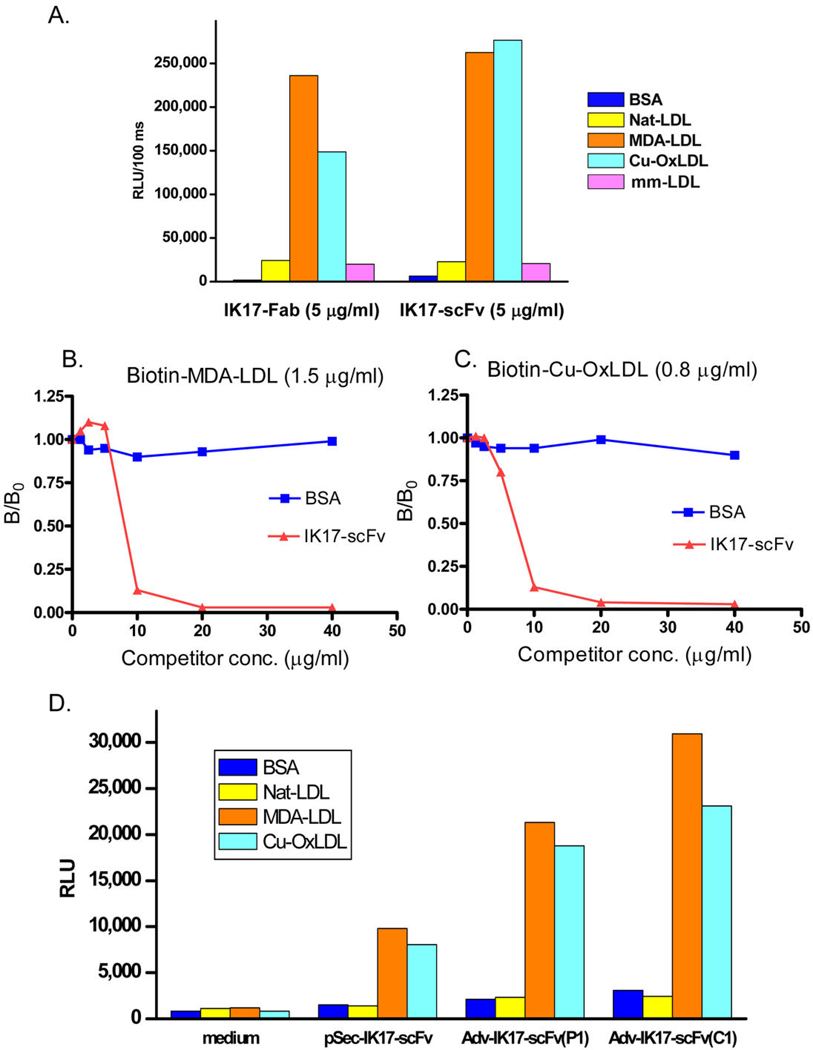
IK17-scFv was generated and purified to near homogeneity as described in detail in Supplement. Similar to the parent IK17-Fab, the purified IK17-scFv displayed the same binding to OxLDL and MDA-LDL when examined by chemiluminescent ELISA (Fig. 2A), and inhibited the binding of both MDA-LDL (Fig. 2B) and OxLDL (Fig. 2C) to J774 macrophages. Examination of the binding of the IK17-scFv gene product from the eukaryotic expression vector pSecTag2A (Fig. 2D) and the adenovirus shuttle vector pDelta-E1Z revealed the same binding properties as purified IK17-Fab. Furthermore, the IK17-scFv recombinant adenovirus (Adv-IK17-scFv) rescued from 293 cells was infectious and cells infected with Adv-IK17-scFv expressed and - secreted IK17-scFv into the culture medium (Supplemental Fig. 3) and retained the binding properties of the parent IK17-Fab (Fig. 2D).
Figure 2. Characterization of Adv-IK17-scFv expression in vitro.
(A) Binding of purified IK17-Fab and IK17-scFv to MDA-LDL and Cu-OxLDL, but not to native LDL (Nat-LDL) or minimally modified LDL (mm-LDL). The ELISA was done on the same antigen coated plates but respective IK17-Fab or IK17-scFv detected with goat-anti-human IgG Fab-AP or anti-c-myc-AP respectively. Extent of binding is expressed as relative light units/100 msec (RLU). (B and C) Purified IK17-scFv inhibits the binding of Cu-OxLDL or MDA-LDL to J774 macrophages. Fixed concentration of biotinylated MDA-LDL (1.5 µg/ml; B) or Cu-OxLDL (0.8 µg/ml; C) were incubated with freshly purified IK17-scFv at indicated concentrations. Data expressed as a ratio of binding in the presence of competitor (B) divided by binding in the absence of competitor (B0). (D) Binding properties of IK17-scFv gene products from the eukaryotic expression vector pSecTag2A and from the culture medium of HEK293 cells infected with Adv-IK17-scFv. Shown are ELISA assays to indicated antigens of control media (no infection) and media from initial plaque (P1), and media from a single clone (C1) (all at 1:50 dilution).
The adenovirus IK17-scFv transgene was expressed and secreted into the circulation
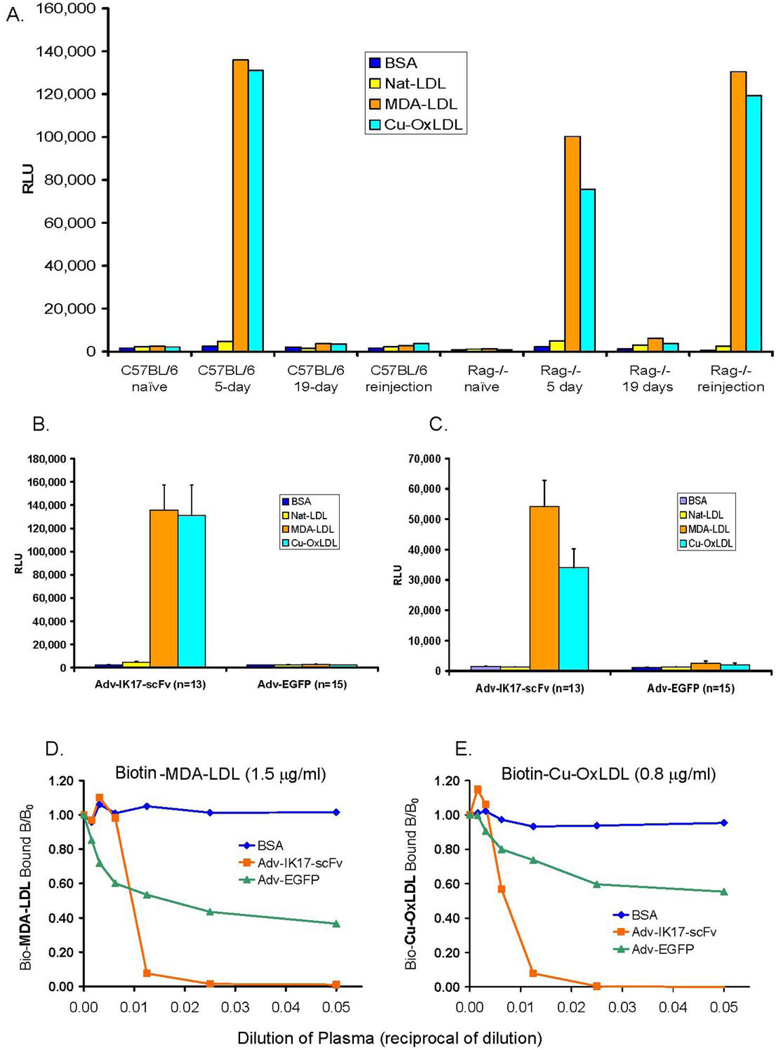
In an initial study, we injected 2.5×1011 vp of Adv-IK17-scFv into wild type C57B6 and LDLR−/−/Rag−/− mice via tail vein and collected blood samples at 5, 19, and 28 days post injection. Functional IK17-scFv, detected by binding to MDA-LDL and Cu-OxLDL, was detectable in plasma at day 5, but was lost after 19 days (Fig. 3A). Repeat injection 21 days after the initial injection did not result in the appearance of plasma IK17-scFv binding activity 7 days later. We reasoned that the host immune response extinguished the adenoviral expression of IK17-scFv, both at the level of the liver and by mounting an antibody response to the human transgene. Indeed, we demonstrated murine IgG and IgM titers to the IK17-scFv gene product from the plasma 19 days post injection of Adv-IK17-scFv in wild type LDLR−/−mice (data not shown). To overcome this problem, we injected Adv-IK17-scFv into LDLR−/−/Rag1−/− mice. However, even in these mice that lack both B and T cells, IK17-scFv expression in plasma was also limited to less than 3 weeks, presumably due to methylation of the CMV promoter in the liver(21). However, in these mice, repeated injections of Adv-IK17-scFv restored the transgene expression each time for approximately 3 weeks (Fig. 3A).
Figure 3. Injection of Adv-IK17-scFv delivers functional IK17-scFv into mouse plasma.
(A) ELISA of plasma from wild type C57BL/6 and the LDLR−/−/Rag1−/− mice at indicated time post-injection with 2.5 × 1011 virus particles of Adv-IK17-scFv. The binding to each antigen is the average of results from two mice from each group assayed, with all determinations in the same assay. Repeat injections of Adv-IK17-scFv restored the expression of transgene in LDLR−/−/Rag1−/− mice, but not in C57BL/6 wt mice. (B and C) IK17-scFv plasma expression in LDLR−/−/Rag1−/− mice throughout 16 weeks of gene transfer in Study 2. Shown are binding to indicated antigens (in RLU/100 msec) using plasma (1:50 dilution) from Adv-IK17-scFv or Adv-EGFP injected mice one week post-injection with 1011 of indicated viral particles (B), as well as the terminal blood after 16 weeks of HC-diet and 9 biweekly injections (C). (D and E) Adenovirus expressed IK17-scFv inhibits the binding of OxLDL to J774 macrophages. Fixed concentration of biotinylated MDA-LDL (1.5 µg/ml; D) or Cu-OxLDL (0.8 µg/ml; E) were incubated with indicated dilutions of pooled plasma from Adv injected animals (graphed as reciprocals, e.g. 1/100 = 0.01). Y axis indicates the biotinylated OxLDL bound to macrophages expressed as a ratio of binding in the presence of competitor (B) divided by binding in the absence of competitor (B0). In D and E, BSA alone is used as a comparator.
Sustained expression of IK17-scFv for 16 week intervention study
By repeatedly injecting Adv-IK17-scFv at two week intervals, we maintained a sustained and high titer of IK17-scFv in plasma over a 16 week period. Using real-time PCR to monitor tissue expression of the injected transgene, we demonstrated that it was predominantly expressed in liver as expected (Supplemental Fig. 4). By monitoring IK17-scFv binding to MDA-LDL and OxLDL by ELISA, we demonstrated sustained expression of functional IK17-scFv in plasma throughout the 16 weeks of gene transfer (Figs. 3B and C). A competitive immunoassay demonstrated that the binding of secreted IK17-scFv to both OxLDL and MDA-LDL was inhibited by both MDA-LDL and OxLDL, but not native-LDL, indicating that the plasma IK17-scFv retained its specific binding properties (Supplemental Fig. 5). Furthermore, the secreted IK17-scFv retained its functional property to inhibit the binding of MDA-LDL and OxLDL to macrophages (Figs. 3D and E). Serial dilutions of plasma from mice injected with Adv-IK17-scFv displayed a dose dependent inhibition of binding of MDA-LDL and OxLDL to J774 macrophages and at a dilution of 1:40 completely abolished binding. This degree of inhibition was much greater than that observed with equivalent dilutions of plasma from control mice injected with the Adv-EGFP vector. Although plasma of the Adv-EGFP mice also showed a mild degree of inhibition, this also occurred in plasma from wild type mice, which is in part nonspecific and in part due to plasma proteins that may bind OxLDL (data not shown).
Plasma from Adv-IK17-scFv injected mice immunostain advanced atherosclerotic lesions
To demonstrate that the secreted IK17-scFv could localize to atherosclerotic lesions, a 1:20 dilution of plasma from an Adv-IK17-scFv injected mouse was used to immunostain atherosclerotic lesions from a human brain artery (Fig. 4). The IK17-scFv preferentially stained the necrotic cores (Fig. 4A), as previously observed with affinity purified intact IK17-Fab(22). The same dilution of plasma from Adv-EGFP injected mouse produced no specific immunostaining (Fig. 4B). The secreted IK17-scFv also localized to its own atherosclerotic lesions as documented at time of sacrifice in aortic sections that were obtained from mice expressing the Adv-IK17-scFv and immunostained for IK17-scFv using an anti-myc antibody. There was specific localization (brown areas) of IK17-scFv to atherosclerotic lesions (Fig. 4C). In contrast, while there was light and diffuse staining of tissue by plasma of Adv-EGFP mice, there was no specific localization to the atherosclerotic lesions (Fig. 4D).
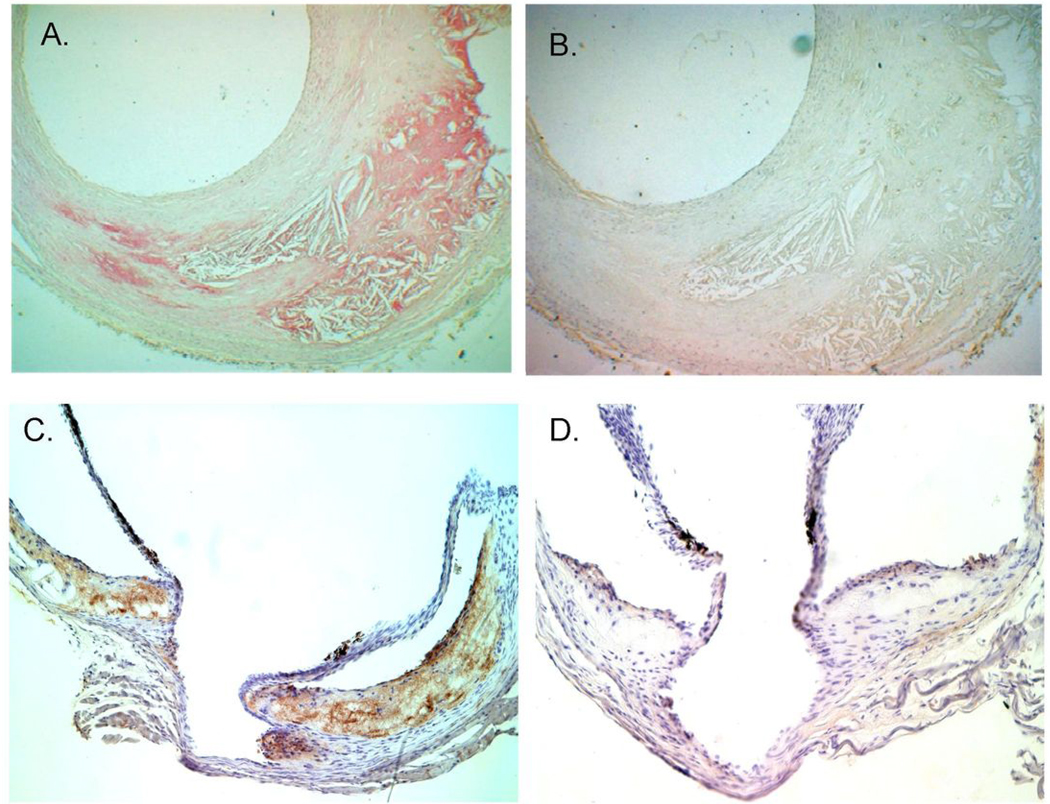
Figure 4. Adv expressed IK17-scFv immunostains atherosclerotic lesion.
Pooled plasma (1:20 dilution) from Adv-IK17-scFv mice (A) or control Adv-EGFP mice (B) were used to immunostain human brain artery atherosclerotic lesion. Plasma samples were obtained from respective mice 5 days post injection. (C and D) demonstrate that the Adenoviral expressed IK17-scFv localizes to atherosclerotic lesions in the cholesterol-fed LDLR−/−/Rag1−/− mice. Cross-sections at the aortic valve obtained from mouse treated with Adv-IK17-scFv (C) or with Adv-EGFP (D). Sections were obtained at time of sacrifice and immunostained with biotinylated-anti-c-myc.
Impact of plasma IK17-scFv on atherosclerosis
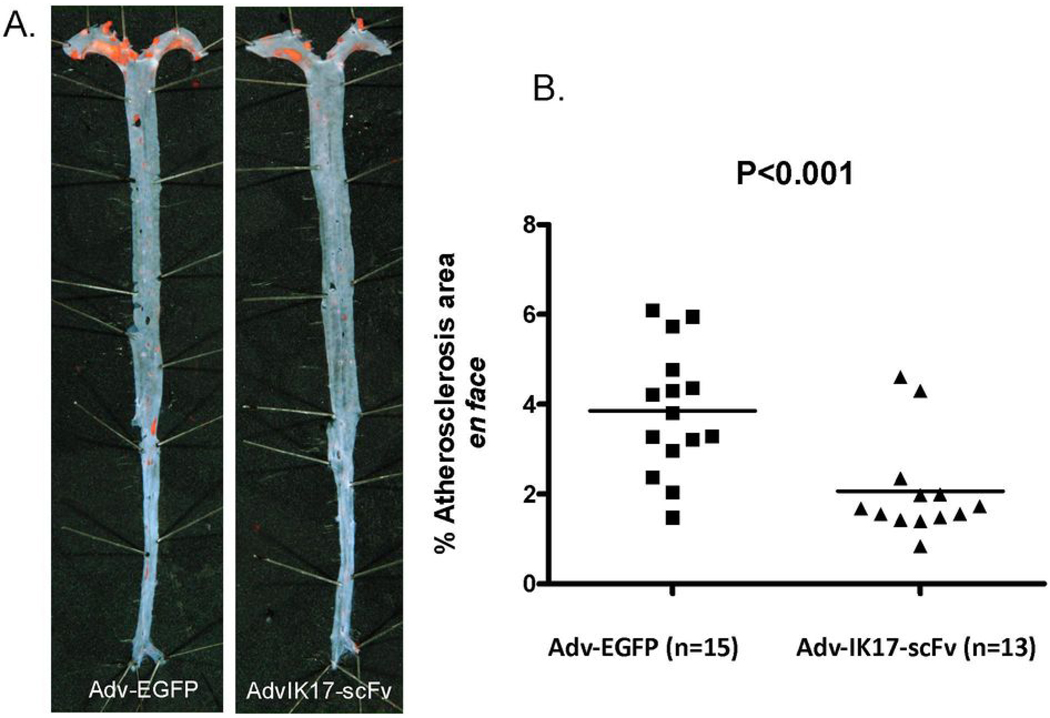
We evaluated the impact of the secreted IK17-scFv on the extent of atherosclerosis in the LDLR−/−//Rag−/− mice during a 16 week period of feeding the HC diet, compared to mice receiving the Adv-EGFP vector under an identical protocol. Both groups of mice gained weight equally and had similar plasma cholesterol and triglyceride levels during the intervention period (Table 1). The extent of en face aortic lesion area was significantly reduced in the Adv-IK17-scFv treated mice compared to the Adv-EGFP treated mice (Fig. 5A), leading to a 46 % reduction in lesion area (2.1% ± 0.3 versus 3.9% ± 0.4, P<0.001, Fig. 5B). Cross-sectional analysis at the aortic origin revealed only small lesions, and no statistically significant difference (0.27 ± 0.05 mm2 versus 0.23 ±0.06 mm2, total lesion area, P=0.62).
Figure 5. Adv-IK17-scFv reduces atherosclerosis in LDLR−/−/Rag1−/− male mice.
(A) Representative Sudan IV stained en face preparations of aortas from mice injected with Adv-IK17-scFv or Adv-EGFP. (B) Quantitative analysis of atherosclerotic surface area in the entire aorta. Data expressed as percentage of Sudan IV stained area of the entire aorta examined.
Effect of Adv-IK17-scFv on macrophage foam cell formation
Previously, Li et al(20) have shown that the extent of foam cell formation observed in elicited peritoneal macrophages reflected the extent of foam cell formation noted in the aorta of mice following various experimental interventions. Therefore, we elicited peritoneal macrophages from Adv-IK17-scFv and Adv-EGFP mice, as well as from LDLR−/−/Rag1−/− mice fed either chow or the HC diet. In contrast to macrophages isolated from mice that were fed a normal chow diet, macrophages isolated from hypercholesterolemic mice exhibited extensive Oil red O droplets (Fig. 6A, chow-only and HC-diet only). Macrophages from the Adv-IK17-scFv treated mice showed a marked reduction of lipid accumulation (Fig. 6A, HC-Diet + AdvIK17-scFv) compared to macrophages isolated from the Adv-EGFP mice or the HC fed diet (Fig. 6A, HC-Diet only and HC-Diet +Adv-EGFP). We also quantified the number of macrophages showing lipid droplets/100 cells counted. Macrophages isolated from Adv-IK17-scFv treated mice had significantly fewer cells with lipid droplets compared to those from Adv-EGFP treated, or cholesterol-fed non-treated LDLR−/−/ Rag1−/− / mice (36.8 % ± 7.2 (n=4) versus 80.5 % ± 3.7 (n=3) or 77.2 % ± 5.4 (n=4), P<0.0001) (Fig. 6B). This observation suggests that the IK17-scFv significantly decreased foam cell formation, consistent with the demonstrated ability of the plasma of the IK17-scFv treated mice to inhibit uptake of OxLDL by macrophages, as shown in Fig. 3D and 3E.
DISCUSSION
This study demonstrates that infusion of the IK17-Fab into LDLR−/− mice inhibits atherosclerotic lesion formation. Furthermore, adenoviral mediated expression of IK17 in vivo as a functional single chain antibody, IK17-scFv, also led to inhibition of foam cell formation and decreased atherosclerosis. These data support the hypothesis that a major mechanism by which oxidation-specific antibodies inhibit atherosclerosis is by blocking OxLDL binding to macrophages, supporting an important role for SR-mediated uptake of OxLDL in the pathogenesis of atherosclerosis. Furthermore, these results suggest the potential of oxidation-specific antibodies in treating cardiovascular disease in humans.
Although atherogenesis is a highly complex process, it is strongly related to elevated plasma LDL cholesterol levels. Once “native” LDL penetrates the intima, it binds to the glycoprotein matrix, which prolongs its half-life and renders it susceptible to a variety of modifications (1,23). Among these, the generation of OxLDL has been widely studied and considered important, as OxLDL is recognized by a variety of SRs on macrophages, leading to unregulated uptake. Indeed, although excellent experimental studies in genetically targeted mice have supported the importance of SR-A and CD36 in atherogenesis(24–26), other such studies have provided opposing data and conclusions(6,27). Aside from differences in experimental conditions(4), the nature of the modified lipoprotein ligands for the SRs induced by diet may be an important variable in such studies(16). In addition, such SRs are innate “pattern recognition receptors” and may well have other vital functions(14,28). Deleting them via genetic targeting may have disrupted other important properties involved in immunity and atherogenesis, and/or led to unknown compensatory functions that may complicate the interpretation of their importance in uptake of OxLDL. Therefore, inhibiting uptake of OxLDL with oxidation-specific antibody fragments lacking immunological properties other than their ability to recognize their respective epitopes allows one to examine the importance of SR-mediated uptake of OxLDL in atherogenesis.
We previously characterized IK17-Fab, the first cloned human antibody to OxLDL. It binds to a different epitope than that recognized by E06(7), one contained on both OxLDL and MDA-LDL, but like E06, it has the ability to block the uptake and degradation of OxLDL by macrophages. To date, we have not fully characterized the exact epitope to which IK17 binds, though it appears to be a complex MDA type structure, present in both the lipid and protein moieties of OxLDL and MDA-LDL. Importantly, IK17-Fab immunostains atherosclerotic lesions in mice, rabbits and humans, especially the necrotic core(22,29), and has been used to successfully image lesion formation in vivo in LDLR−/− and apoE−/− mice(15,30). It thus targets relevant epitopes in lesions and would be strategically positioned to inhibit OxLDL uptake by macrophages in vivo. Indeed, in this study we demonstrate that either the passive transfer of IK17-Fab to LDLR−/− mice, or the adenoviral-mediated expression of IK17-scFv in LDLR−/−/Rag1−/− mice, inhibited atherosclerosis progression. To our knowledge, neither IK17-Fab, nor the IK17-scFv have any immunological properties of intact antibodies other than the ability to bind to oxidation-specific epitopes, and thus inhibit uptake of OxLDL by macrophages. Furthermore, they achieve this inhibition by binding to SR ligands on OxLDL, and not by interfering directly with SR functions. We believe these data strongly support the hypothesis that SR mediated uptake of OxLDL plays an important role in atherogenesis, particularly in the setting of a high cholesterol diet used in these studies(16). Indeed, the demonstration that in vivo foam cell formation in peritoneal macrophage was inhibited by the expression of IK17-scFv provides direct evidence to support this hypothesis.
In Study 1, we injected purified LPS-free IK17-Fab into LDLR−/− mice. The impact of IK17-Fab infusion was limited by the development of murine anti-IK17-Fab antibodies during the latter part of the intervention period, and indeed, at time of sacrifice, the titer of murine anti-IK17-Fab antibodies correlated directly with lesion formation, suggesting that depletion of IK17-scFv was associated with greater lesion formation. Despite these limitations, lesion formation was inhibited by 29%, supporting the hypothesis that macrophage SR uptake plays an important role in early lesion formation. This conclusion was strongly supported by the findings in Study 2, in which a biologically functional IK17-scFv was generated and expressed in plasma for a period of 16 weeks. The IK17-scFv is a linear protein in which the antigen-binding domains of the antibody, the respective CDR3 regions of the VH and VL genes, are joined by a short synthetic linker that allows appropriate folding of the expressed protein to effect the biological properties of the intact IK17-Fab. IK17-scFv lacks any of the effector properties of an intact antibody other than its ability to bind to its target epitope. Sustained expression of IK17-scFv was accomplished by biweekly injections of Adv-IK17-scFv into LDLR−/−/ Rag1−/− mice. Not only was en face lesion formation inhibited substantially, by 46%, but foam cell formation was inhibited in peritoneal macrophages isolated from these mice. Inhibition of foam cell formation was not seen in LDLR−/−/Rag1−/− mice injected in an identical protocol with the same Adv vector but bearing the EGFP gene.
It should be noted that in Study 2 we maintained a small cohort of LDLR−/−/Rag1−/− mice on the same HC diet but performed no interventions. The plasma cholesterol levels in these mice were lower than in both groups of Adv-injected mice and at time of sacrifice, the extent of atherosclerosis was similar to the mice injected with the Adv-IK17-scFv. This suggests that the repeated injections of Adv in this protocol increased both plasma cholesterol levels and extent of lesion formation. Since cholesterol levels of Adv-EGFP and Adv-IK17-scFv injected animals were the same throughout the study, this demonstrates that despite the apparently increased atherogenic stress caused by the repeated Adv administration, IK17-scFv decreased lesion formation. Another caveat is that the IK17 interventions only decreased lesion formation in the en face analyses, and not at the aortic root. We do not know the reasons for this. In our previous study with immunization of LDLR−/− mice with MDA-LDL, we observed the opposite, i.e. decreased lesion formation at the aortic root, but not distally. In those studies we used a high-fat, HC- diet and plasma cholesterol levels exceeded 1,500 mg/dl. In the current studies, we have used a cholesterol-enriched diet that does not have any added fat and produces more modest hypercholesterolemia, and almost devoid of VLDL. High VLDL cholesterol levels observed in high-fat, HC-diet have been associated with enhanced lesion formation at the aortic origin(31). With the HC-diet used in our current study, we have observed smaller lesions at the aortic root, and in particular, in the LDLR−/−/Rag−/− mice, the aortic root lesions were only ~10% of those seen in the LDLR−/− mice.
These studies not only provide evidence to support an important role for SR uptake of OxLDL in macrophage foam cell formation, but demonstrate the potential therapeutic efficacy of interventions that target OxLDL uptake by macrophages, such as oxidation-specific antibodies or antibody fragments. We and others have previously shown that raising the titer of E06/T15 antibodies directed to OxPL by immunization strategies(11,13,32) or by direct infusion(12) inhibited atherosclerosis progression, and the current study, and data of others (32,33) have shown that direct infusion of a Fab or an IgG1 to MDA-type oxidation-specific epitopes also limits lesion formation.
In the current manuscript, we also show the feasibility of utilizing gene transfer techniques to deliver into plasma an antibody fragment capable of influencing macrophage foam cell formation. The adenovirus encoding IK17-scFv produced a significant level of transgene in the plasma 4–5 days post-injection, which displayed the identical binding properties as its parental IK17-Fab(22). It maintained its ability to bind to and block the uptake of OxLDL by macrophages in culture and immunostained oxidation-specific epitopes in atherosclerotic lesions. Most importantly, it decreased foam cell formation in peritoneal macrophages and substantially inhibited the progression of atherosclerosis. Therefore, this gene transfer experiment demonstrates that a scFv gene can be delivered and expressed in tissues, leading to the secretion into plasma of a biologically active antibody fragment.
In our initial gene transfer experiment in wild type LDLR−/− mice, there was the rapid extinguishing of the Adv mediated expression of IK17-scFv, which we thought secondary to adaptive immune responses. However, we were surprised that the Adv-IK17-scFv was also rapidly extinguished after two weeks even in LDLR−/−/Rag1−/− mice, which lacks adaptive responses. This most likely involved inactivation of the CMV promoter by hypermethylation(21). If one were to apply such gene transfer techniques to humans, the use of alternative promoters and the use of fully human antibody fragments would limit such inadequacies. The Adv vector used in this study was selected to afford high titers to allow demonstration of the feasibility of this approach, and obviously more appropriate vectors would be needed for the long-term expression in other animal models, or in eventual application to humans. The recent reports of the long term expression of gene products in humans using lentiviral vectors(34,35) offers promise that one day vectors will be developed that will allow safe and sustained gene transfer, which could then be utilized to express antibodies such as the IK17-scFv described in this report.
Limitations
Immunohistochemistry was performed only in the aortic root where the lesions were very small and there were no differences in treatment effect in the extent of atherosclerosis. Because the remaining aorta was used for extensive processing for Sudan staining and en face atherosclerosis quantitation, it was not available for immunohistochemical analyses to assess differences in cellular, matrix and oxidation-specific epitope content in regions of the aorta where a treatment effect was present. Future studies will focus on changes in plaque characteristics and atherosclerosis regression in response to human oxidation-specific antibodies.
Clinical Implications
Our data demonstrate the feasibility of direct infusion of human oxidation-specific antibodies to inhibit lesion uptake of OxLDL by macrophages, which presumably plays an important role in macrophage activation as well as participating in the induction of macrophage apoptosis and death(36). Oxidation-specific antibodies such as IK17 and E06 have recently been shown to strongly immunostain human vulnerable and ruptured plaques(37). OxPL are also released from clinically relevant human lesions in significant quantities and can be captured by distal protection devices following percutaneous coronary and carotid interventions(38). One might envision the infusion of oxidation-specific antibodies in the setting of unstable or acute coronary syndromes to rapidly stabilize plaques or use prior to percutaneous coronary, carotid and peripheral interventions to neutralize released pro-inflammatory oxidized lipids(39). In addition, these experiments provide a template for utilizing the biologically functional scFv as a reagent targeting the vulnerable plaque for imaging approaches or to mediate delivery of therapeutic agents or drugs(15,30). If this approach is translated to humans, we anticipate its initial application to be by direct infusion of an appropriate dose of the antibody, similar to other antibody therapeutics for inflammatory conditions and cancer treatment. Finally, one might also envision a therapeutic approach leading to a more sustained elevation of titers of oxidation-specific antibodies, which could be accomplished by a variety of techniques, including gene transfer techniques once safe and effective vectors are developed, as well as the use of vaccine approaches.
Supplementary Material
Acknowledgments
We thank Joe Juliano, Karen Bowden and Florence Casanada for their technical assistance in these studies.
Funding Sources: These studies were supported by NIH Grants R01 HL086559 (JW), PO1 HL0888093 (JW, ST, KH), R01 HL087391 (AL), by a grant from the Fondation Leducq (JW,ST) and by AHA Scientist Development Awards to KH and PXS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Drs. Tsimikas, Shaw and Witztum are named as inventors on patents and patent applications for the potential commercial use of antibodies to oxidized LDL held by the University California, San Diego. Drs. Tsimikas and Witztum are directors and have equity interest in Atherotope, Inc. The other authors report no conflicts.
References
- 1.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 2.Boullier A, Bird DA, Chang MK, et al. Scavenger receptors, oxidized LDL, and atherosclerosis. Annals of the New York Academy of Sciences. 2001;947:214–222. doi: 10.1111/j.1749-6632.2001.tb03943.x. discussion 222-3. [DOI] [PubMed] [Google Scholar]
- 3.Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. Journal of Lipid Research. 2009;50 Suppl:S282–S286. doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witztum JL. You are right too! Journal of clinical investigation. 2005;115:2072–2075. doi: 10.1172/JCI26130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuchibhotla S, Vanegas D, Kennedy DJ, et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovascular Research. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore KJ, Kunjathoor VV, Koehn SL, et al. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. Journal of clinical investigation. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw PX, Horkko S, Chang MK, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. Journal of Clinical Investigation. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horkko S, Bird DA, Miller E, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boullier A, Gillotte KL, Horkko S, et al. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. The Journal of biological chemistry. 2000;275:9163–9169. doi: 10.1074/jbc.275.13.9163. [DOI] [PubMed] [Google Scholar]
- 10.Gillotte-Taylor K, Boullier A, Witztum JL, Steinberg D, Quehenberger O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. Journal of lipid research. 2001;42:1474–1482. [PubMed] [Google Scholar]
- 11.Binder CJ, Horkko S, Dewan A, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nature Medicine. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 12.Faria-Neto JR, Chyu KY, Li X, et al. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis. 2006;189(1):83–90. doi: 10.1016/j.atherosclerosis.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Caligiuri G, Khallou-Laschet J, Vandaele M, et al. Phosphorylcholine-targeting immunization reduces atherosclerosis. J Am Coll Cardiol. 2007;50:540–546. doi: 10.1016/j.jacc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 14.Hartvigsen K, Chou MY, Hansen LF, et al. The role of innate immunity in atherogenesis. Journal of Lipid Research. 2009;50 Suppl:S388–S393. doi: 10.1194/jlr.R800100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briley-Saebo KC, Shaw PX, Mulder WJ, et al. Targeted molecular probes for imaging atherosclerotic lesions with magnetic resonance using antibodies that recognize oxidation-specific epitopes. Circulation. 2008;117:3206–3215. doi: 10.1161/CIRCULATIONAHA.107.757120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Tobias R, McClure S, Styba G, Shi Q, Jackowski G. Removal of Endotoxin from Recombinant Protein Preparations. Clinical Biochemistry. 1997;30:455–463. doi: 10.1016/s0009-9120(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 17.Reardon CA, Blachowicz L, Lukens J, Nissenbaum M, Getz GS. Genetic background selectively influences innominate artery atherosclerosis: immune system deficiency as a probe. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:1449–1454. doi: 10.1161/01.ATV.0000079793.58054.2E. [DOI] [PubMed] [Google Scholar]
- 18.Hartvigsen K, Binder CJ, Hansen LF, et al. A diet-induced hypercholesterolemic murine model to study atherogenesis without obesity and metabolic syndrome. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:878–885. doi: 10.1161/01.ATV.0000258790.35810.02. [DOI] [PubMed] [Google Scholar]
- 19.Chou MY. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. Journal of Clinical Investigation. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li AC, Binder CJ, Gutierrez A, et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. The Journal of clinical investigation. 2004;114:1564–1576. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong Q, Wu M, Huan Y, et al. Transgene expression is associated with copy number and cytomegalovirus promoter methylation in transgenic pigs. PLoS One. 2009;4:e6679. doi: 10.1371/journal.pone.0006679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw PX, Horkko S, Tsimikas S, et al. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler Thromb Vasc Biol. 2001;21:1333–1339. doi: 10.1161/hq0801.093587. [DOI] [PubMed] [Google Scholar]
- 23.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy DJ, Kuchibhotla SD, Guy E, et al. Dietary cholesterol plays a role in CD36-mediated atherogenesis in LDLR-knockout mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:1481–1487. doi: 10.1161/ATVBAHA.109.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki H, Kurihara Y, Takeya M, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 26.Febbraio M, Podrez EA, Smith JD, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J ClinInvest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manning-Tobin JJ, Moore KJ, Seimon TA, et al. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. The Journal of Clinical Investigation. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torzewski M, Shaw PX, Han KR, et al. Reduced in vivo aortic uptake of radiolabeled oxidation-specific antibodies reflects changes in plaque composition consistent with plaque stabilization. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:2307–2312. doi: 10.1161/01.ATV.0000149378.98458.fe. [DOI] [PubMed] [Google Scholar]
- 30.Briley-Saebo KC, Cho YS, Shaw PX, et al. Targeted iron oxide particles for in vivo magnetic resonance detection of atherosclerotic lesions with antibodies directed to oxidation-specific epitopes. Journal of the American College of Cardiology. 2011;57:337–347. doi: 10.1016/j.jacc.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanderLaan PA, Reardon CA, Thisted RA, Getz GS. VLDL best predicts aortic root atherosclerosis in LDL receptor deficient mice. Journal of Lipid Research. 2009;50:376–385. doi: 10.1194/jlr.M800284-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeibig S, Li Z, Wagner S, et al. Effect of the oxLDL Binding Protein Fc-CD68 on Plaque Extension and Vulnerability in Atherosclerosis. Circulation Research. 2011 doi: 10.1161/CIRCRESAHA.111.240515. [DOI] [PubMed] [Google Scholar]
- 33.Schiopu A, Bengtsson J, Soderberg I, et al. Recombinant Human Antibodies Against Aldehyde-Modified Apolipoprotein B-100 Peptide Sequences Inhibit Atherosclerosis. Circulation. 2004;110:2047–2052. doi: 10.1161/01.CIR.0000143162.56057.B5. [DOI] [PubMed] [Google Scholar]
- 34.Aiuti A, Cattaneo F, Galimberti S, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. The New England Journal of Medicine. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 35.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 36.Seimon TA, Nadolski MJ, Liao X, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke A, Cresswell N, Kolodgie FD, Virmani R, Tsimikas S. Increased expression of oxidation-specific epitopes and Lp(a) reflect unstable plaques in human coronary arteries. J Am Coll Cardiol. 2008;49:A299. [Google Scholar]
- 38.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nature Cell Biology. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsimikas S, Lau HK, Han KR, et al. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109:3164–3170. doi: 10.1161/01.CIR.0000130844.01174.55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.