Summary
Purpose
Past studies reported more widespread structural brain abnormalities in patients with left compared to right temporal lobe epilepsy (TLE), but the profile of these differences remain unknown. This study investigated the relationship between cortical thinning, white matter compromise, epilepsy variables, and the side of seizure onset, in patients with TLE.
Methods
We performed diffusion tensor imaging tractography and cortical thickness analyses of 18 patients with left TLE (LTLE), 18 patients with right TLE (RTLE), and 36 controls. We investigated the relationship between brain structural abnormalities, side of seizure onset, age of seizure onset, and disease duration.
Key findings
TLE groups displayed cortical thinning and white matter compromise, predominately on the side ipsilateral to the seizure onset. Relative to RTLE, patients with LTLE showed more widespread abnormalities, particularly in white matter fiber tracts. Greater compromise in white matter integrity was associated with earlier age of seizure onset, while cortical thinning was marginally associated with disease duration.
Significance
These data support previous findings of LTLE showing greater structural compromise than RTLE, and suggest that mechanisms may not be uniform for gray and white matter compromise in patients with LTLE and RTLE. These results may indicate that LTLE is different than RTLE, possibly due to greater vulnerability of the left hemisphere to early injury and the progressive effects of seizures.
Keywords: epilepsy, MRI, diffusion tensor imaging, cortical thickness
INTRODUCTION
Patients with left temporal lobe epilepsy (LTLE) generally show greater impairment in learning and memory than those with right temporal lobe epilepsy (RTLE), both before and after surgical treatment (Bell & Davies, 1998; Helmstaedter & Elger, 2009). Although this finding may partly relate to the higher sensitivity of neuropsychological measures for detecting verbal than nonverbal impairments, there is accumulating evidence to suggest that patients with LTLE show a more distributed, bilateral pattern of structural changes compared to those with RTLE (Bonilha, et al., 2007; Riederer, et al., 2008; Coan, et al., 2009). A number of studies have shown that patients with temporal lobe epilepsy (TLE) generally demonstrate gray matter volume loss and cortical thinning beyond hippocampal sclerosis (HS; Bonilha, et al., 2003; Bernasconi, et al., 2004; Keller & Roberts, 2008; McDonald, et al., 2008; Bernhardt, et al., 2010), and that patients with LTLE exhibit more pronounced atrophy in ipsilateral and contralateral regions than those with RTLE (Bonilha, et al., 2007; Keller & Roberts, 2008). White matter disruptions have also been reported in patients with TLE (Gross, et al., 2006; Focke, et al., 2008; Lin, et al., 2008; Concha, et al., 2009; Bonilha, et al., 2010; Riley, et al., 2010), and there is evidence that those with LTLE exhibit a more widespread pattern of white matter atrophy (Ahmadi, et al., 2009).
The mechanisms underlying the different patterns of abnormalities in LTLE versus RTLE are not well understood. Developmental factors may explain differences in brain vulnerability in these two groups. In particular, because the left hemisphere matures later and more slowly than the right hemisphere, it may be more vulnerable to early insults from febrile and early-onset seizures for a longer period of time (Corballis & Morgan, 1978). This could disrupt white matter development to a greater extent than gray matter, given the accelerated trajectory of frontotemporal white matter myelination during the first several years of life (Pujol, et al., 2006). Indeed, children with recent-onset epilepsy already exhibit abnormal white matter volume and integrity at the time of diagnosis, despite normal gray matter volumes (Hermann, et al., 2010; Hutchinson, et al., 2010).
In addition, the excitotoxic effect of uncontrolled and repetitive seizures may lead to progressive changes in the neocortex and white matter in patients with TLE, due to more extensive patterns of white matter connectivity within the left hemisphere in individuals who are left hemisphere dominant for language (Powell, et al., 2007). Left hemisphere seizures may propagate more diffusely, leading to greater damage over more widely distributed brain regions. Supporting this hypothesis, cortical thinning in patients with TLE has been shown to exceed the rate of atrophy associated with normal aging (Bernhardt, et al., 2009). Also, the extent of extratemporal gray matter reduction in TLE is closely related to disease duration in brain regions that are functionally and anatomically connected to the hippocampus (Bernasconi, et al., 2005; Bonilha, et al., 2006). Importantly, this reduction is greater in those with LTLE (Riederer, et al., 2008).
Few studies have focused on evaluating whether the patterns of structural compromise in TLE are different depending on the side of seizure onset. The current study analyzed cortical thickness and white matter compromise (as measured by fractional anisotropy; FA), in a cohort of patients with medically refractory TLE. We also investigated the hypothesis that early seizures or duration of epilepsy may have different impacts on brain structures of patients with LTLE and RTLE. We speculated that patients with LTLE have more widespread white matter compromise and cortical thinning than controls and those with RTLE. Furthermore, we predicted that earlier age of seizure onset would have a greater impact on white matter integrity, whereas longer disease duration would contribute more to cortical thinning in both patient groups.
METHODS
Participants
Thirty-six patients with medically refractory TLE and 36 age- and gender-matched controls participated (Table 1). All patients were under evaluation for surgical treatment at the UCSD Epilepsy Center. They were diagnosed with medically refractory epilepsy by experienced epileptologists (E.S.T and V.J.I.), according to the criteria defined by the International League Against Epilepsy (1989). Patients were classified into LTLE (n=18) or RTLE (n=18) based on seizure onsets recorded by video-EEG, seizure semiology, and neuroimaging results. Where clinically indicated, patients underwent Phase II video-EEG monitoring using 5-contact foramen ovale electrodes (n = 34) to exclude bilateral independent seizure onsets. Clinical MRI scans were available on all patients (i.e., T1-weighted, T2-weighted, and coronal FLAIR sequences with 1mm slices through the medial temporal lobe). MRIs were visually inspected by a board-certified neuroradiologist for detection of HS and the exclusion of contralateral temporal lobe structural abnormalities. In 25 patients (14 LTLEs, 11 RTLEs), MRI findings suggested the presence of ipsilateral HS. No patients showed evidence of contralateral HS or extra-hippocampal pathology on clinical MRI. Eleven patients had a history of febrile seizures (5 LTLE, 6 RTLE). Control participants were screened for neurological or psychiatric conditions.
Table 1.
Demographic Characteristics of the Patients and Control Samples
| LTLE (n = 18) |
RTLE (n = 18) |
Controls (n = 36) |
|
|---|---|---|---|
| Age (Y) | 36.56 (11.40) | 37.58 (11.21) | 37.07 (11.13) |
| Education (Y) | 13.83 (1.86) | 13.28 (1.78) | 14.83 (1.92) |
| Gender (F/M) | 11/7 | 10/8 | 19/17 |
| Left Hippocampal volume (mm3) | 3031.28 | 4068.22 | 4026.78 |
| Right Hippocampal volume (mm3) | 4009.44 | 3553.89 | 4128.53 |
| Age of seizure onset (Y) | 16.72 (12.89) | 13.28 (11.53) | - |
| Duration of illness (Y) | 19.50 (11.85) | 23.72 (14.89) | - |
| Seizure frequency (/mo) | 9.94 (9.96) | 5.06 (4.02) | - |
| Number of anticonvulsant meds | 2.22 (0.81) | 2.39 (0.61) | - |
| Number of patients with HS | 14 | 11 | - |
| History of febrile seizure | 5 | 6 | - |
Note. TLE = temporal lobe epilepsy; F = female; M = male; meds = medications; /mo = per month; Y = years. Unless otherwise noted, the data represent means followed by standard deviations in parentheses. Seizure frequency refers to the number of self-reported complex-partial seizures per month. HS refers to the number of patients who showed sclerosis of the ipsilateral hippocampus as diagnosed by clinical MRI. History of febrile seizure refers to the number of patients who had a history of febrile seizures.
Procedure
Image Acquisition
Magnetic resonance imaging was performed on a General Electric (GE) 1.5T EXCITE HD scanner with an 8-channel phased-array head coil. Image acquisitions included a conventional 3-plane localizer, GE calibration scan, two T1-weighted 3D structural scans (TE=3.8ms, TR=10.7ms, flip angle=8 degrees, bandwidth=31.25 Hz/pixel, FOV=25.6 cm, matrix=192×256, slice thickness=1.0mm), and three diffusion-weighted (DW) sequences. Diffusion data were acquired using single-shot echo-planar imaging with isotropic 2.5 mm voxels (matrix size=96 × 96, FOV=24 cm, 47 axial slices, slice thickness=2.5 mm, partial k-space acquisition, TE=75.6 msec, TR=12.3 sec), covering the entire cerebrum and brainstem without gaps. One volume series was acquired with 51 diffusion gradient directions using a b-value of 1000 mm2/s with an additional b=0 volume. For use in nonlinear B0 distortion correction, two additional b=0 volumes were acquired with either forward or reverse phase-encode polarity. All patients were seizure-free per self-report for a minimum of 24 hours prior to the MRI scan (Yogarajah & Duncan, 2008).
Image Processing
Image files in DICOM format were transferred to a Linux workstation for further processing.
Structural MRI processing
Two T1-weighted images were rigid body registered to each other, averaged, and reoriented into a common space, similar to alignment based on the AC-PC line. Images were corrected for non-linear warping caused by non-uniform fields created by the gradient coils (Jovicich, et al., 2006). Image intensities were corrected for spatial sensitivity inhomogeneities in the 8-channel head coil by normalizing with the ratio of a body coil scan to a head coil scan.
Diffusion image processing
Five pre-processing steps were performed 1.) Head motion between scans was removed by rigid body registration between the b=0 images of each DW scan. 2.) Within-scan motion was removed by calculating diffusion tensors, synthesizing of DW volumes from those tensors, and rigid body registering each data volume to its corresponding synthesized volume. 3.) Image distortion in the DW volumes caused by eddy currents was minimized by nonlinear optimization 4.) Image distortion caused by magnetic susceptibility artifacts was minimized with a nonlinear B0-unwarping method using paired images with opposite phase-encode polarities (Chang & Fitzpatrick, 1992; Morgan, et al., 2004; Reinsberg, et al., 2005). 5.) Images were resampled using cubic interpolation to 1.875 mm3 isotropic voxels.
Fiber Tracking and Calculations
Fiber tract FA values were derived using a probabilistic diffusion tensor atlas that was developed using in-house software written in Matlab and C++. A full description of the atlas and the steps used to create the atlas are described elsewhere (Hagler, et al., 2009). In brief, the atlas was originally derived from a training set of healthy controls and patients with TLE. FA and the first Eigenvector volumes for each training participant were exported to DTI Studio (John Hopkins University, Baltimore, MD) where manual tracings were performed according to the multiple region of interest (ROI) procedure described by Wakana et al. (2007), excluding voxels with FA < 0.15. FA was calculated for each tract from the Eigenvalues obtained from the diffusion images as described in Pierpaoli, et al. (1996) and Nucifora, et al. (2007). Data from this manual training set were used to create a probabilistic fiber atlas that consisted of averaged information about the locations and local orientations of 13 chosen fiber tracts.
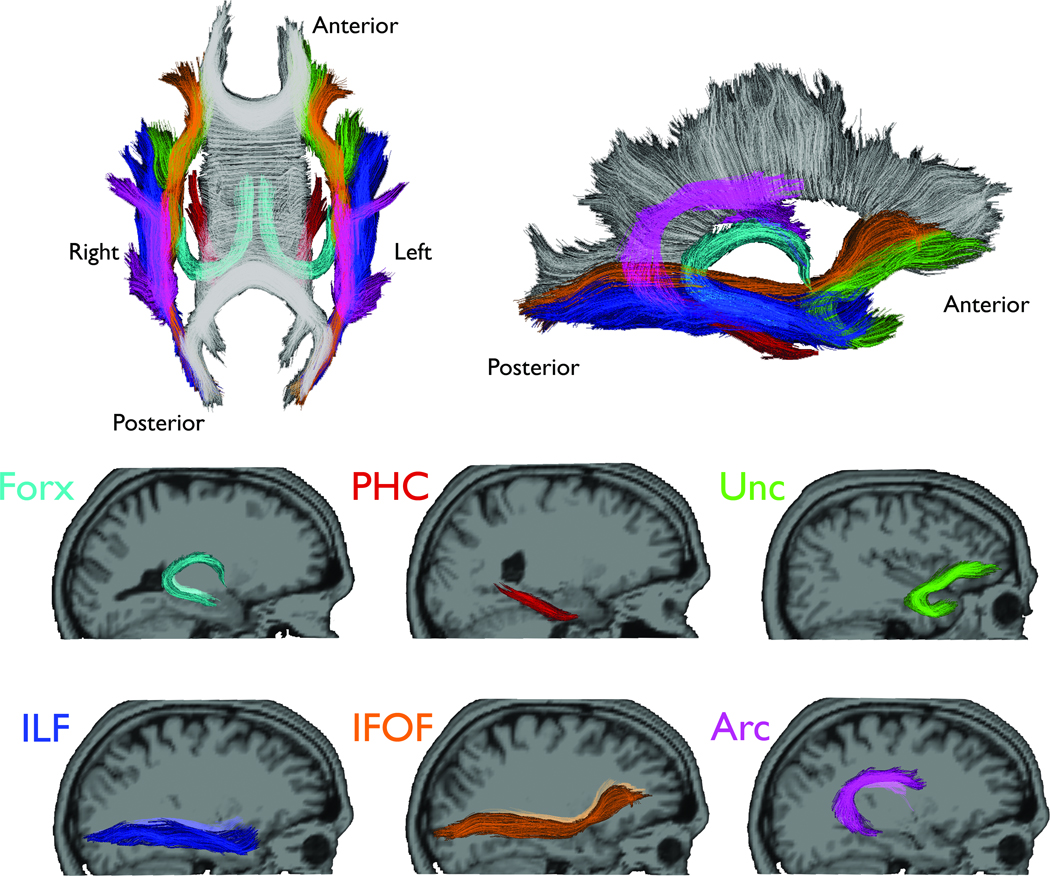
For each participant, T1-weighted images were used to nonlinearly register the brain to a common space, and diffusion tensor orientation estimates were compared to the atlas to obtain a map of the relative probability that a voxel belongs to a particular fiber given the location and similarity of diffusion orientations. Voxels identified with FreeSurfer’s automated brain segmentation (Fischl, et al., 2002) as cerebrospinal fluid (CSF) or gray matter were excluded from the fiber ROIs. Average FA was calculated for each fiber ROI, weighted by fiber probability, so that voxels with low probability of belonging to a given fiber contributed minimally to average FA values. In the current study, this probabilistic atlas-based method was used to obtain the following fiber tracts due to their projections from the temporal lobe, and therefore, likely involvement in TLE (Figure 1): fornix (FORX), parahippocampal cingulum (PHC), uncinate fasciculus (UNC), inferior longitudinal fasciculus (ILF), inferior fronto-occipital fasciculus (IFOF), and arcuate fasciculus (ARC).
Figure 1.
Sagittal views of the selected fiber tracts. Individual fiber tracts are shown projected on their corresponding T1-weighted images using Tractoview software. Color-coding is included to assist with identification of the fibers in the superimposed images.
Surface reconstruction and parcellation
Individual T1-weighted images were used to construct models of each participant’s cortical surfaces using FreeSurfer software 4.5.0 (http://surfer.nmr.mgh.harvard.edu). From this reconstructed surface, measures of cortical thickness were obtained using the procedure described by Fischl and Dale (2000). Sulcal and gyral features across individual participants were aligned by morphing each participant’s brain to an average spherical representation that allows for accurate matching of cortical thickness measurement locations among participants, while minimizing metric distortion. To improve signal to noise ratio, thickness estimates were smoothed on the average surface using a 15-mm full width at half maximum Gaussian kernel.
Cortical thickness estimates were computed at each vertex (~1mm spacing) across the cortical mantle and within gyral-based ROIs, as described by Desikan et al. (2006). Mean thickness for each ROI was calculated by averaging the cortical thickness measurements based on the unsmoothed data at each vertex within a given ROI. In this study, these ROI measures were further combined and averaged to produce lobar (frontal, lateral temporal, medial temporal, parietal, and occipital) ROIs.
Statistical Analysis
Statistical analyses were conducted with SPSS Statistics 17.0 (http://www.spss.com/). Analyses were evaluated at p < 0.01 to control for Type I errors.
Region of interest analysis
To examine group differences in FA values of fiber tracts ROIs, repeated measures analyses of variance (RM ANOVAs) were performed, with ROI (six levels) and side (left vs. right) as within-subject factors, and group (control, LTLE, and RTLE) as a between-subject factor.
Surface-based analysis
Vertex-wise estimates of cortical thickness were obtained for each group and used to create group difference maps. From the mean group difference maps, t-statistical maps were calculated at each vertex, and cluster based-thresholding was performed according to previously described procedures (Hagler, et al., 2006). Gaussian Random Field Theory was used to model the distribution (Worsley, et al., 1996) and the intrinsic smoothness of the data was estimated from normalized residuals. This yielded significant clusters of thickness differences between groups corrected for multiple comparisons (FWHM=23.5 mm; t-statistics thresholded at t > 2.0; cortical surface clusters < 344 mm2 excluded; corrected cluster p < 0.05).
Relationship with disease duration and age of seizure onset
Z-scores were calculated for each fiber tract FA and lobar cortical thickness in each patient group based on the mean and standard deviation of the controls. Data were organized in terms of “ipsilateral” and “contralateral” structures, relative to the seizure onset. Pearson’s correlations were calculated between disease-related variables (i.e., age of seizure onset/disease duration) and the brain structural measurements (i.e., FA/cortical thickness) using ipsilateral and contralateral z-scores.
Bivariate correlations were performed between brain structural measurements, and demographic (age, education) and two disease-related variables. To determine the contributions of the disease-related variables, hierarchical regression analyses were then performed separately for the two patient groups. For FA values, age, age of seizure onset, and disease duration were entered at steps 1, 2, and 3 respectively. For cortical thickness, age and disease duration were entered at steps 1 and 2, respectively. The order of steps was based on the assumption that factors associated with early onset precede chronic duration effects. Age was included at step one to control for its significant associations with age of seizure onset and disease duration. Age of onset was not entered as a predictor for cortical thickness prediction, due to its lack of correlation with cortical thickness.
RESULTS
There were no statistically significant differences among the controls, LTLEs, or RTLEs in age, or years of education (F[2,71] = .04, p > .10, F[2,70] = 2.99, p > .05, respectively). The distribution of gender across the three groups was comparable (χ2[2] = .34, p = .85). There were no statistically significant differences between the two patient groups in disease duration (t =-.94, p >.10), age at seizure onset (t = .85, p > .10), or the number of patients with HS (χ2[1] = 1.93, p = .16). The volume of the ipsilateral hippocampus did not differ between the two patient groups, although there was a trend for those with LTLE to have smaller ipsilateral volumes (t[34] = −1.99, p = .054). Both patient groups’ ipsilateral hippocampal volumes were smaller when compared to the corresponding side of the controls hippocampal volume (t[52] = 5.82, p = .001, and t[52] = 2.58, p = .02, LTLE and RTLE, respectively), but their contralateral hippocampal volumes were not statistically different from the controls (t[52] = .916, p > .10, and t[52] = −.32, p > .10, LTLE and RTLE, respectively).
Group differences in FA values
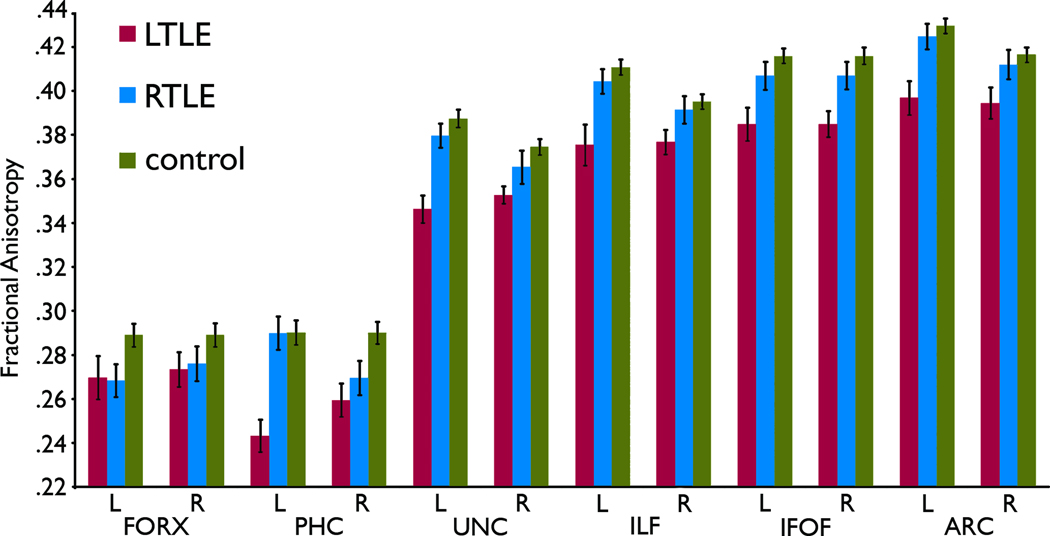
Figure 2 shows raw FA values of fiber tracts studied, and mean FA z-values are provided in Supplemental Table 2. The RM ANOVA on FA z-score revealed main effects of group (F[2,68] = 16.31, p < .001) and side (F[1,68] = 7.34, p = .009), as well as a group by side interaction (F[2,68]= 6.95, p = .002). Patients with LTLE overall showed smaller FA z-scores relative to controls and patients with RTLE, and the FA z-scores of the left hemisphere were smaller than those of the right. However, the group by side interaction indicated that this left-right difference was especially driven by the lower FA z-scores in the ipsilateral hemisphere of the LTLE group. Pair-wise comparisons involving the two patient groups indicated that patients with LTLE demonstrated smaller FA z-scores in the ipsilateral hemisphere of the UNC (ipsilateral; −1.647, contralateral: −0.997, p = .007) and ILF (ipsilateral; −1.679, contralateral; −0.866, p = .009). A non-significant but similar trend was observed for the PHC, ARC, and IFOF, p < .025. Patients with RTLE showed a trend of smaller ipsilateral FA values only in the PHC (p = .012).
Figure 2.
White matter fractional anistropy (FA) of selected fiber tract ROIs in patients with LTLE and RTLE and healthy controls. Error bars represent standard errors.
Group differences in cortical thickness
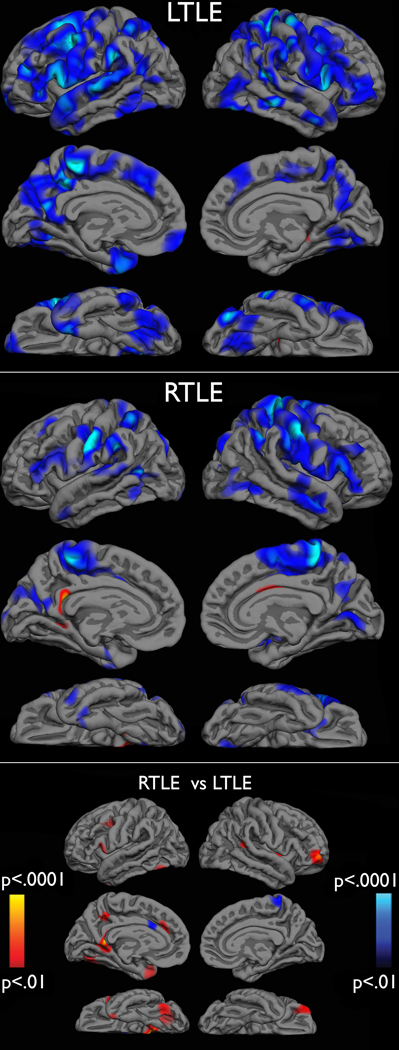
Figure 3 displays cluster-size thresholded t-statistic surface maps of cortical thinning in patients with LTLE versus controls, RTLE versus controls, and LTLE versus RTLE. Both patient groups showed cortical thinning across frontal, temporal, and parietal cortex, when compared to healthy controls, especially in the ipsilateral hemisphere. Visual inspection of these results suggest that patients with LTLE showed more widespread, bilateral thinning, while patients with RTLE showed thinning that was greatest in ipsilateral frontoparietal regions. Direct comparison of LTLE and RTLE revealed suprathreshold clusters of greater thinning in LTLE in the left medial and inferior temporal regions, retrosplenial cortex, and in the right orbital frontal and frontal polar regions. Group means of the lobar ROIs are provided in Supplemental Table 1.
Figure 3.
Cluster-based t-statistic surface maps of cortical thinning in patients with LTLE versus controls (top), RTLE versus controls (middle), and RTLEs versus LTLEs (bottom). Cluster maps are thresholded at t > 2, corrected p-values < .05. In the top and middle graphs, blue areas represent areas of thinner cortex in patients compared to controls and red areas represent areas of thinner cortex in controls compared to patients. In the bottom graph red areas represent thinner cortex in patients with LTLE compared to patients with RTLE. Areas of blue represent thinner cortex in patients with RTLE.
Hierarchical regression analyses
Initial correlation analyses demonstrated no statistically significant relationship between demographic variables (i.e., age and years of education) and FA values (rs ranged between −.556 and .302, and −.445 and .380, respectively). Years of education did not correlate with cortical thickness in any of the groups (rs ranged between −.286 and .374), while age significantly correlated with cortical thickness of the right lateral temporal lobe (r = −.501, p = .002) and right frontal lobe (r = −.525, p = .001) of healthy controls, and bilateral lateral temporal lobes (r = −.623, p = .006, r = −.789, p < .001, left and right, respectively) and right occipital lobe (r = −.597, p = .009) of the LTLE group.
In patients with LTLE, lower FA of the ipsilateral PHC was associated with an earlier age at seizure onset (r = .629, p < .01) and longer disease duration (r = −.775, p < .01). Lower FA of the ipsilateral UNC was also associated with longer disease duration (r = −.682, p < .01). There were no significant correlations between these disease-related variables and FA values in patients with RTLE (rs ranged from −.313 to .453). Zero-order correlations also revealed that longer disease duration in patients with LTLE was associated with thinner cortex in the ipsilateral parietal lobe (r = −.598, p = .009) and a strong trend for thinning in the contralateral frontal lobe (r = −.545, p = .019). Patients with RTLE did not show any significant correlations between disease duration and cortical thickness (rs ranged from −.498 to −.231). Age at seizure onset did not correlate with thickness measures in either patient group (rs ranged from −.287 to .354).
Tables 2 and 3 show the results of the hierarchical regression analyses. After controlling for age, age at seizure onset was a significant predictor of FA values of the ipsilateral PHC and bilateral UNC, and marginally significant as a predictor of FA values of the ipsilateral ILF, while the RTLE group did not show such relationship. For cortical thickness, disease duration was a marginally significant predictor of the ipsilateral parietal lobe in patients with LTLE.
Table 2.
Proportion of Variance (R2Δ) of Fiber Fractional Anisotropy Accounted For by Age of Onset and Disease Duration After Covarying for Age
| LTLE | RTLE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age of Seizure Onset |
Disease Duration |
Age of Seizure Onset |
Disease Duration |
||||||
| R2Δ | p | R2Δ | p | R2Δ | p | R2Δ | p | ||
| FORX | |||||||||
| Ipsilateral | .03 | .45 | .01 | .07 | .10 | .22 | .01 | .67 | |
| Contralateral | .02 | .51 | .01 | .19 | .10 | .23 | .03 | .52 | |
| PHC | |||||||||
| Ipsilateral | .63 | <.001 | .01 | .67 | .05 | .04 | .07 | .79 | |
| Contralateral | .17 | .08 | .00 | .98 | .02 | .58 | .08 | .28 | |
| UNC | |||||||||
| Ipsilateral | .49 | .002 | .04 | .27 | .22 | .06 | .03 | .44 | |
| Contralateral | .36 | .010 | .15 | .05 | .02 | .55 | .03 | .49 | |
| ILF | |||||||||
| Ipsilateral | .31 | .02 | .01 | .66 | .06 | .33 | .03 | .46 | |
| Contralateral | .19 | .08 | .01 | .69 | .05 | .38 | .02 | .61 | |
| IFOF | |||||||||
| Ipsilateral | .27 | .03 | .05 | .33 | .09 | .25 | .00 | .88 | |
| Contralateral | .10 | .19 | .07 | .27 | .05 | .37 | .04 | .41 | |
| ARC | |||||||||
| Ipsilateral | .15 | .12 | .01 | .76 | .18 | .09 | .05 | .37 | |
| Contralateral | .02 | .55 | .00 | .97 | .01 | .73 | .02 | .65 | |
Note. ARC = Arcuate Fasiculus; FORX = Fornix; IFOF = Inferior Fronto-Occipital Fasiculus; ILF = Inferior Longitudinal Fasiculus; PHC = Parahippocampal Cingulum; SD = Standard Deviation; LTLE = left temporal lobe epilepsy; RTLE = right temporal lobe epilepsy; UNC = Uncinate Fasiculus.
Bold font indicates R2 statistically significant at p < .01. Underline indicates R2 marginally significant.
Table 3.
Proportion of Variance (R2Δ) of Lober Cortical Thickness Accounted For by Disease Duration After Covarying for Age
| LTLE | RTLE | ||||
|---|---|---|---|---|---|
| Disease Duration |
Disease Duration |
||||
| R2Δ | p | R2Δ | p | ||
| Medial Temporal | |||||
| Ipsilateral | .03 | .50 | .08 | .26 | |
| Contralateral | .02 | .58 | .03 | .50 | |
| Lateral Temporal | |||||
| Ipsilateral | .05 | .25 | .10 | .18 | |
| Contralateral | .05 | .15 | .15 | .12 | |
| Frontal | |||||
| Ipsilateral | .08 | .22 | .02 | .70 | |
| Contralateral | .15 | .07 | .02 | .55 | |
| Parietal | |||||
| Ipsilateral | .23 | .029 | .09 | .21 | |
| Contralateral | .13 | .09 | .02 | .54 | |
| Occipital | |||||
| Ipsilateral | .03 | .46 | .04 | .42 | |
| Contralateral | .00 | .85 | .13 | .16 | |
Note. TLE = temporal lobe epilepsy. Underline indicates R2 marginally significant.
DISCUSSION
The current study investigated (1) the degree of white matter compromise and regional neocortical thinning in patients with RTLE and LTLE, and (2) how two disease-related variables, i.e., age of seizure onset and disease duration, differentially contribute to structural abnormality in each group. Our data suggest that patients with LTLE showed significant reduction in white matter integrity relative to those with RTLE and to healthy controls in fiber tracts that project from or traverse the temporal lobe. In addition, patients with LTLE showed a significant left-right asymmetry in their FA values, with ipsilateral fiber tracts showing smaller FA values than contralateral ones. Patients with RTLE did not demonstrate this asymmetry.
In addition, we observed widespread neocortical thinning in patients with TLE, a finding that is supported by previous imaging studies (Bonilha, et al., 2003; Bernasconi, et al., 2004; Bonilha, et al., 2004; Bonilha, et al., 2007; Keller & Roberts, 2008; Bernhardt, et al., 2010), and by recent postmortem evidence of bilateral neocortical and white matter pathology in TLE (Blanc, et al., 2011). In the current study, patients with LTLE demonstrated significant thinning of the cortical gray matter relative to controls. In contrast, patients with RTLE showed a pattern that suggests more restricted regional thinning. Direct comparison between LTLE and RTLE indicate areas of greater thinning in left TLE. Therefore, although the differences in cortical atrophy between our patient groups were small, they consistently favor more widespread, pronounced thinning in patients with LTLE. Of note, the greater neocortical thinning and white matter abnormality in LTLE group, when compared to RLTE group, cannot be accounted for by known clinical variables. Specifically, the two groups have comparable age of seizure onset, epilepsy duration, seizure frequency, anticonvulsant medication exposure, and proportion of patients with a history of febrile seizure (see Table 1).
Regression analyses demonstrated that patients with LTLE and RLTE showed different relationships between disease-related variables and brain structural measures. In LTLE, early age at seizure onset was a strong predictor of more disrupted fiber tract integrity. Conversely, longer disease duration was associated with more marked cortical thinning in LTLE. These associations were not observed in patients with RTLE. The lack of significant correlations in patients with RTLE could possibly be explained by the generally smaller differences from controls in their brain structural measurement. It has also been suggested that patients with RTLE are a more heterogeneous group than those with LTLE in terms of hippocampal pathology (Cheung, et al., 2009). Although our brain structural measurements did not support this hypothesis, perhaps there are other aspects of heterogeneity in patients with RTLE that were not captured by our measures, but attenuate the clinico-anatomic relationships that were observed in our LTLE group.
Another important finding is that white matter compromise and cortical thinning may be mediated by different mechanisms. Our results within patients with LTLE indicated that an earlier age of seizure onset was associated with greater white matter compromise in frontotemporal fiber tracts, but that it was not associated with greater cortical thinning. This observation is consistent with past studies of children with epilepsy of recent onset, which showed that white matter compromise was observed early in the course of the disease and may be related to early insults or developmental anomalies that likely precede the effects from refractory seizures (Hutchinson, et al., 2010). In addition, Hermann et al. (2010) have shown that the trajectory of white matter development in children with epilepsy lags behind the course observed in healthy children, whereas cortical volumes did not differ between the groups. In another study comparing individuals with early and late onset TLE, the early onset group had significantly greater reduction in white matter volume, which also correlated with lower neuropsychological scores (Hermann, et al., 2002). Our data support these findings and further demonstrate that the association between age at seizure onset and white matter compromise is particularly apparent in patients with LTLE, indicating that the left hemisphere may in fact be more vulnerable to early insults in patients with TLE.
Past studies point to multiple factors that may result in a greater vulnerability of the left hemisphere to various developmental insults (Njiokiktjien, 2006). It was postulated that the left hemisphere is immature at birth and then undergoes a rapid, but prolonged maturation process, rendering it more vulnerable (especially the white matter) to early brain insults (Corballis & Morgan, 1978). Furthermore, due to perinatal vascular asymmetry in the two hemispheres, the left hemisphere is shown to be more vulnerable to hypoxic-ischemic insults (Mullaart, et al., 1995) that are known to have devastating effects on the hippocampus and surrounding regions (Schmidt-Kastner & Freund, 1991). If the left hemisphere is indeed vulnerable for a longer period of time relative to the right, then left temporal lobe seizures during this critical time of development may disrupt more white matter maturation both ipsilaterally and contralaterally.
Unlike age of seizure onset, disease duration was not associated with fiber tract FA values once age and age of seizure onset were taken into consideration. Rather, longer disease duration was related to cortical thinning in the ipsilateral parietal and contralateral frontal lobes, and this relationship only emerged in LTLE. Our statistical approach of controlling for effects of age, and evidence from past longitudinal studies (Bernhardt, et al., 2009; Coan, et al., 2009; Bernhardt, et al., 2010), indicate that this relationship is likely above and beyond normal aging processes. Although the reason for more pronounced thinning in LTLE is unclear, Keller et al. (2002) have hypothesized that there are inherent differences between LTLE and RTLE in extrahippocampal brain structures, with LTLE resulting in a more bilateral, extensive pattern of atrophy. Potentially more intricate connections of the hippocampus with the rest of the brain in the dominant hemisphere can give rise to more excitotoxic damage from seizures, or to more neuronal loss from deafferentation secondary to hippocampal atrophy (Bonilha, et al., 2007; Coan, et al., 2009). Furthermore, vascular differences in blood perfusion to the left versus right hemisphere may result in more frequent and extreme damage to the left hemisphere in both children and adults (Njiokiktjien et al., 2006). Together, these studies suggest that patients with LTLE are more susceptible to bilateral neocortical atrophy resulting from refractory seizures, and that this pattern may be accentuated in their more vulnerable, ipsilateral hemisphere.
In summary, the current study used multiple neuroimaging measures to unveil unique patterns of white matter compromise and cortical thinning in a well-characterized group of patients with refractory LTLE and RTLE. Our study is limited by both its cross-sectional design and modest sample size. Longitudinal studies that track large cohorts of normal developing children and children with TLE will be quite valuable, but have their own limitations (i.e., cohort effects, changes in scanning protocols). In addition, although our LTLE and RTLE patients did not statistically differ in their ipsilateral hippocampal volumes, inspection of Table 1 reveals that the mean ipsilateral volume for the LTLE patients was numerically smaller than the mean for the RTLE patients, and three more patients with LTLE were identified as having MTS. Therefore, it is possible that modest differences in hippocampal pathology could have contributed to some of the observed findings. However, a post-hoc analysis including only LTLE and RTLE patients with MTS revealed the same pattern of results, despite similar levels of ipsilateral hippocampal atrophy (LTLE ipsilateral hippocampal volume = 2766 cm; RLTE = 3120; t[23] = −1.3; p = .26). Our analysis of the fiber tracts may be also limited by partial voluming effects, which may differ between fibers because of relative size and proximity to ventricles. For instance, we observed lower mean FA values in the FORX and PHC relative to the other fiber tracts. Although voxels identified as CSF and gray matter were excluded from fiber tract ROIs, voxels on the outer boundary of the fiber tracts were likely affected to some extent by partial voluming. Inclusion of signals from CSF and gray matter would reduce the average FA, an effect more pronounced for small diameter fiber bundles surrounded by CSF (Concha, et al., 2005). However, because partial voluming of these fibers reduced FA across all three groups, it is unlikely to have accounted for the between-group differences in FA. Another limitation is the difficulty inherent in patients’ self-report. It is often challenging to pinpoint the age at which seizures began because many patients do not report or recognize seizures until they have become problematic. It is also difficult to quantify the frequency and magnitude of generalized tonic-clonic seizures based on patient self-report, although this could also account for differences in brain atrophy. Nevertheless, our results highlight differential contributions of developmental factors versus progressive refractory epilepsy on white and gray matter compromise, as well as a more pronounced pattern of atrophy in patients with LTLE. In addition, our patient group was carefully selected to exclude patients with bilateral temporal or extratemporal onsets, as are seen in up to 24% of patients with unilateral MTS (Kansal, et al., 2010). Whether TLE is a progressive disorder has been previously debated in the literature (Sutula, 2004; Kuzniecky, et al., 2009), as have the possible developmental precursors necessary to develop TLE (Hermann, et al., 2010; Roper, et al., 2011). Our study provides new insights into the putative mechanisms that result in brain structural compromise in TLE, and reveals different patterns in LTLE versus RTLE, which in turn begins to disentangle the contributions of early cerebral insults, differential vulnerability of the two hemispheres, and the impact of refractory seizures in TLE.
Supplementary Material
ACKNOWLEDGMENTS
The project was supported by NIH Grant K23NS05609 (CRM) and R01NS065838 (CRM). BCB was funded by the Savoy Foundation for Epilepsy. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. We greatly acknowledge support from GE Healthcare. Authors thank Erkut Kucukboyaci for helpful discussions.
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. Eric Halgren has equity interest in CorTechs Labs, Inc, and also serves on its Board of Directors. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. The remaining authors have no conflict of interest.
References
- Ahmadi ME, Hagler DJ, Jr, McDonald CR, Tecoma ES, Iragui VJ, Dale AM, Halgren E. Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. AJNR Am J Neuroradiol. 2009;30:1740–1747. doi: 10.3174/ajnr.A1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell BD, Davies KG. Anterior temporal lobectomy, hippocampal sclerosis, and memory: recent neuropsychological findings. Neuropsychol Rev. 1998;8:25–41. doi: 10.1023/a:1025679122911. [DOI] [PubMed] [Google Scholar]
- Bernasconi N, Duchesne S, Janke A, Lerch J, Collins DL, Bernasconi A. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage. 2004;23:717–723. doi: 10.1016/j.neuroimage.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology. 2005;65:223–228. doi: 10.1212/01.wnl.0000169066.46912.fa. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Bernasconi N, Concha L, Bernasconi A. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology. 2010;74:1776–1784. doi: 10.1212/WNL.0b013e3181e0f80a. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Worsley KJ, Kim H, Evans AC, Bernasconi A, Bernasconi N. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology. 2009;72:1747–1754. doi: 10.1212/01.wnl.0000345969.57574.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc F, Martinian L, Liagkouras I, Catarino C, Sisodiya SM, Thom M. Investigation of widespread neocortical pathology associated with hippocampal sclerosis in epilepsy: a postmortem study. Epilepsia. 2011;52:10–21. doi: 10.1111/j.1528-1167.2010.02773.x. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Edwards JC, Kinsman SL, Morgan PS, Fridriksson J, Rorden C, Rumboldt Z, Roberts DR, Eckert MA, Halford JJ. Extrahippocampal gray matter loss and hippocampal deafferentation in patients with temporal lobe epilepsy. Epilepsia. 2010;51:519–528. doi: 10.1111/j.1528-1167.2009.02506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Appenzeller S, Coan AC, Cendes F, Li LM. Gray matter atrophy associated with duration of temporal lobe epilepsy. Neuroimage. 2006;32:1070–1079. doi: 10.1016/j.neuroimage.2006.05.038. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Castellano G, Pereira F, Rio PA, Cendes F, Li LM. Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol. 2004;61:1379–1384. doi: 10.1001/archneur.61.9.1379. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Halford JJ, Eckert M, Appenzeller S, Cendes F, Li LM. Asymmetrical extra-hippocampal grey matter loss related to hippocampal atrophy in patients with medial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2007;78:286–294. doi: 10.1136/jnnp.2006.103994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Kobayashi E, Montenegro MA, Guerreiro MM, Li LM, Cendes F. Voxel based morphometry study of partial epilepsies. Arq Neuropsiquiatr. 2003;61 Suppl 1:93–97. [PubMed] [Google Scholar]
- Chang H, Fitzpatrick JM. A technique for accurate magnetic resonance imaging in the presenceof field inhomogeneities. IEEE Trans Med Imaging. 1992;11:319–329. doi: 10.1109/42.158935. [DOI] [PubMed] [Google Scholar]
- Cheung MC, Chan AS, Lam JM, Chan YL. Pre- and postoperative fMRI and clinical memory performance in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2009;80:1099–1106. doi: 10.1136/jnnp.2009.173161. [DOI] [PubMed] [Google Scholar]
- Coan AC, Appenzeller S, Bonilha L, Li LM, Cendes F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology. 2009;73:834–842. doi: 10.1212/WNL.0b013e3181b783dd. [DOI] [PubMed] [Google Scholar]
- Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- Concha L, Beaulieu C, Collins DL, Gross DW. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:312–319. doi: 10.1136/jnnp.2007.139287. [DOI] [PubMed] [Google Scholar]
- Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol. 2005;26:2267–2274. [PMC free article] [PubMed] [Google Scholar]
- Corballis MC, Morgan M. On the Biological Basis of Human Laterality: I. Evidence for a Maturational Left-Right Gradiant. Behav. Brain Sci. 1978;2:261–269. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage. 2008;40:728–737. doi: 10.1016/j.neuroimage.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006;47:1360–1363. doi: 10.1111/j.1528-1167.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Ahmadi ME, Kuperman J, Holland D, McDonald CR, Halgren E, Dale AM. Automated white-matter tractography using a probabilistic diffusion tensor atlas: Application to temporal lobe epilepsy. Hum Brain Mapp. 2009;30:1535–1547. doi: 10.1002/hbm.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C, Elger CE. Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain. 2009;132:2822–2830. doi: 10.1093/brain/awp182. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Bell B, Rutecki P, Sheth R, Ruggles K, Wendt G, O'Leary D, Magnotta V. The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia. 2002;43:1062–1071. doi: 10.1046/j.1528-1157.2002.49901.x. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Dabbs K, Becker T, Jones JE, Myers y, Gutierrez A, Wendt G, Koehn MA, Sheth R, Seidenberg M. Brain development in children with new onset epilepsy: a prospective controlled cohort investigation. Epilepsia. 2010;51:2038–2046. doi: 10.1111/j.1528-1167.2010.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E, Pulsipher D, Dabbs K, Myers y, Gutierrez A, Sheth R, Jones J, Seidenberg M, Meyerand E, Hermann B. Children with new-onset epilepsy exhibit diffusion abnormalities in cerebral white matter in the absence of volumetric differences. Epilepsy Res. 2010;88:208–214. doi: 10.1016/j.eplepsyres.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kansal L, Tecoma E, Iragui V. The utility of presurgical evaluation with foramen ovale electrodes; 64th American Epilepsy Society Annual Meeting; San Antonio, TX: 2010. [Google Scholar]
- Keller SS, Mackay CE, Barrick TR, Wieshmann UC, Howard MA, Roberts N. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage. 2002;16:23–31. doi: 10.1006/nimg.2001.1072. [DOI] [PubMed] [Google Scholar]
- Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008;49:741–757. doi: 10.1111/j.1528-1167.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- Kuzniecky R, Thesen T, Devinsky O. Epilepsy: is localization-related epilepsy a progressive disorder? Maybe. Nat Rev Neurol. 2009;5:356–357. doi: 10.1038/nrneurol.2009.82. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Riley JD, Juranek J, Cramer SC. Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Res. 2008;82:162–170. doi: 10.1016/j.eplepsyres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Hagler DJ, Jr, Ahmadi ME, Tecoma E, Iragui V, Gharapetian L, Dale AM, Halgren E. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia. 2008;49:794–803. doi: 10.1111/j.1528-1167.2008.01539.x. [DOI] [PubMed] [Google Scholar]
- Morgan PS, Bowtell RW, McIntyre DJ, Worthington BS. Correction of spatial distortion in EPI due to inhomogeneous static magnetic fields using the reversed gradient method. J Magn Reson Imaging. 2004;19:499–507. doi: 10.1002/jmri.20032. [DOI] [PubMed] [Google Scholar]
- Mullaart RA, Daniels O, Hopman JC, de Haan AF, Stoelinga GB, Rotteveel JJ. Asymmetry of the cerebral blood flow: an ultrasound Doppler study in preterm newborns. Pediatr Neurol. 1995;13:319–322. doi: 10.1016/0887-8994(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Njiokiktjien C. Differences in vulnerability between the hemispheres in early childhood and adulthood. Fiziol Cheloveka. 2006;32:45–50. [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Lee SK, Melhem ER. Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology. 2007;245:367–384. doi: 10.1148/radiol.2452060445. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Barker GJ, Koepp MJ, Duncan JS. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage. 2007;36:209–221. doi: 10.1016/j.neuroimage.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Ortiz H, Sebastian-Galles N, Losilla JM, Deus J. Myelination of language-related areas in the developing brain. Neurology. 2006;66:339–343. doi: 10.1212/01.wnl.0000201049.66073.8d. [DOI] [PubMed] [Google Scholar]
- Reinsberg SA, Doran SJ, Charles-Edwards EM, Leach MO. A complete distortion correction for MR images: II. Rectification of static-field inhomogeneities by similarity-based profile mapping. Phys Med Biol. 2005;50:2651–2661. doi: 10.1088/0031-9155/50/11/014. [DOI] [PubMed] [Google Scholar]
- Riederer F, Lanzenberger R, Kaya M, Prayer D, Serles W, Baumgartner C. Network atrophy in temporal lobe epilepsy: a voxel-based morphometry study. Neurology. 2008;71:419–425. doi: 10.1212/01.wnl.0000324264.96100.e0. [DOI] [PubMed] [Google Scholar]
- Riley JD, Franklin DL, Choi V, Kim RC, Binder DK, Cramer SC, Lin JJ. Altered white matter integrity in temporal lobe epilepsy: association with cognitive and clinical profiles. Epilepsia. 2010;51:536–545. doi: 10.1111/j.1528-1167.2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SN, Moseley BD, Cascino GD. Do subtle cortical structure changes indicate a developmental basis for temporal lobe epilepsy? Neurology. 2011;76:117–118. doi: 10.1212/WNL.0b013e318205d545. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Sutula TP. Mechanisms of epilepsy progression: current theories and perspectives from neuroplasticity in adulthood and development. Epilepsy Res. 2004;60:161–171. doi: 10.1016/j.eplepsyres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yogarajah M, Duncan JS. Diffusion-based magnetic resonance imaging and tractography in epilepsy. Epilepsia. 2008;49:189–200. doi: 10.1111/j.1528-1167.2007.01378.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.