Abstract
Anatomical, clinical and imaging findings suggest that the cerebellum is engaged in cognitive and affective functions as well as motor control. Evidence from converging modalities also indicates that there is a functional topography in the human cerebellum for overt control of movement vs. higher functions, such that the cerebellum can be divided into zones depending on connectivity with sensorimotor vs. multimodal association cortices. Using functional MRI, we show that regions active during overt movement differ from those involved in higher-level language, spatial processing and working memory tasks. Nine healthy participants each completed five tasks in order to determine the relative activation patterns for the different paradigms. Right-handed finger-tapping activated right cerebellar lobules IV-V and VIII, consistent with descriptions of the cerebellar homunculi. Verb generation engaged right cerebellar lobules VI-Crus I and a second cluster in lobules VIIB-VIIIA. Mental rotation activation peaks were localized to medial left cerebellar lobule VII (Crus II). A 2-back working memory task activated bilateral regions of lobules VI-VII. Viewing arousing vs. neutral images did not reliably activate the cerebellum or cerebral limbic areas in this study. The cerebellar functional topography identified in this study reflects the involvement of different cerebro-cerebellar circuits depending on the demands of the task being performed: overt movement activated sensorimotor cortices along with contralateral cerebellar lobules IV-VI and VIII, whereas more cognitively demanding tasks engaged prefrontal and parietal cortices along with cerebellar lobules VI and VII. These findings provide further support for a cerebellar role in both motor and cognitive tasks, and better establish the existence of functional subregions in the cerebellum. Future studies are needed to determine the exact contribution of the cerebellum – and different cerebro-cerebellar circuits – to task performance.
Keywords: cerebellum, functional MRI, cognition, sensorimotor, topography
1. Introduction
The understanding of human cerebellar function has undergone a paradigm shift. No longer considered purely devoted to motor control, a wider role for the cerebellum in cognitive and affective functions is supported by anatomical, clinical and functional neuroimaging data. However, clinical findings are inconsistent, and cerebellar activation in neuroimaging studies is commonly reported, but often not interpreted, potentially ignoring an important component of functional neural systems. Recent evidence from functional connectivity studies in humans indicates that the cerebellum participates in functional networks with sensorimotor areas engaged in motor control and with association cortices that are involved in cognitive processes (Habas et al., 2009; Krienen and Buckner, 2009; O’Reilly et al., 2010).
We have proposed that there is a functional topography of the cerebellum, based on its linkages with sensorimotor and higher-order brain areas (see Schmahmann, 1991, 2004; Stoodley and Schmahmann, 2010), such that different cerebellar regions process sensorimotor, cognitive and affective information. This concept is of vital importance to the interpretation of lesion-symptom correlations in clinical studies and cerebellar activation patterns in functional neuroimaging data.
Extensive connections between the cerebellum, spinal cord, and sensorimotor and association areas of the cerebral cortex provide the anatomical substrates for the cerebellar contribution to both movement (Holmes, 1939) and cognition (see Schmahmann and Pandya, 1997). The cerebellum is comprised of ten lobules, grouped as the anterior lobe (lobules I through V); posterior lobe (lobules VI through IX); and the flocculonodular lobe (lobule X). Physiological experiments in cats (Adrian, 1943; Snider and Eldred, 1951) and functional MRI (fMRI) studies in humans (see Grodd et al., 2005) reveal the presence of sensorimotor homunculi in lobules III-VI and lobule VIII. In contrast, association area projections (prefrontal areas, posterior parietal, and superior temporal, posterior parahippocampal and cingulate areas) are mainly localized to lobules VI and VII (for overview, see Kelly and Strick, 2003; Stoodley and Schmahmann, 2010).
Focal lesions in stroke patients also provide insights into cerebellar structure-function relationships. Following cerebellar stroke the expected motor syndrome (gait impairment, incoordination of the extremities, disordered eye movements and slurring of speech) is present in some, but not all, patients (Schmahmann et al., 2009). Similarly, not all patients experience the cerebellar cognitive affective syndrome (CCAS; Schmahmann and Sherman, 1998), characterized by deficits in executive function, visual spatial processing, selected aspects of language, and affect. If there are different functional regions in the cerebellum, then one would predict that different clinical symptoms may be present depending on the location of cerebellar damage, and depending on which cerebellar circuits are affected. There is evidence of this in the sensorimotor domain, where the cerebellar motor syndrome is associated with anterior lobe damage (Schmahmann et al., 2009) and dysarthria is associated with damage to the representation of the articulatory apparatus (cerebellar lobule VI; Urban et al., 2003). The CCAS occurs more often following damage to the posterior lobe of the cerebellum (Schmahmann and Sherman, 1998), and damage to right posterolateral cerebellar regions has been linked with language deficits (e.g., Marien et al., 2001). Finally, affective symptoms in children are more likely to be present when cerebellar lesions (Levisohn et al., 2000) or malformations (Tavano et al., 2007) affect the posterior midline vermal regions. Therefore, these clinical findings suggest that different regions of the cerebellum are involved in different functional domains.
Further support for cerebellar functional topography comes from functional neuroimaging data. Resting-state functional connectivity studies have shown that activity in sensorimotor regions correlates with the contralateral cerebellar anterior lobe and lobule VIII, whereas activity in prefrontal, posterior parietal, and superior and middle temporal association areas, as well as the cingulate gyrus and retrosplenial cortex, correlates with activity in cerebellar lobules VI and VII (Krienen and Buckner, 2009; O’Reilly et al., 2010). In a recent meta-analysis of cerebellar activation patterns reported in functional imaging studies, it was apparent that sensorimotor tasks activated the anterior lobe and lobule VIII, whereas language tasks activated right cerebellar regions in lobules VI and VII, and spatial tasks tended to lateralize to the left cerebellar hemisphere (Stoodley and Schmahmann, 2009). These findings are consistent with the contralateral connections between the cerebral cortex and cerebellar hemispheres, and suggest that cerebellar activation patterns in imaging studies reflect the involvement of different cerebro-cerebellar loops in a task-dependent manner.
However, meta-analyses are limited by the combination of data from many different studies, acquired on scanners of different strengths, while subjects completed different task paradigms, yielding data that were analyzed with a variety of techniques and statistical thresholds. Therefore, we used fMRI to investigate cerebellar activation patterns for various tasks within individual subjects, in order to examine the topography of activation peaks for sensorimotor, cognitive and affective tasks. Further, these data provide important information about cerebellar participation in the distributed neural circuits subserving sensorimotor as well as higher-order functions.
In the scanner, nine healthy male young adults performed a set of tasks assessing sensorimotor, linguistic, spatial, working memory and affective processing. Each participant completed five tasks which previously had been shown to engage the cerebellum, including: finger tapping with the right index finger (sensorimotor); generating verbs in response to common nouns (language); mental rotation of letter stimuli (spatial); a 2-back task (working memory); and viewing images from the International Affective Picture Scale (IAPS, affective processing; Lang et al., 2005). Reported task activations represent contrast images controlling for the motor responses associated with each task, with the exception of the finger-tapping paradigm, in which the goal was to highlight activation related to overt movement.
Based on the anatomical projections between the cerebellum and cerebral cortices (see Schmahmann and Pandya, 1997; Stoodley and Schmahmann, 2010), we hypothesized that the overt motor task (finger tapping) would activate regions of the cerebellum to which sensorimotor regions project, namely lobules IV-VI and lobule VIII. We predicted that the cognitive tasks (verb generation, mental rotation, 2-back task), would predominantly activate lobules VI and VII, and that language activation would be right-lateralized and spatial activation left-lateralized. Finally, we anticipated that viewing images from the IAPS scale would engage the posterior vermis of the cerebellum, which on the basis of clinical (Heath, 1977; Levisohn et al., 2000; Schmahmann and Sherman, 1998; Tavano et al., 2007), behavioral (Berman et al., 1978), electrophysiological (Heath and Harper, 1974; Snider and Maiti, 1976), and stimulation studies (Demirtas-Tatlidede et al., 2010), is thought to connect with limbic and autonomic regions of the brain.
2. MATERIALS AND METHODS
2.1 Participants
Nine healthy, right-handed adult males (mean age 25 years, 6 months) with no history of neurological illness or injury participated in the study. Data from one participant has been reported as a proof-of-principle, single case study (Stoodley et al., 2010). The project was approved by the Institutional Review Board of the Massachusetts General Hospital and all participants provided written, informed consent. Handedness was confirmed by a score > 40 on the Edinburgh Handedness Inventory (Oldfield, 1971; mean ± standard deviation handedness score 75.7 ± 20.8).
2.2 Cognitive ability
Participants cognitive abilities were assessed with the abbreviated form of the Wechsler Adult Intelligence Scale-III (WAIS-III; The Psychological Corporation, 1997). This standardized scale of intelligence includes seven subtests (Similarities, Arithmetic, Digit span, Information, Picture completion, Digit symbol and Block design) designed to measure both verbal and non-verbal cognitive ability.
2.3 Tasks
Participants were familiarized with the tasks before the functional MRI (fMRI) session. The tapping task was run using Matlab 7.0.4 (Mathworks, Sherborn, MA) on a Sony Vaio laptop; all other tasks were presented using MacStim software (White Ant Occasional Publishing, West Melbourne, Australia) and run on a Mac iBook laptop. In the scanner, the participants completed five different tasks that were chosen because they reliably activate the cerebellum in imaging studies. These included:
2.3.1 Finger-tapping (sensorimotor processing)
Participants were required to tap a button with the right index finger in time with cued beeps at a pace of 2 Hz. Beeps were presented at a comfortable level via MR-compatible headphones; task instructions were presented visually. After an initial fixation period, instructions to “Tap along” were given for 3.5 s, followed by 15 s of cued tapping. “Stop” instructions were then followed by the “Listen only” (2.5 s) condition, which consisted of 15 s of the cueing beeps during which the participant rested. A 15 s fixation period separated the blocks. Each 36-s block (tapping followed by listen only followed by fixation) was repeated 6 times, leading to a total run time of 5:36. Accuracy and response time data were measured.
2.3.2 N-back task (working memory, executive function)
Participants were asked to respond with a button press when the current letter on the screen was the same as that which appeared “N” items previously; an X task, in which the participants responded to the letter X, was used for comparison. In this study, we used a 2-back task. Following an initial fixation period of 20 s, “Respond to X” appeared for 4 s, followed by 16 letters (0.2 s presentation with 1.8 s inter-stimulus-interval [ISI]), leading to a block time of 56 s. Participants responded by pressing a button with the right index finger when the letter “X” appeared, which was approximately 25% of the time. Following a 20 s fixation period, “2 back” appeared for 4 s, to cue the participant to perform the 2-back task. Again, 16 letters appeared in succession and the participant was asked to press a button when the letter presented was the same as that appearing 2-back (approximately 25% of trials). The fixation-X task-fixation-2 back sequence repeated three times, leading to a total run time of 5:36. Two runs of the task were performed. Performance accuracy and response times were recorded.
2.3.3 Verb generation (language)
In this task, participants covertly generated a verb when presented with a concrete noun (e.g., beer → drink). After an initial instruction period, “Read only” appeared for 5 s, followed by 10 nouns, each of which appeared for 2 s with no ISI. During this time, the participant was instructed to silently read the nouns as they appeared. After a 5 s fixation period, “Generate a verb” appeared for 5 s, cueing the verb generation task. Again, 10 nouns appeared and the participants covertly generated a verb for each noun. Each run comprised initial instructions and four blocks of read only interleaved with four blocks of verb generation, leading to a total run time of 4:18. Each 30-s block consisted of the fixation period, followed by instructions (“Read only” or “Generate a verb”), followed by the presentation of nouns. The same stimuli were used for the read only and verb generation conditions, appearing in randomized order. Participants performed two runs of the task, and after the scan session provided verbs for a sample set of the nouns presented in the study as a measure of accuracy.
2.3.4 Mental rotation (visual-spatial processing)
The participants were required to judge whether a single rotated letter was presented in its normal form or as a mirror image by pressing the button under the right index finger if the letter was in its normal orientation, and the button under the right middle finger if the letter was mirror-oriented. After 18 s of initial instructions, blocks of non-rotated and rotated letters (F, G, J and R) alternated. The rotated letters appeared at angles of 40, 120, 200, and 280 degrees. Each block comprised 8 black letter stimuli which appeared on a gray background for 1500 ms with an ISI of 1500 ms; a 6 s rest period followed stimuli presentation, leading to a block length of 30 s. Each run comprised four blocks with non-rotated letters alternating with four blocks with rotated letters, leading to a total run time of 4:18. Participants completed two runs of the task. Behavioral performance in the scanner was analyzed for accuracy and response times.
2.3.5 Viewing of pictures from the International Affective Picture System (IAPS; Lang et al., 2005; affective processing)
These images are similar with regard to visual features (complexity, color, etc.) but differ in emotional content. Participants viewed images rated as “highly arousing” (average standard valency for negative images was 2.9, and for positive images 6.7; arousal ratings were 6.4 and 6.3, respectively) and “neutral” (average valency, 5.2; average arousal rating, 2.6) while in the scanner. Images with extreme arousal content were excluded. Participants completed one run of the task. After an initial instruction period of 11 s, 8 blocks comprised of neutral images alternated with 8 blocks of arousing images. Each block consisted of 8 images appearing for 2 s with an ISI of 500 ms, resulting in a 20-s block length. The total run time was 5:31, and participants completed one run of the task. After the scan session, the participants viewed a subset of the images and rated them on a scale of 1-9 to assess the degree to which they found them negative/positive or neutral/arousing.
2.4 fMRI scanning
Scanning was performed at the Athinoula A. Martinos Center for Biomedical Imaging using a 3T TimTrio (Siemens, Erlanger, Germany) MRI with a 12-channel head coil. During scanning, the participant s head was immobilized using tight but comfortable foam padding. Stimuli were presented via a liquid crystal display (LCD) projector onto a screen located in the scanner bore and viewed through a mirror attached to the head coil; auditory stimuli were presented through MR-compatible headphones. Each participant underwent a sagittal T1-weighted structural MR scan (magnetization prepared rapid gradient echo, MPRAGE), with 128 1.33 mm-thick slices, 1.3 × 1.0 × 1.3 mm voxel size, TR 2530 ms, TE 3.39 ms, flip angle 7°, and field of view (FOV) 256 × 256 mm. Functional echo-planar imaging (EPI) runs were performed with the following parameters: 42 interleaved axial slices at a TR 2900 ms, TE 30 ms, voxel size 3 × 3 × 3 mm, flip angle 90°, FOV 288 mm. One run each of the tapping (112 measurements) and affective (110 measurements) tasks were run. Participants completed two runs of the verb generation (85 measurements per run), mental rotation (85 measurements per run) and 2-back task (112 measurements per run).
2.5 Data analysis
Analysis of fMRI data was performed using Statistical Parametric Mapping, version 8 (SPM8; Wellcome Department of Cognitive Neurology, London). Data were realigned for motion correction by registration to the mean image. Artifact detection was performed using the Artifact Detection Tools (ART) toolbox (http://www.nitrc.org/projects/artifact_detect/); global mean intensity (> 2 standard deviations from mean) and motion (> 2 mm) outliers were identified and entered as regressors of no interest in the General Linear Models. Each participant s T1 anatomical scan was co-registered to the mean functional image of each run, and the gray and white matter segmented to produce modulated, normalized images. The spatial normalization parameters from the segmentation process were used to normalize the realigned functional images to MNI space. This procedure has been shown to more accurately align the cerebellum, without introducing the elongation that can occur with the standard “normalize” procedure in SPM (Diedrichsen et al., 2009). Data were then smoothed using a 6 mm full width half maximum filter. The realigned, normalized and smoothed data were modeled using a boxcar function convolved with a canonical hemodynamic response function. A 128s high-pass filter was used. At the 1st level, general linear modeling (GLM) was employed to form statistical parametric maps of the T-statistic. The contrasts were: 1. Tapping vs. Listen only; 2. 2-back vs. X-task; 3. Verb generation vs. Read only; 4. Rotated vs. Non-rotated letters; 5. Arousing vs. Neutral images. Individual contrast images for each task were entered into a 2nd level random effects analysis to make inferences at the group level. The SPM T-maps were thresholded at a voxel-level threshold of P<0.0001 with a cluster-level threshold of P<0.05 (False Discovery Rate [FDR] corrected) unless otherwise noted.
In order to assess areas which were significantly activated in all tasks, the conjunction null was evaluated using a full factorial design with 5 levels (each representing one of the 5 tasks). The lack of independence between the activation related to each task paradigm was accounted for in the model. An uncorrected P<0.001 and cluster size of k>15 was used to search for significant regions of conjunction at the whole brain level. This model also allowed us to examine relative activation patterns for the sensorimotor (finger tapping) vs. cognitive tasks (verb generation, n-back and mental rotation).
The Spatially Unbiased Atlas Template (SUIT) of the cerebellum and brainstem (Diedrichsen, 2006; Diedrichsen et al., 2009) for the SPM Segmentation method and the MRI Atlas of the Human Cerebellum (Schmahmann et al., 2000) were used to localize activation patterns within the cerebellum. The SUIT atlas was developed to improve both the registration and anatomical detail of the cerebellum for structural and functional images. While our images are in MNI space and not SUIT space, we used the SPM Segmentation method so that the data would be unbiased relative to the SUIT atlas template (see Diedrichsen et al., 2009). The MRI Atlas provides detailed anatomy for one individual brain and was used to guide analysis of individual activation patterns. Activation patterns in the cerebral hemispheres were localized using the AAL atlas (Tzourio-Mazoyer et al., 2002). The activation patterns were visualized using MRIcroGL software (http://www.cabiatl.com/mricrogl/).
3. RESULTS
3.1 Behavioral Performance
Participants (n=9, mean age 25 years, 6 months) scored in the average to above-average range on all subtests of the abbreviated form of the Wechsler Adult Intelligence Scale-III (WAIS-III; The Psychological Corporation, 1997; mean ± standard deviation = 10 ± 3 for each subtest). Mean scores for the Similarities (13.1 ± 2.1), Arithmetic (12.9 ± 1.3), Digit Symbol (13.3 ± 2.7), Information (15.2 ± 1.9), Block design (13.2 ± 3.0), Digit span (13.0 ± 3.0) and Picture completion (10.6 ± 2.5) subtests indicated that all participants were of above-average cognitive ability.
3.1.1 Tapping
On the finger tapping task, the cues were paced every 500 msec. On average, the participants tapped every 501.4 ± 0.7 msec, indicating excellent performance on the task. None of the participants made erroneous taps during the “listen only” condition.
3.1.2 N-back
Due to technical difficulties with data collection, behavioral data from 3 participants were analyzed for the n-back task. These participants were 100% accurate when performing the X-task, and performed the 2-back task at a mean accuracy of 90.2 ± 9.9%. Mean RTs were 438.2 ± 30.8 msec for the X-task, and 553.1 ± 44.7 msec for the 2-back task.
3.1.3 Verb generation
A sample of 40 nouns from the verb generation task was presented to the participants during post-scan testing, and participants generated appropriate verbs for the nouns. The mean score was 39/40 with a standard deviation of 1.2, indicating that the participants were able to successfully complete the task.
3.1.4 Mental Rotation
Behavioral response data from 4 participants were analyzed for the mental rotation task. Mean accuracy during the trials in which the letters were upright was 99.1 ± 1.9%, and accuracy during the rotated trials was 96.7 ± 4.4%. Mean RTs during the upright trials were 817.0 ± 125.3 msec and 924.2 ± 210.3 during the rotated trials.
3.1.5 Viewing IAPS pictures
Post-testing indicated that the participants had normal responses to the IAPS images. A subset of images were shown to the participants and they were rated on a scale of 1-9 for their degree of arousal (1=extremely low to 9=extremely high) and valency (1=extremely negative, 5=neutral, 9=extremely positive). Based on the IAPS ratings for the images, our participants average ratings were very close to the published norms – the mean z-score for the arousal ratings was 0.08, and for the valency ratings the mean z-score was -0.07 for all images presented in post-testing.
3.2 fMRI Results
The fMRI analyses were conducted at the whole-brain level, although here we focus on the cerebellar activation patterns (see Tables 1-5 and Figure 2 for more detail on whole-brain activation patterns). Activation clusters surviving a voxel-level threshold of P<0.0001 and a cluster-level threshold of P<0.05 (False Discovery Rate [FDR] corrected) are reported.
Table 1. Tapping vs. Listen only.
The tapping task activated the insula, pre- and post-central gyri, and the supplementary motor area, as well as thalamic, cingulate, and parietal regions. Cerebellar activation peaks were in right lobules IV-V and right lobules VIIIA and VIIIB.
| Location | Cluster size (voxels) | Max T | MNI Coordinates |
|---|---|---|---|
| R Insula | 179 | 36.1 | 34 20 8 |
| R Insula (BA 13), precentral gyrus (Rolandic perculum) | 69 | 25.6 | 44 4 4 |
| Cingulate, superior medial frontal gyrus | 77 | 17.8 | 6 22 38 |
| L Rolandic operculum, superior temporal gyrus | 68 | 17.5 | -44 -28 18 |
| L VPL Thalamus | 129 | 14.3 | -18 -20 6 |
| Cerebellum R lobules VIIIA, VIIIB | 43 | 13.3 | 12 -66 -48 |
| L Precentral gyrus (Rolandic operculum), insula (BA 13) | 67 | 12.5 | -46 4 8 |
| L Postcentral gyrus | 158 | 12.2 | -46 -24 32 |
| L Putamen | 100 | 12.0 | -26 -10 6 |
| L Precentral gyrus (BA 9), inferior frontal gyrus (operculum) | 34 | 10.9 | -56 4 24 |
| R Inferior parietal lobule, supramarginal gyrus | 48 | 10.9 | 60 -38 30 |
| L Insula | 42 | 9.9 | -40 10 4 |
| Cingulate cortex | 61 | 9.8 | 0 8 36 |
| Cerebellum R lobules IV, V | 19 | 9.0 | 14 -48 -10 |
| L Postcentral gyrus (BAs 2, 3), precentral gyrus | 90 | 8.5 | -42 -20 56 |
| R Superior frontal gyrus, supplementary motor area | 13 | 8.4 | 20 8 60 |
| L Supplementary motor area | 12 | 8.1 | -10 4 50 |
| R Putamen, pallidum | 11 | 7.7 | 24 -4 2 |
| Cerebellum R lobule VIIIB | 13 | 7.7 | 20 -50 -50 |
| R Supplementary motor area | 10 | 7.3 | 8 8 66 |
Key: R = Right; L = Left; BA = Brodmann s area. MNI coordinates = x, y, z coordinates of cluster peaks. Clusters meeting a height threshold of P<0.0001 with cluster-level threshold of P<0.05 (FDR corrected) are reported.
Table 5. IAPS.
Viewing “emotional” vs. “neutral” images strongly activated occipital-temporal regions of the brain bilaterally.
| Location | Cluster size (voxels) | T-value | MNI Coordinates |
|---|---|---|---|
| R Lingual gyrus, calcarine cortex, middle temporal, inferior temporal | 3437 | 21.2 | 16 -90 -6 |
| L Fusiform gyrus, inferior occipital gyrus, middle temporal, inferior temporal | 1500 | 12.1 | -34 -68 -12 |
| L Calcarine cortex, lingual gyrus, cerebellum lobule VI | 301 | 8.4 | -10 -90 -8 |
| R Superior temporal gyrus, middle temporal | 96 | 6.8 | 48 -40 18 |
| Cerebellum R lobule VIIB** | 9 | 5.7 | 20 -70 -42 |
Key: R = Right; L = Left. MNI coordinates = x, y, z coordinates of cluster peaks. Clusters meeting a height threshold of P<0.002 with cluster-level threshold of FDR-corrected P<0.05 are reported.
This cluster does not meet the cluster-level threshold.
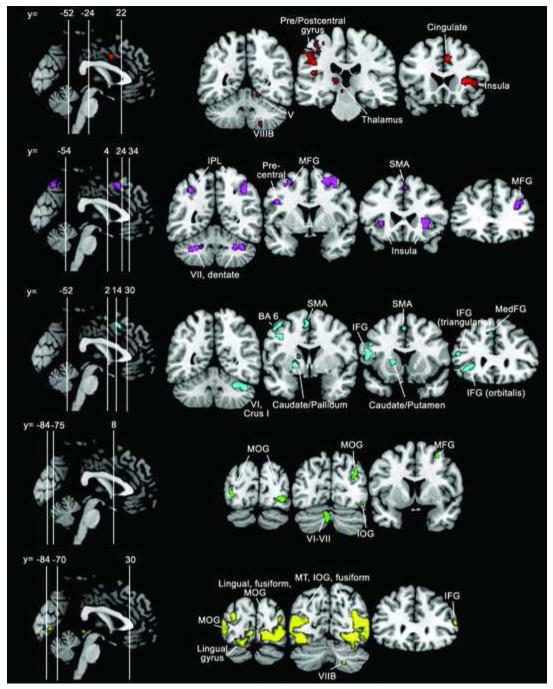
Figure 2.
fMRI results. Whole-brain activation patterns for (from top to bottom) finger tapping (red); n-back (violet); verb generation (cyan); mental rotation (green); and IAPS (yellow) tasks. Activation maps are thresholded at a voxel-level threshold of P < 0.0001 (uncorrected) with a cluster-level correction of P < 0.05 (corrected for false-discovery rate [FDR]). Left is shown on the left. IFG, inferior frontal gyrus; IOG, inferior occipital gyrus; IPL, inferior parietal lobule; MFG, middle frontal gyrus; MedFG, medial frontal gyrus; MOG, middle occipital gyrus; MT, middle temporal; SMA, supplementary motor area.
Figure 1 shows the functional activation patterns in the cerebellum for the different tasks. Both topographic and lateralization effects are evident. While overt movement activated known sensorimotor representations in lobules IV-V and VIII, the cognitive tasks showed activation peaks in lobules VI and VII. In terms of laterality, finger tapping with the right index finger engaged the right cerebellum, reflecting the connectivity patterns of the cerebellum with spinal cord (ipsilateral) and cerebral cortex (contralateral). Similarly, verb generation activation patterns were right-lateralized, whereas the activation during the mental rotation paradigm was left-lateralized, consistent with the broad classification of language as a left cerebral hemisphere function and spatial tasks as engaging more right cerebral hemisphere areas.
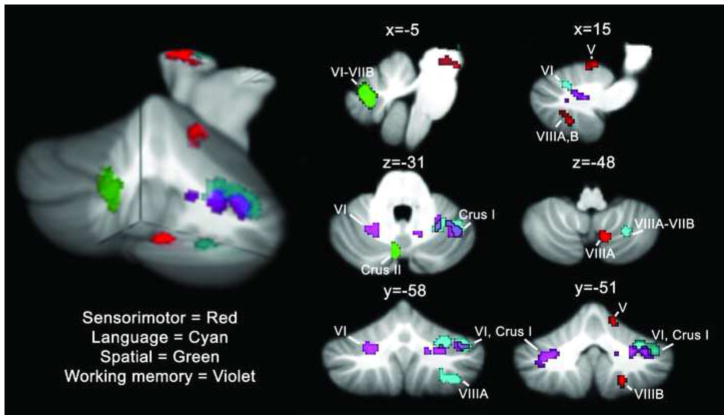
Figure 1.
fMRI results. Cerebellar activation patterns for finger tapping (red), verb generation (cyan), mental rotation (green) and the n-back task (violet) are shown on sagittal (top row), axial (middle row) and coronal (bottom row) slices through the cerebellum. Regions where verb generation and n-back activation overlap are outlined in black. A 3-D rendering of the activation patterns is shown on the left, with a cutout at x = -5, y = -56, z = -45 (MNI coordinates). Activation maps are thresholded at a voxel-level threshold of P < 0.0001 (uncorrected) with a cluster-level correction of P < 0.05 (corrected for false-discovery rate [FDR]). Left is shown on the left.
Consistent with previous findings (e.g., Grodd et al., 2005), right-handed finger tapping activated right cerebellar lobules IV-V and lobule VIII (tapping activation is shown in red, Figure 1). Working memory (2-back vs. X-task contrast; shown in violet) engaged bilateral cerebellar regions including lobules VI and VII (Crus I). The main right cerebellar peak was in Crus I with a second peak in lobule VI. The left cerebellar peak was in Crus I and the cluster extended into lobule VI. Verb-for-noun generation (verb generation vs. reading nouns; shown in cyan) activated right cerebellar hemisphere regions of lobules VI and VII, extending into VIIIA; there were two right cerebellar clusters, one with a peak in Crus I and the second with a max peak in VIIIA. Mental rotation (the contrast of rotated vs. upright letters) involved left cerebellar lobule VII at the midline (shown in green). The IAPS task did not reliably activate the cerebellum in this study as it has in previous reports, nor were non-cerebellar areas in the amygdala, insula, cingulate gyrus and orbital, medial and lateral prefrontal cortices activated (Bermpohl et al., 2006; Northoff et al., 2000). At a more lenient threshold (P<0.002, uncorrected at the cluster level) a small cluster in right VIIB was evident, however.
The conjunction analysis of all five task paradigms did not reveal any significant regions of overlap for all tasks (no significant clusters of k>15 in the whole brain at P<0.001, uncorrected). Our paradigms could be broadly grouped as “sensorimotor” (finger tapping), “cognitive” (verb generation, mental rotation, n-back task), and “affective” (IAPS image viewing). The conjunction of the cognitive paradigms revealed significant overlap in frontal (supplementary motor area [MNI coordinates x y z] 2 16 50, T=5.44, k=222; left precentral gyrus, -50 4 28, T=4.42, k=42; left middle frontal gyrus, -24 6 58, T=3.77, k=40), parietal (left inferior parietal lobule, -40 -42 42, T=5.06, k=219; left superior parietal lobule, -24 -64 50, T=4.62, k=222) and insular (34 24 -2, T=5.22, k=64) cortices, but there was no significant overlap in the cerebellum.
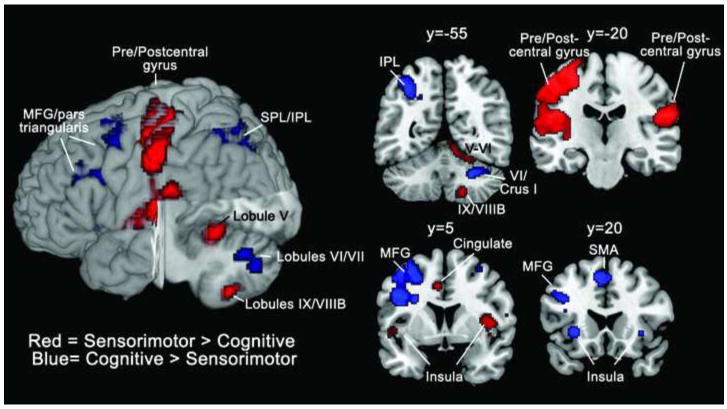
The contrast of finger tapping > cognitive paradigms revealed statistically significant differences in right cerebellar lobules V and IX/VIIIB, as well as sensorimotor cortices, the supplementary motor area, and the right superior medial frontal gyrus, extending into the anterior cingulate (threshold was set at P<0.0001, FDR-corrected P<0.05 at the cluster level; see Table 6 and Figure 3). The contrast of cognitive > finger tapping tasks revealed greater activation in right cerebellar lobules VI and VII, including Crus I and VIIB, along with various cortical regions.
Table 6. Sensorimotor vs. cognitive tasks.
| Location | Cluster size (voxels) | T-value | MNI Coordinates |
|---|---|---|---|
| Tapping > cognitive | |||
| L Postcentral gyrus, rolandic operculum, precentral gyrus | 3774 | 10.94 | -42 -20 54 |
| R Rolandic operculum, supramarginal gyrus | 985 | 7.46 | 56 -14 18 |
| L Supplementary motor area (area 6), middle cingulate cortex | 183 | 6.28 | -4 -6 54 |
| Cerebellum lobules R lobules V, VI | 292 | 5.85 | 18 -46 -16 |
| R Insula | 118 | 5.48 | 44 4 6 |
| Cerebellum R lobules IX, VIIIB | 92 | 5.34 | 10 -60 -50 |
| R Superior medial gyrus, anterior cingulate | 87 | 5.29 | 2 54 2 |
| Cognitive > Tapping | |||
| L Inferior parietal lobule, superior parietal lobule (7A) | 1273 | 7.22 | -26 -70 42 |
| L Supplementary motor area (area 6) | 402 | 6.91 | 0 16 50 |
| Cerebellum R lobules Crus I, VI | 271 | 6.82 | 38 -62 -30 |
| L Precentral gyrus, middle frontal gyrus including pars triangularis | 1387 | 6.55 | -30 0 48 |
| R Insula | 46 | 6.28 | 34 24 -2 |
| Cerebellum R lobules Crus II, VI | 69 | 5.56 | 8 -76 -34 |
| L Insula | 119 | 5.44 | -30 20 -2 |
| R Middle frontal gyrus, superior frontal gyrus | 53 | 4.89 | 32 0 54 |
| L Inferior frontal gyrus (pars triangularis) | 77 | 4.76 | -40 42 0 |
Key: R = Right; L = Left. MNI coordinates = x, y, z coordinates of cluster peaks. Clusters meeting a height threshold of P<0.0001 with cluster-level threshold of FDR-corrected P<0.05 are reported.
Figure 3.
Sensorimotor vs. cognitive activation patterns. Regions where finger tapping > cognitive tasks (verb generation, n-back and mental rotation) are shown in red; regions where cognitive > finger tapping are shown in blue. A 3-D rendering of the activation patterns is shown on the left, with a cutout at x = 9, y = -19, z = 18 (MNI coordinates). Activation maps are thresholded at a voxel-level threshold of P < 0.0001 (uncorrected) with a cluster-level FDR-corrected P < 0.05. Left is shown on the left. Labeling is based on entire activation cluster, not all of which may appear in a given slice (see Table 6). IPL, inferior parietal lobule; MFG, middle frontal gyrus; SPL, superior parietal lobule.
4. DISCUSSION
Our aim was to determine whether the functional topography suggested by the anatomical connectivity of different regions of the cerebellum was evident when participants performed different types of tasks. As all participants performed the full set of tasks, we were able to look at the relative activation patterns for different tasks using the same data acquisition parameters and analysis methods. This is a step beyond previous work surveying the imaging literature and determining whether different categories of tasks (e.g., language, spatial, executive, sensorimotor) engage different cerebellar regions (Stoodley and Schmahmann, 2009).
It is clear from the results, as well as many previous investigations, that the cerebellum is active during both motor and cognitive tasks (see Stoodley, 2011, for review). Furthermore, different regions of the cerebellum are engaged depending on the nature of the task being performed. Finger tapping activated sensorimotor circuits between the cerebral cortex, cerebellar lobules IV-V and VIII, and the spinal cord. In contrast, cognitive tasks engaged different cerebro-cerebellar circuits, including cerebellar lobules VI and VII and the prefrontal and parietal cortices.
These findings support the cerebellar functional topography that was evident in a meta-analysis of cerebellar activation patterns during cognitive and affective tasks (Stoodley and Schmahmann, 2009). Language tasks activated predominantly right-hemisphere regions of lobules VI, Crus I and Crus II, and spatial task activation peaks were predominantly left-lateralized and localized to lobule VI. Working memory paradigms activated bilateral regions of lobules VI, Crus I, and VIIIA; other executive function tasks showed converging activation in lobules VI, Crus I and left VIIB. Emotional processing paradigms activated bilateral clusters in VI-Crus I as well as a midline posterior region in VIIAt. The meta-analysis data, which highlight converging findings from a number of published studies in the literature, indicate that activity during cognitive tasks tends to be localized to lobules VI and VII. Further, both the lobular and lateralization patterns seen in the meta-analysis study are consistent with the results of the current study, as are the results of a preliminary case study investigating intra-individual cerebellar topography (see Figure 4; Stoodley et al., 2010).
Figure 4.
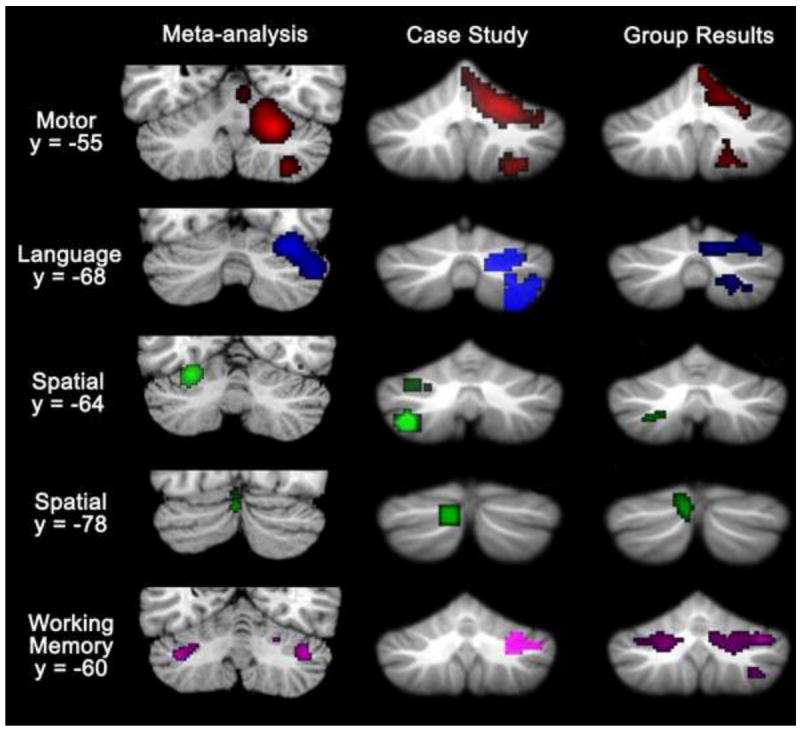
Converging evidence of cerebellar topography from a meta-analysis of published imaging data (Stoodley and Schmahmann, 2009), a single-case study (Stoodley et al., 2010), and the present study. Consistently active clusters during motor (red; right-handed finger movement), language (blue), spatial (green) and working memory (purple) paradigms are shown on coronal cerebellar slices. Left is shown on the left.
4.1 Activation patterns relative to previous studies
BOLD signal patterns during the finger-tapping task – including a peak in ipsilateral lobule V and a second cluster in lobules VIIIA/B – were consistent with those found in previous studies during simple sensorimotor tasks (Bushara et al., 2001; Grodd et al., 2001; Rijntjes et al., 1999; Stoodley and Schmahmann, 2009). For example, Grodd and colleagues showed that right-hand movement engaged right lobule V with a secondary representation in right lobule VIII (Grodd et al., 2001). Similarly, right lobules V-VI and lobules VIIIA-B were consistently activated across studies of right-handed finger movements (Stoodley and Schmahmann, 2009). These findings also correspond with resting-state functional connectivity studies which show that activity in sensorimotor cortices correlates with activity in cerebellar lobules V, VI and VIII (Habas et al., 2009; Krienen and Buckner, 2009; O’Reilly et al., 2010). When sensorimotor tasks are more complex, involving sequenced movements, there is functional topography for hand vs. foot movements in lobule VI (Schlerf et al., 2010).
Cerebellar activation is consistently reported during working memory paradigms, starting with early fMRI and PET studies (e.g., Desmond et al., 1997; Fiez et al., 1996). The cerebellum is involved in both verbal and non-verbal working memory tasks, such as the paced auditory serial addition task (PASAT), the n-back task, and the Sternberg paradigm (e.g., Beneventi et al., 2007; Cardinal et al., 2008; Chen and Desmond, 2005a, b; Forn et al., 2008; Hautzel et al., 2009; Hayter et al., 2007; Honey et al., 2000; Kirschen et al., 2005; Kirschen et al., 2010; LaBar et al., 1999; Marvel and Desmond, 2010; Stoodley et al., 2010; Tomasi et al., 2005). Our finding of bilateral cerebellar activation in lobules VI and Crus I during the n-back task is consistent with other studies showing cerebellar posterior lobe activation during working memory tasks (e.g., Honey et al., 2000; LaBar et al., 1999; Tomasi et al., 2005; Valera et al., 2005). Based on activation patterns during the Sternberg paradigm, Desmond and colleagues have proposed that the superior lobule VI/Crus I activation is related to the activation of a cerebello-frontal loop between the cerebellum and Broca s area, which is involved in articulatory rehearsal and the visual-to-phonological encoding necessary for visually-presented stimuli; further, they suggest that a cerebello-parietal loop (right lobules VIIB and VIIIA and the inferior parietal lobule) is involved in the maintenance and storage of information (Chen and Desmond, 2005a). While we did not see the inferior (lobule VIII) cluster that is often seen during the Sternberg paradigm, the n-back task used here did not enable us to extract activation patterns related specifically to maintenance and storage during working memory.
In the current study, verb generation activated right cerebellar hemisphere regions of lobules VI and VII, extending into VIIIA. The lateralization of the verb generation activation is consistent with studies showing that right posterior lateral regions of the cerebellum are involved in language tasks, including semantic and phonological processing tasks, word stem completion, and verbal fluency (see Stoodley and Schmahmann, 2009, for review). Clinical findings also suggest that language deficits, including verbal fluency (Neau et al., 2000; Richter et al., 2007; Schweizer et al., 2010), are related to damage to the right posterior cerebellar hemisphere. Several studies have shown that the cerebellum is part of the “language network”; for example, dynamic causal modeling of activity during a rhyming task revealed that right lobules VI and Crus I are reciprocally connected with language areas in the left inferior frontal gyrus and left lateral temporal cortex (Booth et al., 2007). From this study, as well as others, it is clear that cerebellar activation is not dependent on movement of the articulatory muscles – i.e., overt speech – but rather is also present when there is no motor response. Indeed, overt and covert speech may engage different cerebellar regions due to the differential demands on motor control. For example, Frings and colleagues (Frings et al., 2006) found that articulation engaged bilateral paravermal regions of lobule VI, whereas a verb generation condition specifically activated right hemisphere regions of lobule VI and Crus I. Ackermann and colleagues (Ackermann et al., 2007) have suggested the formation of a “pre-articulatory verbal code (inner speech)” underlies the cerebellum s role in both speech production and perception.
Several previous studies have reported cerebellar activation during mental rotation or spatial transformation tasks (Bonda et al., 1995; Creem-Regehr et al., 2007; Jordan et al., 2001; Parsons et al., 1995; Stoodley et al., 2010; Vingerhoets et al., 2002; Weiss et al., 2009; Zacks et al., 2002). The mental rotation task in this study activated a cluster in medial left cerebellar lobule VII. This cluster is consistent with one of the “spatial” clusters reported in the meta-analysis by Stoodley and Schmahmann (Stoodley and Schmahmann, 2009). Although the meta-analysis cluster was more superior to the activation peak in the current results (the meta-analysis peak was located at -4 -70 -18 vs. -4 -72 -32 here), this could be due to differences in the registration methods, as standard normalization procedures can stretch the cerebellum in the z-direction (see Diedrichsen, 2006). This region of the cerebellum may constitute part of the “oculomotor vermis” and is active during the control of eye movements (see Glickstein et al., 2011). We attempted to minimize eye movement differences in the mental rotation task by presenting only one letter on the screen at a time, although it is still possible that this activation is related to oculomotor factors inherent in the mental rotation task.
In this study, the IAPS task did not reliably activate the cerebellum. There are several potential explanations for this finding. We performed only one run of this task, which may have resulted in insufficient power. It has been reported that negative stimuli activate the cerebellum (and occipito-temporal regions) more significantly than positive stimuli (Lane et al., 1997; Paradiso et al., 1999; Takahashi et al., 2004), and the combination of positive and negative arousing stimuli used in this study also may have limited the cerebellar activation. It is worth noting that the task also did not activate traditional limbic regions (Papez, 1937) and paralimbic cortices (Yeterian and Pandya, 1985) such as the amygdala, insula, cingulate gyrus or neocortical areas including the orbital, medial, and lateral prefrontal cortices that have been shown to activate in the IAPS task (Bermpohl et al., 2006; Northoff et al., 2000).
Finally, our findings are consistent with the functional connectivity between the cerebellum and association cortices, which suggests that hemispheric regions of VI and VII participate in functional networks with prefrontal and parietal regions, whereas sensorimotor cortices show functional correlations with lobules V and VIII (e.g., Habas et al., 2009; Krienen and Buckner, 2009; O’Reilly et al., 2010). The contrast of finger tapping > cognitive tasks revealed peaks in the left postcentral gyrus (-42 -20 54) and cerebellar lobules V (18 -46 -16) and VIIIB (18 -54 -50), which correspond closely with the functional connectivity findings of Krienen and Buckner (2009), where a seed at -42 -24 60 led to correlations in lobules V (22 -52 -22) and VIIIB (20 -58 -54). The cognitive > finger tapping comparison led to peaks in cerebellar lobules VI (26 -60 -30), Crus I (38 -62 -30) and Crus II (8 -76 -34), as well as parietal and frontal cortices; these findings correspond to the functional connectivity data showing correlations in Crus I (-32 -64 -32 and 28 -70 -30) for the prefrontal mask and Crus II (-10 -88 -34 and 14 -86 -42) for the parietal mask reported by O Reilly and colleagues (2010). These functional subdivisions of the cerebellum are also supported by structural findings in humans, which indicate that separate cerebro-ponto-cerebellar and cerebello-thalamic-cerebral tracts exist for Crus I/II (“cognitive”) and lobules V-VI (“sensorimotor”) (Salmi et al., 2010).
4.2 Why is the cerebellum involved in these tasks?
Our results demonstrate the “where” of cerebellar activation patterns, but they do not explain “what” the cerebellum is contributing to the performance of each of these tasks. Because we were interested in establishing whether tasks utilizing different categories of information (e.g., language, spatial) engaged different cerebellar regions, we specifically chose tasks that have been shown to reliably produce cerebellar activation in previous studies, rather than targeting a particular processing mechanism per se. It has been proposed that the computational contribution which underlies the role of the cerebellum in motor control can also be applied to the information that the cerebellum receives from association cortices, be it linguistic, spatial, or required for working memory (internal model theory, see Ito, 2006; the universal cerebellar transform, see Schmahmann, 1991, 2000). There is currently no consensus as to “what” the cerebellum is doing, but several proposed theories provide options that could be applicable to both motor and non-motor tasks, including: the development and refinement of internal models for both motor and mental operations (Ito, 2008); the idea that the cerebellum is involved in the monitoring of expected and observed outcomes (e.g., Ben-Yehudah et al., 2007), such as expected events in a working memory paradigm; a cerebellar role in the timing (e.g., Ivry et al., 2002) or sequencing of stimuli (see Ackermann, 2008; Molinari et al., 2008), which is relevant to working memory (encoding the sequence of stimuli) or language (the sequencing and ordering of syllables; e.g. Bohland and Guenther, 2006); and the dysmetria of thought theory that the cerebellum integrates multiple internal representations with external stimuli and self generated responses in an implicit (automatic / non-conscious) manner, serving as an oscillation dampener to optimize performance according to context (Schmahmann, 1991, 2000, 2004; Schmahmann and Pandya, 1997; Schmahmann and Sherman, 1998). It should be acknowledged that the cerebellum could be engaged in tasks that have some relevance to current or future movement – this could apply to finger tapping, verbal rehearsal during working memory tasks, inner speech, motor imagery, or facial expressions. In this case, increased demands on motor planning or rehearsal mechanisms could be responsible for cerebellar activation during cognitive task paradigms in which overt movement is either controlled for or absent. Even with the most cautious and narrow interpretation, however, the fact that the cerebellum is engaged in the absence of overt appendicular movement represents a radical departure from earlier notions that the cerebellum is involved solely in motor control. Regardless of the underlying mechanism driving cerebellar activation, these data clearly show that there is functional separation within the cerebellum – and related cerebral cortical areas – according to task demands.
4.3 Conclusion
Establishing functional subregions of the cerebellum has potentially wide-ranging implications. It helps explain the presence of both the classic cerebellar motor syndrome as well as the CCAS in patients with cerebellar lesions. In addition, structural and functional cerebellar differences have been found in a range of disorders, from schizophrenia to autism, and deeper understanding of functional topography in the human cerebellum may lead to new insights into the anatomical underpinnings, pathophysiology and clinical manifestations of these disorders. Further, our results may help to interpret neuroimaging findings in the context of cerebellar involvement in a wide range of motor and cognitive functions. The present data contribute to the growing body of evidence indicating a role for the human cerebellum in tasks beyond those requiring overt control of movement, and reinforce the new conceptualization of the cerebellum as part of the distributed neural circuits subserving cognition and emotion.
Table 2. N-BACK Task.
The 2-back vs. x-task contrast showed widespread, bilateral frontal and parietal activation. The cerebellum similarly showed bilateral activation peaks, located in lobules VI-VII.
| Location | Cluster size (voxels) | T-value | MNI Coordinates |
|---|---|---|---|
| R Insula | 100 | 13.9 | 32 22 4 |
| R Inferior parietal lobule, supramarginal gyrus | 388 | 13.8 | 38 -40 40 |
| Cerebellum R lobules VII (Crus I), VI including dentate | 136 | 12.7 | 36 -52 -32 |
| Precuneus | 90 | 11.9 | -4 -68 54 |
| L Superior parietal lobule | 88 | 11.4 | -22 -66 48 |
| R Superior frontal gyrus, middle frontal gyrus | 237 | 10.8 | 30 4 60 |
| Cerebellum L lobules VII (Crus I), VI | 103 | 10.5 | -32 -52 -36 |
| R Middle frontal gyrus | 97 | 10.5 | 36 36 22 |
| Supplementary motor area | 83 | 9.8 | 2 16 50 |
| L Caudate | 21 | 9.6 | -12 -2 18 |
| L Insula, inferior frontal gyrus | 36 | 9.4 | -30 26 0 |
| R Angular gyrus, middle occipital gyrus | 89 | 9.1 | 38 -72 38 |
| L Middle frontal gyrus, superior frontal gyrus | 68 | 9.1 | -28 8 58 |
| Cerebellum R lobules V, VI | 19 | 8.9 | 30 -34 -36 |
| R Middle frontal gyrus, superior frontal gyrus | 20 | 7.9 | 26 16 52 |
| L Inferior parietal lobule | 39 | 7.9 | -32 -50 44 |
| L Precentral gyrus, inferior frontal gyrus (operculum) | 17 | 7.5 | -46 4 30 |
Key: R = Right; L = Left. MNI coordinates = x, y, z coordinates of cluster peaks. Clusters meeting a height threshold of P<0.0001 with cluster-level threshold of P<0.05 (FDR corrected) are reported.
Table 3. Mental rotation.
The contrast of rotated vs. upright letters activated occipital and parietal areas. In the cerebellum, the activation was found in left lobule VII.
| Location | Cluster size (voxels) | T-value | MNI Coordinates |
|---|---|---|---|
| Cerebellum L lobule VII (Crus II), extending from VI to VIIB at the midline | 81 | 16.7 | -4 -74 -32 |
| R Middle occipital gyrus, precuneus | 108 | 16.5 | 32 -70 20 |
| L Fusiform gyrus | 46 | 14.7 | -34 -62 -6 |
| R Inferior occipital gyrus, middle occipital gyrus | 73 | 14.6 | 34 -82 -4 |
| R Inferior temporal gyrus, fusiform gyrus | 79 | 14.1 | 40 -58 -8 |
| R Superior parietal lobule | 96 | 13.4 | 26 -64 58 |
| R Inferior parietal lobule | 52 | 12.2 | 36 -40 40 |
| L Caudate | 16 | 10.9 | -22 2 26 |
| R Middle temporal gyrus, inferior temporal gyrus | 26 | 10.7 | 50 -46 -6 |
| R Inferior parietal lobule, supramarginal gyrus | 12 | 10.0 | 40 -40 32 |
| L Superior parietal lobule | 60 | 9.9 | -24 -62 52 |
| L Middle occipital gyrus, inferior occipital gyrus | 26 | 9.3 | -36 -84 0 |
| L Precuneus (BA 7), superior parietal lobule | 15 | 9.3 | -14 -62 54 |
| R Superior occipital gyrus, middle occipital, cuneus, calcarine | 9 | 9.2 | 22 -92 10 |
| R Precuneus (BA 7), superior parietal lobule | 26 | 9.0 | 12 -66 48 |
| R Calcarine, lingual gyrus | 15 | 8.7 | 12 -90 2 |
| R Middle frontal gyrus, superior frontal gyrus | 15 | 8.1 | 30 10 54 |
| L Inferior parietal lobule | 19 | 7.9 | -34 -46 42 |
Key: R = Right; L = Left; BA = Brodmann s area. MNI coordinates = x, y, z coordinates of cluster peaks. Clusters meeting a height threshold of P<0.0001 with cluster-level threshold of P<0.05 (FDR corrected) are reported.
Table 4. Verb Generation.
Verb generation vs. read only activated frontal cortices. In the cerebellum, activation peaks were found in right lobules VI and VII (Crus I) with a second cluster involving lobules VIIB and VIIIA.
| Location | Cluster size (voxels) | T-value | MNI Coordinates |
|---|---|---|---|
| Supplementary motor area, medial (superior) frontal gyrus, superior frontal gyrus | 134 | 15.6 | -8 22 48 |
| Cerebellum R lobules VII (Crus I), VI | 236 | 14.9 | 42 -56 -30 |
| L Inferior frontal gyrus (operculum, pars triangularis) | 90 | 13.9 | -48 12 14 |
| L Precentral gyrus | 194 | 12.3 | -46 -2 50 |
| L Inferior frontal gyrus (orbitalis, pars triangularis) | 115 | 10.6 | -44 34 -6 |
| Cerebellum R lobules VIIIA, VIIB | 65 | 10.5 | 36 -58 -52 |
| L Putamen, pallidum | 152 | 10.2 | -18 4 0 |
| Supplementary motor area | 41 | 9.3 | -4 2 56 |
| L Inferior frontal gyrus (pars triangularis) | 42 | 9.1 | -50 32 20 |
| L Inferior frontal gyrus (operculum, pars triangularis) | 25 | 8.0 | -46 12 28 |
| L Thalamus | 29 | 7.9 | -10 -12 6 |
Key: R = Right; L = Left. MNI coordinates = x, y, z coordinates of cluster peaks. Clusters meeting a height threshold of P<0.0001 with cluster-level threshold of P<0.05 (FDR corrected) are reported.
Highlights.
We investigated cerebellar functional topography for motor and cognitive tasks.
Subjects completed motor, language, working memory, spatial, and affective tasks.
Overt movement activated the anterior lobe and lobule VIII.
Cognitive measures activated topographically distinct areas in lobules VI and VII.
Findings are consistent with the localization of cerebro-cerebellar loops.
Acknowledgments
This study was supported in part by The National Center for Research Resources (P41RR14075); the Massachusetts General Hospital Fund for Medical Discovery (CJS); the National Institutes of Health (071535, EMV); the Birmingham Foundation (JDS) and the MINDlink foundation (JDS). The authors would like to thank Larry Seidman for the use of the nback task, Peter Hansen for providing stimuli for the mental rotation task, Janet Sherman for help with the psychometric test battery, and Joanna Willms and Jason MacMore for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann H. Cerebellar contributions to speech production and speech perception: psycholinguistic and neurobiological perspectives. Trends Neurosci. 2008;31:265–272. doi: 10.1016/j.tins.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Mathiak K, Riecker A. The contribution of the cerebellum to speech production and speech perception: clinical and functional imaging data. Cerebellum. 2007;6:202–213. doi: 10.1080/14734220701266742. [DOI] [PubMed] [Google Scholar]
- Adrian ED. Afferent areas in the cerebellum connected with the limbs. Brain. 1943;66:289–315. [Google Scholar]
- Ben-Yehudah G, Guediche S, Fiez JA. Cerebellar contributions to verbal working memory: beyond cognitive theory. Cerebellum. 2007;6:193–201. doi: 10.1080/14734220701286195. [DOI] [PubMed] [Google Scholar]
- Beneventi H, Barndon R, Ersland L, Hugdahl K. An fMRI study of working memory for schematic facial expressions. Scand J Psychol. 2007;48:81–86. doi: 10.1111/j.1467-9450.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Berman AF, Berman D, Prescott JW. The effect of cerebellar lesions on emotional behavior in the rhesus monkey. In: Cooper IS, Riklan M, Snider RS, editors. The Cerebellum, Epilepsy and Behavior. New York: Plenum Press; 1978. pp. 277–284. [Google Scholar]
- Bermpohl F, Pascual-Leone A, Amedi A, Merabet LB, Fregni F, Gaab N, Alsop D, Schlaug G, Northoff G. Dissociable networks for the expectancy and perception of emotional stimuli in the human brain. NeuroImage. 2006;30:588–600. doi: 10.1016/j.neuroimage.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32:821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Frey S, Evans A. Neural correlates of mental transformations of the body-in-space. Proc Natl Acad Sci U S A. 1995;92:11180–11184. doi: 10.1073/pnas.92.24.11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J, Wood L, Lu D, Houk J, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007;1133:136–144. doi: 10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushara K, Wheat J, Khan A, Mock B, Turski P, Sorenson J, Brooks BR. Multiple tactile maps in the human cerebellum. Neuroreport. 2001;12:2483–2486. doi: 10.1097/00001756-200108080-00039. [DOI] [PubMed] [Google Scholar]
- Cardinal KS, Wilson SM, Giesser BS, Drain AE, Sicotte NL. A longitudinal fMRI study of the paced auditory serial addition task. Mult Scler. 2008;14:465–471. doi: 10.1177/1352458507084263. [DOI] [PubMed] [Google Scholar]
- Chen S, Desmond J. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 2005a;24:332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Chen S, Desmond J. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005b;43:1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Creem-Regehr SH, Neil JA, Yeh HJ. Neural correlates of two imagined egocentric transformations. Neuroimage. 2007;35:916–927. doi: 10.1016/j.neuroimage.2006.11.057. [DOI] [PubMed] [Google Scholar]
- Demirtas-Tatildede A, Freitas C, Pascual-Leone A, Schmahmann JD. Modulatory effects of theta burst stimulation on cerebellar nonsomatic functions. Cerebellum Dec. 2010;7 doi: 10.1007/s12311-010-0230-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond J, Gabrieli J, Wagner A, Ginier B, Glover G. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006;33:127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Fiez J, Raife E, Balota D, Schwarz J, Raichle M, Petersen S. A positron emission tomography study of the short-term maintenance of verbal information. J Neurosci. 1996;16:808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forn C, Ventura-Campos N, Belenguer A, Belloch V, Parcet MA, Avila C. A comparison of brain activation patterns during covert and overt paced auditory serial addition test tasks. Hum Brain Mapp. 2008;29:644–650. doi: 10.1002/hbm.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings M, Dimitrova A, Schorn C, Elles H-G, Hein-Kropp C, Gizewski E, Diener H, Timmann D. Cerebellar involvement in verb generation: An fMRI study. Neurosci Lett. 2006;409:19–23. doi: 10.1016/j.neulet.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Sultan F, Voogd J. Functional localization in the cerebellum. Cortex. 2011;47:59–80. doi: 10.1016/j.cortex.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hulsmann E, Ackermann H. Functional MRI localizing in the cerebellum. Neurosurg Clin N Amer. 2005;16:77–99. doi: 10.1016/j.nec.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hulsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp. 2001;13:55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzel H, Mottaghy FM, Specht K, Muller HW, Krause BJ. Evidence of a modality-dependent role of the cerebellum in working memory? An fMRI study comparing verbal and abstract n-back tasks. Neuroimage. 2009;47:2073–2082. doi: 10.1016/j.neuroimage.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Hayter A, Langdon D, Ramnani N. Cerebellar contributions to working memory. Neuroimage. 2007;36:943–954. doi: 10.1016/j.neuroimage.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Heath RJ. Modulation of emotion with a brain pacemaker. J Nerv Ment Dis. 1977;165:300–317. [PubMed] [Google Scholar]
- Heath R, Harper JW. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala and other temporal lobe sites: Evoked potential and histological studies in monkeys and cats. Exp Neurol. 1974;45:2682–2687. doi: 10.1016/0014-4886(74)90118-6. [DOI] [PubMed] [Google Scholar]
- Holmes G. The cerebellum of man. Brain. 1939;62:1–30. [Google Scholar]
- Honey G, Bullmore E, Sharma T. Prolonged reaction time to a verbal working memory task predicts increased power of posterior parietal cortical activation. Neuroimage. 2000;12:495–503. doi: 10.1006/nimg.2000.0624. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Ann N Y Acad Sci. 2002;978:302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. [DOI] [PubMed] [Google Scholar]
- Jordan K, Heinze H-J, Lutz K, Kanowski M, Jancke L. Cortical activations during the mental rotation of different visual objects. Neuroimage. 2001;13:143–152. doi: 10.1006/nimg.2000.0677. [DOI] [PubMed] [Google Scholar]
- Kelly R, Strick P. Cerebellar loops with motor cortex and prefrontal cortex. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen M, Chen S, Schraedley-Desmond P, Desmond J. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: an fMRI study. Neuroimage. 2005;24:462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Kirschen MP, Chen SH, Desmond JE. Modality specific cerebro-cerebellar activations in verbal working memory: an fMRI study. Behav Neurol. 2010;23:51–63. doi: 10.3233/BEN-2010-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. Neuroimage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert B. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. University of Florida; Gainsville, FL: 2005. [Google Scholar]
- Levisohn L, Cronin-Golomb A, Schmahmann J. Neuropsychological consequences of cerebellar tumour resection in children: Cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123:1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- Marien P, Engelborghs S, Fabbro F, De Deyn PP. The lateralized linguistic cerebellum: a review and a new hypothesis. Brain Lang. 2001;79:580–600. doi: 10.1006/brln.2001.2569. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. The contributions of cerebro-cerebellar circuitry to executive verbal working memory. Cortex. 2010;46:880–895. doi: 10.1016/j.cortex.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Chiricozzi FR, Clausi S, Tedesco AM, De Lisa M, Leggio MG. Cerebellum and detection of sequences, from perception to cognition. Cerebellum. 2008;7:611–615. doi: 10.1007/s12311-008-0060-x. [DOI] [PubMed] [Google Scholar]
- Neau J-P, Arroyo-Anllo E, Bonnaud V, Ingrand P, Gil R. Neuropsychological disturbances in cerebellar infarcts. Acta Neurol Scand. 2000;102:363–370. doi: 10.1034/j.1600-0404.2000.102006363.x. [DOI] [PubMed] [Google Scholar]
- Northoff G, Richter A, Gessner M, Schagenhauf F, Fell J, Baumgart F, Kaulisch T, Kotter R, Stephan KE, Leschinger A, Hagner T, Bargel B, Witzel T, Hinrichs H, Bogerts B, Scheich H, Heinze HJ. Functional dissociation between medial and lateral prefrontal cortical spatiotemporal activation in negative and positive emotions: A combined fMRI/MEG study. Cereb Cortex. 2000;10:93–107. doi: 10.1093/cercor/10.1.93. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Architecture and connections of cortical association areas. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 4. New York: Plenum Press; 1985. pp. 3–61. [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Arch Neurol Psychiatry. 1937;38:725–743. [Google Scholar]
- Paradiso S, Johnson DL, Andreasen NC, O’Leary DS, Watkins GL, Ponto LL, Hichwa RD. Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. Am J Psychiatry. 1999;156:1618–1629. doi: 10.1176/ajp.156.10.1618. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB, Martin CC, Jerabek PA, Lancaster JL. Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature. 1995;375:54–58. doi: 10.1038/375054a0. [DOI] [PubMed] [Google Scholar]
- Richter S, Gerwig M, Aslan B, Wilhelm H, Schoch B, Dimitrova A, Gizewski ER, Ziegler W, Karnath HO, Timmann D. Cognitive functions in patients with MR-defined chronic focal cerebellar lesions. J Neurol. 2007;254:1193–1203. doi: 10.1007/s00415-006-0500-9. [DOI] [PubMed] [Google Scholar]
- Rijntjes M, Buechel C, Kiebel S, Weiller C. Multiple somatotopic representations in the human cerebellum. Neuroreport. 1999;10:3653–3658. doi: 10.1097/00001756-199911260-00035. [DOI] [PubMed] [Google Scholar]
- Salmi J, Pallesen KJ, Neuvonen T, Brattico E, Korvenoja A, Salonen O, Carlson S. Cognitive and motor loops of the human cerebro-cerebellar system. J Cogn Neurosci. 2010;22:2663–2676. doi: 10.1162/jocn.2009.21382. [DOI] [PubMed] [Google Scholar]
- Schlerf JE, Verstynen TD, Ivry RB, Spencer RM. Evidence of a novel somatopic map in the human neocerebellum during complex actions. J Neurophysiol. 2010;103:3330–3336. doi: 10.1152/jn.01117.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. An emerging concept: The cerebellar contribution to higher function. Arch Neurol. 1991;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The role of the cerebellum in affect and psychosis. J Neuroling. 2000;13:189–214. [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga A, Petrides M, Evans A. MRI Atlas of the Human Cerebellum. Academic Press; San Diego: 2000. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Macmore J, Vangel M. Cerebellar stroke without motor deficit: clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience. 2009;162:852–861. doi: 10.1016/j.neuroscience.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. In: Schmahmann J, editor. The Cerebellum and Cognition. Academic Press; San Diego: 1997. pp. 31–60. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Alexander MP, Susan Gillingham BA, Cusimano M, Stuss DT. Lateralized cerebellar contributions to word generation: a phonemic and semantic fluency study. Behav Neurol. 2010;23:31–37. doi: 10.3233/BEN-2010-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider R, Eldred E. Electro-anatomical studies on cerebro-cerebellar connections in the cat. J Comp Neurol. 1951;95:1–16. doi: 10.1002/cne.900950102. [DOI] [PubMed] [Google Scholar]
- Snider RS, Maiti A. Cerebellar contribution to the Papez circuit. J Neurosci Res. 1976;2:133–146. doi: 10.1002/jnr.490020204. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ. The cerebellum and cognition: Evidence from functional imaging studies. Cerebellum. 2011 doi: 10.1007/s12311-011-0260-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. An fMRI study of intra-individual functional topography in the human cerebellum. Behav Neurol. 2010;23:65–79. doi: 10.3233/BEN-2010-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Koeda M, Oda K, Matsuda T, Matsushima E, Matsuura M, Asai K, Okubo Y. An fMRI study of differential neural response to affective pictures in schizophrenia. Neuroimage. 2004;22:1247–1254. doi: 10.1016/j.neuroimage.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, Borgatti R. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130:2646–2660. doi: 10.1093/brain/awm201. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Caparelli EC, Chang L, Ernst T. fMRI-acoustic noise alters brain activation during working memory tasks. Neuroimage. 2005;27:377–386. doi: 10.1016/j.neuroimage.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Urban PP, Marx J, Hunsche S, Gawehn J, Vucurevic G, Wicht S, Massinger C, Stoeter P, Hopf HC. Cerebellar speech representation: lesion topography in dysarthria as derived from cerebellar ischemia and functional magnetic resonance imaging. Arch Neurol. 2003;60:965–972. doi: 10.1001/archneur.60.7.965. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:439–447. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, Lange Fd, Vandemaele P, Deblaere K, Achten E. Motor imagery in mental rotation: An fMRI study. Neuroimage. 2002;17:1623–1633. doi: 10.1006/nimg.2002.1290. [DOI] [PubMed] [Google Scholar]
- Weiss MM, Wolbers T, Peller M, Witt K, Marshall L, Buchel C, Siebner HR. Rotated alphanumeric characters do not automatically activate frontoparietal areas subserving mental rotation. Neuroimage. 2009;44:1063–1073. doi: 10.1016/j.neuroimage.2008.09.042. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Ollinger JM, Sheridan MA, Tversky B. A parametric study of mental spatial transformations of bodies. Neuroimage. 2002;16:857–872. doi: 10.1006/nimg.2002.1129. [DOI] [PubMed] [Google Scholar]