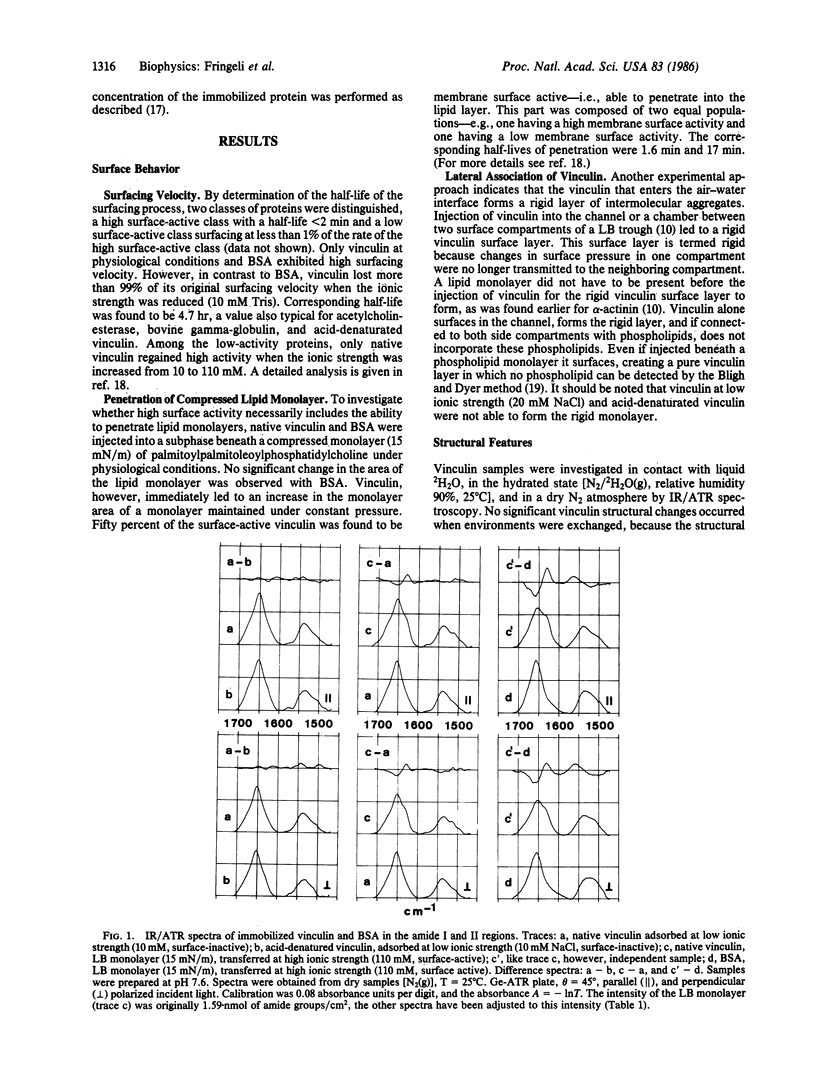
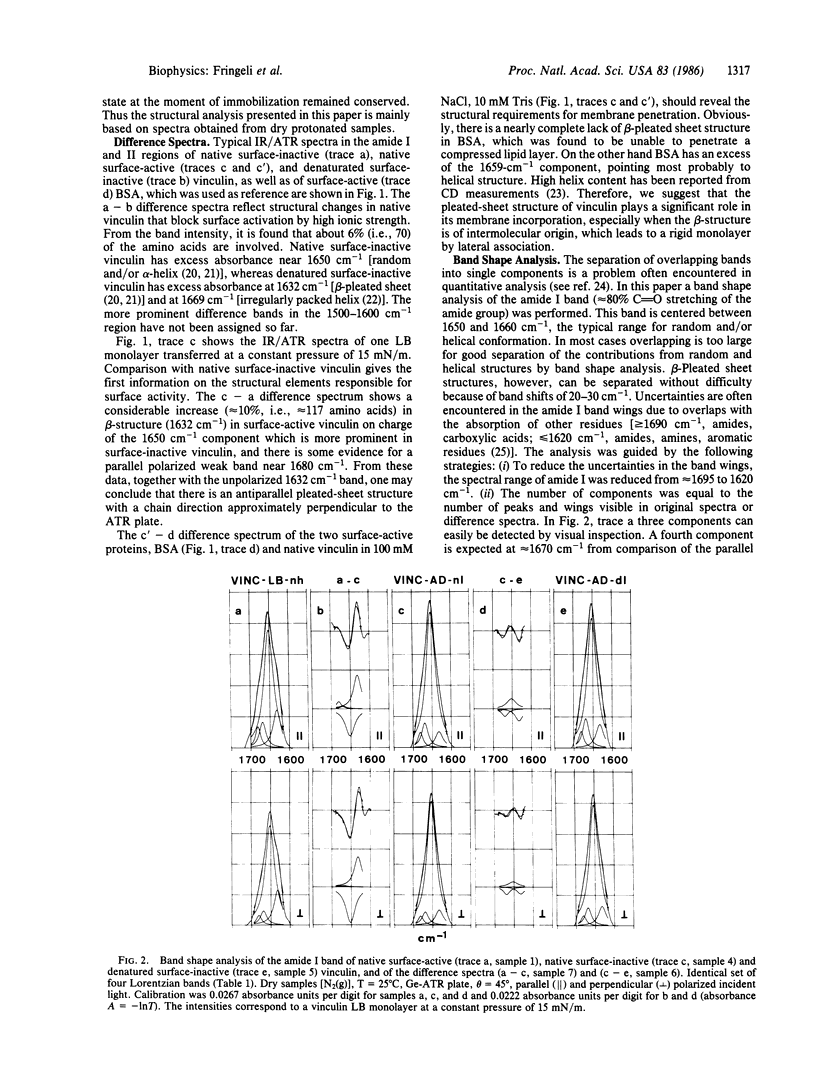
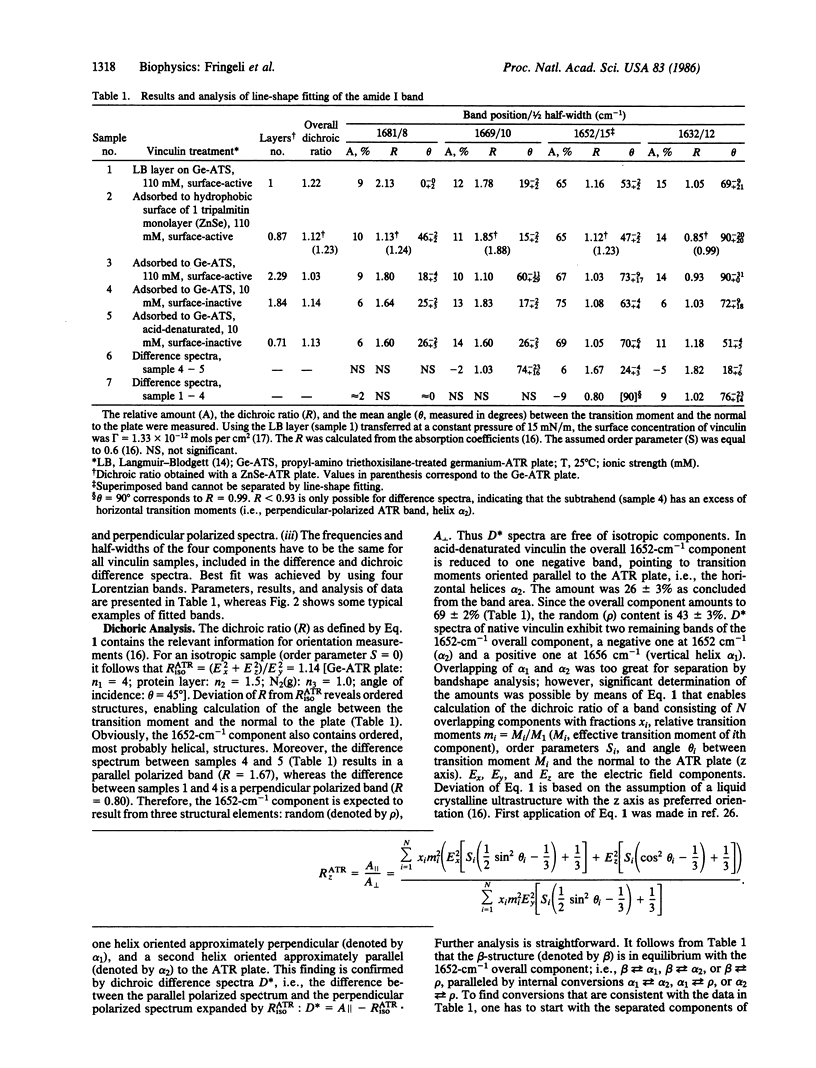
Abstract
Surfacing and membrane-penetrating ability of vinculin and bovine serum albumin have been studied on a macroscopic level by means of a Langmuir film balance and on a molecular level by means of infrared attenuated total reflection spectroscopy. It is suggested that the driving force of the nonspontaneous process of membrane penetration by native vinculin is the spontaneous formation of rigid vinculin monolayers in the membrane. Lateral adhesion of vinculin molecules results from the formation of intermolecular pleated-sheet structures. Vinculin surface activity was found to result from an alpha-helical segment oriented approximately perpendicular to plane of the membrane. There is a conformational equilibrium between this helix and random structure. High ionic strength (110 mM) favors helix formation that leads to the greater than 100-fold enhancement of surfacing velocity relative to the velocity observed at a lower ionic strength (10 mM). Vinculin has a second helical segment oriented parallel to the plane of the membrane that is in a conformational equilibrium with the pleated-sheet structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antler A. M., Greenberg M. E., Edelman G. M., Hanafusa H. Increased phosphorylation of tyrosine in vinculin does not occur upon transformation by some avian sarcoma viruses. Mol Cell Biol. 1985 Jan;5(1):263–267. doi: 10.1128/mcb.5.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Burn P., Rotman A., Meyer R. K., Burger M. M. Diacylglycerol in large alpha-actinin/actin complexes and in the cytoskeleton of activated platelets. Nature. 1985 Apr 4;314(6010):469–472. doi: 10.1038/314469a0. [DOI] [PubMed] [Google Scholar]
- Burridge K., Feramisco J. R. Microinjection and localization of a 130K protein in living fibroblasts: a relationship to actin and fibronectin. Cell. 1980 Mar;19(3):587–595. doi: 10.1016/s0092-8674(80)80035-3. [DOI] [PubMed] [Google Scholar]
- David-Pfeuty T., Singer S. J. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6687–6691. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Burridge K. A rapid purification of alpha-actinin, filamin, and a 130,000-dalton protein from smooth muscle. J Biol Chem. 1980 Feb 10;255(3):1194–1199. [PubMed] [Google Scholar]
- Fringeli U. P. Distribution and diffusion of alamethicin in a lecithin/water model membrane system. J Membr Biol. 1980 Jun 15;54(3):203–212. doi: 10.1007/BF01870236. [DOI] [PubMed] [Google Scholar]
- Fringeli U. P., Fringeli M. Pore formation in lipid membranes by alamethicin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3852–3856. doi: 10.1073/pnas.76.8.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Dutton A. H., Tokuyasu K. T., Singer S. J. Immunoelectron microscope studies of membrane-microfilament interactions: distributions of alpha-actinin, tropomyosin, and vinculin in intestinal epithelial brush border and chicken gizzard smooth muscle cells. J Cell Biol. 1981 Dec;91(3 Pt 1):614–628. doi: 10.1083/jcb.91.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Tokuyasu K. T., Dutton A. H., Singer S. J. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4127–4131. doi: 10.1073/pnas.77.7.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremlich H. U., Fringeli U. P., Schwyzer R. Conformational changes of adrenocorticotropin peptides upon interaction with lipid membranes revealed by infrared attenuated total reflection spectroscopy. Biochemistry. 1983 Aug 30;22(18):4257–4264. doi: 10.1021/bi00287a015. [DOI] [PubMed] [Google Scholar]
- Hofer P., Fringeli U. P. Structural investigation of biological material in aqueous environment by means of infrared-ATR spectroscopy. Biophys Struct Mech. 1979 Dec;6(1):67–80. doi: 10.1007/BF00537596. [DOI] [PubMed] [Google Scholar]
- Jockusch B. M., Isenberg G. Interaction of alpha-actinin and vinculin with actin: opposite effects on filament network formation. Proc Natl Acad Sci U S A. 1981 May;78(5):3005–3009. doi: 10.1073/pnas.78.5.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. K., Schindler H., Burger M. M. alpha-Actinin interacts specifically with model membranes containing glycerides and fatty acids. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4280–4284. doi: 10.1073/pnas.79.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. G., Feldhoff R. C., Clute O. L., Peters T., Jr Fragments of bovine serum albumin produced by limited proteolysis. Conformation and ligand binding. Biochemistry. 1975 Oct 21;14(21):4578–4583. doi: 10.1021/bi00692a004. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Shriver K., Rohrschneider L. Organization of pp60src and selected cytoskeletal proteins within adhesion plaques and junctions of Rous sarcoma virus-transformed rat cells. J Cell Biol. 1981 Jun;89(3):525–535. doi: 10.1083/jcb.89.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger S., Brodbeck U., Reber B., Brunner J. Hydrophobic labeling of the membrane binding domain of acetylcholinesterase from Torpedo marmorata. FEBS Lett. 1984 Mar 26;168(2):231–234. doi: 10.1016/0014-5793(84)80252-5. [DOI] [PubMed] [Google Scholar]


