Abstract
High cardiorespiratory fitness (CRF) is an important protective factor reducing the risk of cardiac-related disability and mortality. Recent research suggests that high CRF also has protective effects on the brain’s macrostructure and functional response. However, little is known about the potential relationship between CRF and the brain’s white matter (WM) microstructure. This study explored the relationship between a comprehensive measure of CRF (VO2 peak, total time on treadmill, and 1-minute heart rate recovery) and multiple diffusion tensor imaging measures of WM integrity. Participants were 26 healthy community dwelling seniors between the ages of 60 and 69 (mean = 64.79 years, SD = 2.8). Results indicated a positive correlation between comprehensive CRF and fractional anisotropy (FA) in a large portion of the corpus callosum. Both VO2 peak and total time on treadmill contributed significantly to explaining the variance in mean FA in this region. The CRF-FA relationship observed in the corpus callosum was primarily characterized by a negative correlation between CRF and radial diffusivity in the absence of CRF correlations with either axial diffusivity or mean diffusivity. Tractography results demonstrated that portions of the corpus callosum associated with CRF primarily involved those interconnecting frontal regions associated with high-level motor planning. These results suggest that high CRF may attenuate age-related myelin declines in portions of the corpus callosum that interconnect homologous premotor cortex regions involved in motor planning.
Keywords: Fitness, Diffusion Tensor Imaging, White Matter, Brain Imaging, Aging, Exercise
Introduction
Normal human aging is associated with declines in brain structure and function. Growing evidence suggests that physical activity or exercise training might attenuate some age-related cerebral and cognitive declines (Colcombe and Kramer, 2003; Colcombe et al., 2003; Kramer and Erickson, 2007; van Gelder et al., 2004). In particular, aerobic training has been shown to increase gray matter (GM) volume (Colcombe et al., 2006), reduce GM loss (Colcombe et al., 2003), and attenuate white matter (WM) volume reductions in the corpus callosum (Colcombe et al., 2006). In addition, improvements in cardiorespiratory fitness (CRF) can boost functional activation of frontal cortex in seniors (Colcombe et al., 2004). Collectively, these findings suggest that CRF may offset some declines in the macrostructure and functioning of the aging brain.
While there is a rapidly accumulating body of data suggesting a relationship between CRF and cerebral macrostructure relevant to aging, relatively little is known about the potential relationship between CRF and the brain’s WM microstructure. A greater understanding of this potential relationship is important because microstructural integrity of cerebral WM is required for proper transmission of information between cortical regions. The relatively recent advent of diffusion tensor imaging (DTI) has made it possible to assess the microstructural integrity of the brain’s WM tracts in vivo (Basser et al., 2000; Basser and Pierpaoli, 1996; Le Bihan, 2003). DTI is sensitized to the random motion of water molecules as they interact within tissues, thus reflecting characteristics of their immediate structural surroundings. In WM, the motion of water molecules is hindered more in directions orthogonal to the main fiber direction than along the fiber, and thus diffusion tends to be anisotropic. Diffusion anisotropy can be measured by DTI by means of fractional anisotropy (FA), an index of overall tissue microstructural integrity (Pierpaoli and Basser, 1996).
To date, only one study has explored the relationship between an objective measure of CRF (determined using peak oxygen consumption measures; VO2 peak) and WM microstructural integrity using DTI. Marks and colleagues (2010) used a region of interest (ROI) approach to explore the relationship of VO2 peak and FA in portions of the cingulum in healthy seniors. These authors reported a moderate positive correlation (r = 0.573) between VO2 peak and FA in a middle portion of the left cingulum bundle. This ROI-based finding provides preliminary support for a moderate relationship between one component of CRF and WM microstructure in one of the brain’s WM tracts. However, several important knowledge gaps remain. One knowledge gap concerns the lack of current understanding about which of the brain’s WM tracts are most strongly correlated with CRF. Unbiased voxelwise analyses across the brain’s WM can be used to address this knowledge gap.
A second knowledge gap concerns the potential neurobiological bases of a CRF-WM integrity relationship. The diffusion properties of mean diffusivity (MD), radial diffusivity (DR), and axial diffusivity (DA), are thought to reflect different components of WM integrity. For example, MD is dependent on the density of biological barriers, such as cell membranes, and represents the diffusion of water in intra- and extracellular compartments (Beaulieu, 2002; Sen and Basser, 2005). Thus, higher MD values represent increased diffusion suggestive of tissue breakdown, atrophy, and increased brain water content (Pierpaoli and Basser, 1996). Component diffusivities (DR and DA), however, provide more specific information about the integrity of axons and the surrounding myelin sheath. Specifically, increases in DR have been linked with a loss of myelin in multiple sclerosis (Klawiter et al., 2011) and in animal models of experimentally induced myelin loss (Song et al., 2002; Song et al., 2005), whereas decreases in DA have been linked to axonal damage that is associated with axonal swelling and fragmentation (Concha et al., 2006; Sun et al., 2006).
Thus, one approach to begin to characterize the neurobiological bases of CRF-WM integrity relationships would be to identify correlations between CRF and MD/DR/DA within regions showing a CRF-FA relationship. This approach allows for a more detailed understanding about WM integrity than separate considerations of FA, MD, DR and DA (Assaf and Pasternak, 2008; Burzynska et al., 2009). For example, regions showing a CRF-FA correlation and overlapping CRF-MD or CRF-DA correlations would suggest a relationship between CRF and gross tissue characteristics (Sen and Basser, 2005). In contrast, regions showing a positive CRF-FA correlation and an overlapping negative CRF-DR correlation would suggest a relationship between CRF and myelin integrity (Ciccarelli et al., 2006).
A third knowledge gap concerns the relationship between brain structure and measures of CRF beyond VO2 peak. The large majority of studies in this area have used VO2 peak (assessed either directly or indirectly) because it provides a measure of functional aerobic capacity and is a predictor of cardiac-related events (Kodama et al., 2009; Laukkanen et al., 2004). However, total treadmill exercise time and one minute heart rate recovery are also strong predictors of cardiac related events (Ekelund et al., 1988; Hsich et al., 2009) (Cole et al., 1999; Nishime et al., 2000; Watanabe et al., 2001). In addition, the prognostic accuracy of cardiac-related events increases when these physiological responses are combined in a single fitness score or index (Gulati et al., 2005; Mark et al., 1987; Michaelides et al., 2009; Myers et al., 2008; Shaw et al., 1998). Consequently, the combined use of multiple measures of CRF (VO2 peak, total time on treadmill, and 1-minute HR recovery) are thought to represent a more comprehensive metric of CRF that reflects functional aerobic capacity and individual differences in physical activity (D'Amore and Mora, 2006). However, little is known about the relationship between a comprehensive measure of CRF and brain structure.
In the present study, we addressed these three knowledge gaps. Specifically, we explored potential relationships between a comprehensive measure of CRF (based on VO2 peak, total time on treadmill, and 1-minute HR recovery) and FA across the brain’s WM using an unbiased voxelwise approach. Next, we determined the relative contributions of each of the physical fitness metrics to voxelwise results. We then explored potential relationships between CRF and MD/DR/DA within regions showing a CRF-FA relationship. Finally, we used tractography methods to determine the anatomical connectivity patterns of WM tract clusters showing a correlation with CRF in the voxelwise results.
Methods
Participants
Thirty-two community dwelling healthy volunteers (19 females) participated in this study (mean age = 64.98 years, SD = 2.7). Participants provided written informed consent in a manner approved by the University of Kentucky Institutional Review Board and were monetarily rewarded for participating. Six of these 32 participants (1 male) were excluded from the study. Of these six participants, one terminated the exercise test early due to difficulty swallowing and leg pain and another demonstrated prolonged ventricular bigeminy resulting in supervising physician termination of the exercise test for safety reasons. The remaining 4 participants were excluded because they failed to achieve VO2 peak (described below).
The 26 remaining participants (14 females) ranged in age from 60 to 69 (mean age = 64.79 years, SD = 2.8). Participants met all criteria for participating in a magnetic resonance imaging (MRI) study. Exclusion for the MRI study included history of a major head injury and/or concussion, neurological disorder (e.g., stroke, seizure), or the presence of metal fragments and/or metallic implants that could cause bodily injury or disrupt the magnetic field. All participants also met all criteria for participating in a graded exercise test. Exclusion for the graded exercise test included a diagnosis of any major medical conditions (e.g., heart, lung, or kidney), a history of uncontrolled high blood pressure, uncontrolled diabetes, a history of heart complications (e.g., heart murmur or coronary artery disease), pulmonary dysfunction (e.g., severe asthma, chronic obstructive pulmonary disease, emphysema), or orthopedic limitations (e.g., foot, knee, or hip problems) that would result in bodily injury or limit performance. A modified version of the Physical Activity Readiness Questionnaire (PAR-Q) was used to screen participants prior to participation in the study. Additionally, physician clearance was obtained for each participant.
The Hollingshead Two-Factor Index of Social Position (ISP) was used as a measure of socioeconomic states (SES; (Hollingshead, 1958). The ISP is based on an individual’s occupation and highest level of formal education. It is calculated by assigning numeric values, from 1–7, to an individual’s occupation and education. Lower values represent higher earning occupations and more years of education. Scores are then weighted by multiplying by 7 (occupation) and 4 (education). Values are then summed to produce a social index.
Cardiorespiratory Fitness Assessment
All participants completed a physician-supervised maximal graded exercise test to assess VO2 peak. Total time on treadmill and 1-minute HR recovery were also collected. The exercise test was conducted at the University of Kentucky’s Clinical Research Development and Operations Center’s (CR-DOC) Functional Assessment and Body Composition Core Laboratory using an indirect calorimetry system with integrated 12-lead electrocardiogram (ECG; Sensormedics Vmax229 metabolic cart; Yorba Linda, CA). A multistage stepwise treadmill protocol, including 3-minute stages, was used to assess CRF. Briefly, each participant started at a treadmill speed of 2.2 miles per hour (mph) and a 0% grade (incline). The treadmill speed was increased by 0.4 mph at the beginning of each 3-minute stage. Treadmill grade was increased by 2% at the beginning of the third stage and continued to increase by 2% at the beginning of each successive stage.
Continuous heart rate and dynamic heart function was measured and monitored via 12-lead ECG. Oxygen consumption was measured breath-by-breath and later averaged over minute intervals and expressed relative to body weight (ml/kg/min) during the test and recovery period. Manual blood pressure and rating of perceived exertion (RPE; using the modified Borg Scales; (Borg, 1970) were collected at the end of each 3-minute stage. All tests were terminated upon participant reported volitional fatigue, the presence of any absolute or relative indications for terminating exercise testing in accordance with the American College of Sports Medicine’s Guidelines for Exercise Testing and Prescription (Thompson et al., 2010), or any symptom the supervising physician considered hazardous to the well-being of the participant. All exercise tests were performed within 15 days of the acquisition of the MRI (mean = 7.68, SD = 4.4).
VO2 peak was defined as meeting ≥ 2 of the following 3 criteria: 1) achievement of an age-predicted maximum HR; 2) self-reported RPE scores ≥ 17; and 3) a respiratory exchange ratio of ≥ 1.1. Additionally, the highest observed VO2 value was used for all analyses. The standard 220-age equation was used to calculate the age-predicted maximum HR in the male participants. For female participants, a sex specific equation was used to calculate the age-predicted maximum HR (Gulati et al., 2010).
The primary purpose of the study was to characterize the relationship between a comprehensive measure of CRF and WM structural integrity. Thus, a composite score was calculated using VO2 peak, total time on treadmill (seconds), and 1-minute HR recovery (percent of peak HR; [1 – (Post-Exercise HR at 1 Minute/Peak HR)] × 100). Briefly, values for VO2 peak, total time on treadmill, and 1-minute HR recovery were normalized across each aerobic fitness metric [e.g., normalized value A = (A-min)/(max-min); range from 0 to 1 for each metric] and then summed to create a single value between 0 and 3 (i.e., composite-CRF). Values closer to 0 represent those participants with lower CRF levels and values closer to 3 represent those participants with higher CRF levels. In addition, relationships between each of the three individual fitness measures and WM integrity were also explored.
Diffusion Tensor Imaging Acquisition
Data were collected on a 3 Tesla Siemens TIM scanner at the University of Kentucky. An 8-channel imaging coil was used. Comfortable, disposable foam earplugs were used to dampen scanner noise. Comfortable foam padding was used to limit head motion within the coil and to further dampen scanner noise. Whole-brain diffusion tensor images (40 contiguous 3mm thick axial slices) were acquired with 36 non-collinear encoding directions (b = 1000 s/mm2) and five images without diffusion weighting (b = 0 , s/mm2, b0) using a double spin echo EPI sequence and the following parameters: repetition time (TR) = 6900 ms, echo time (TE) = 84 ms, inversion time (TI) = 2500 ms, flip angle = 90°, acquisition matrix = 128 × 128, field of view (FOV) = 224 mm, in-plane resolution = 1.75 × 1.75 mm voxels. In addition, a double-echo gradient-echo sequence (TE1 = 5.19 ms, TE2 = 7.65 ms) with slice position and spatial resolution matching those of the EPI acquisition was used to map the spatial inhomogeneity of the B0 field. Total scan time was 7 minutes.
Diffusion Tensor Imaging Processing and Analysis
All diffusion tensor imaging (DTI) data was processed and analyzed using the Functional MRI of the Brain (FMRIB) software library (FSL v4.1.5). Raw images were pre-processed to correct for motion and residual eddy current distortion using a 12-parameter affine alignment to the corresponding b0 image via FMRIB’s Linear Image Registration Tool (FLIRT: http://www.fmrib.ox.ac.uk/fsl). Images were then corrected for static field inhomogeneity distortions using B0 field maps. Brain masks were then generated from each b0 image using FMRIB’s brain extraction tool (BET v2.1) to exclude non-brain voxels from further consideration (Smith et al., 2006). Next, FMRIB’s Diffusion Toolbox (FDT v2.0) was used to fit the diffusion tensor and calculate eigenvalues, FA, MD, DA and DR.
Registration of FA images into MNI152 space and subsequent voxelwise analyses followed a series of procedures known as Tract-Based Spatial Statistics [TBSS v1.2; (Smith et al., 2006 http://www.fmrib.ox.ac.uk/fsl/tbss/), as described in our previous work (Gold et al., 2010a; Smith et al., 2010). Briefly, the first step in this process was to remove likely outliers from the fitted tensor by eroding brain edge artifacts and zeroing the end slices. Second, all subjects’ FA images were aligned to a target (the one to which the least amount of warping is required) using a nonlinear registration approach based on free-form deformations and B-Splines (Rueckert et al., 1999). FA datasets were then affine registered and resampled to 1 × 1 × 1 mm MNI152 space. The exact transformations derived from the FA maps were then applied to the other diffusivity maps (MD/DR/DA) for matched processing of all image volumes. All subsequent processing was carried out in this standardized space.
All MNI-transformed FA images were then averaged to generate a mean FA image that was used to create a common WM tract skeleton. This skeleton was then thresholded at an FA value of 0.2 in order to minimize partial voluming effects after warping across subjects. Each participant’s aligned FA image was subsequently projected onto the FA skeleton in order to account for residual misalignments between participants after the initial nonlinear registration. Each subject’s MD, DA and DR maps in MNI space were then projected onto the common tract skeleton, using the pipeline for non-FA data provided by TBSS, which employs the projection vectors from each individual’s FA-to-skeleton transformation (Smith et al., 2006).
Voxelwise analyses
Multiple regression analysis was performed to explore potential relationships between composite-CRF and FA. Age, sex, and socioeconomic status (SES) were included as covariates of no interest in all analyses. A voxelwise permutation nonparametric test (using 5000 permutations) was employed using a threshold-free cluster enhancement (TFCE) in order to avoid the use of an arbitrary threshold in the initial cluster formation. Results were then thresholded at P < 0.05 (corrected for multiple comparisons). Three subsequent voxelwise analyses using the same parameters described above were run, with MD, DR and DA as dependent variables. To characterize the neurobiological bases of significant FA results, MD, DR and DA regression maps were masked by regions showing a significant relationship between FA and fitness. All statistical maps were dilated using the FSL function tbss_fill for visualization purposes.
Region-wise analyses
Region of interest (ROI) analyses were conducted to explore the unique contributions of each individual fitness measure used in the composite (VO2 peak, total time on treadmill, and 1-minute HR recovery) to the voxelwise WM integrity results. Significant clusters from the voxelwise TFCE results were binarized to create cluster masks using FSL’s ‘fslmaths’ utility command. The means from these clusters (i.e., mean FA and DR values within the significant corpus callosum cluster) were then extracted using FSL’s utility ‘fslmeants’. Separate multiple regressions were then run for each ROI (i.e., FA and DR within the corpus callosum), with individual fitness measures as predictor variables and age, sex, and SES as covariates of no interest.
Diffusion Tensor Imaging Probabilistic Tractography
Probabilistic tractography was performed to determine the anatomical connectivity patterns of the corpus callosum cluster that was correlated with CRF in the voxelwise results. Tractrography was performed using FSL’s Bayesian Estimation of Diffusion Parameters Obtained using Sampling Techniques (BEDPOSTX) and probabilistic tracking (PROBTRACKX) tools (Behrens et al., 2003b), both part of FMRIB’s Diffusion Toolbox (FDT v2.0; http://www.fmrib.ox.ac.uk/fsl/fdt/). Bedpostx uses Markov Chain Monte Carlo sampling to generate distributions on diffusion parameters in each voxel. Two fibers were modeled in each voxel and default parameters were used for weighting and burn-in options (Behrens et al., 2007).
The cluster identified in the voxelwise results corresponded to a large portion of the corpus callosum. Target masks were created in MNI space to determine the connectivity strengths of voxels within the corpus callosum cluster to different regions of prefrontal and sensorimotor cortices. The Harvard-Oxford Cortical Structural Atlas provided by FSL’s software package was used for masks of the frontal pole (FP), middle frontal gyrus (MFG), superior frontal gyrus (SFG), precentral gyrus (PrCG), and postcentral gyrus (PoCG). Since posterior portions of the MFG and SFG masks correspond to portions of premotor cortex, a region with an established role in motor planning, the premotor cortex mask from the Juelich Histological Atlas provided by FSL was used to mask out premotor portions of the MFG and SFG masks (using the ‘fslmaths’ utility command). This served to create separate masks for MFG, SFG, and premotor (PrM) cortex regions.
In order to explore the connection strengths of subject’s native space voxels to different structural target masks in MNI space, FLIRT was used to generate transformation matrices, and their inverses, between subject’s native diffusion space and their T1 image, and between their T1 image and MNI space (using the FMRIB’s suggested parameters: http://www.fmrib.ox.ac.uk/fsl/fdt/fdt_reg.html. Finally, MNI space to native diffusion space matrices were used to ensure that each of the previously generated target masks corresponded to their respective cortical region within each subject’s native space.
The tractography analysis was conducted using FSL’s ProbtrackX software (Behrens et al., 2003a; Behrens et al., 2003b). ProbtrackX repetitively samples from the distributions on voxelwise principle diffusion directions and assigns a probability distribution to each fiber direction. Uncertainty in the data, and the existence of multiple fiber directions in each voxel, are accounted for in the form of probability density functions (Behrens et al., 2007; Behrens et al., 2003b). For each subject, the number of individual samples that were drawn through the probability distributions on the principle fiber direction was 5000 (Behrens et al., 2007). The curvature threshold (0.2) was kept at a value that minimizes the chances of back tracking streamlines (Behrens et al., 2003a; Behrens et al., 2003b), and the maximum number of steps (2000) and step length (0.5mm) corresponded to a distance of 1 meter (http://www.fmrib.ox.ac.uk/fsl/fdt/fdt_probtrackx).
A hard segmentation was performed between the seed cluster identified in the DTI voxelwise analysis and the six cortical target masks in order to classify seed cluster voxels according to the target mask with which they show the highest probability of connection (http://www.fmrib.ox.ac.uk/fsl/fdt/fdt_biggest.html). A single volume was produced with the value of each voxel within the seed cluster now corresponding to one of the six target regions. Voxels corresponding to different target regions were then isolated using FSL’s ‘fslmaths’ utility, resulting in six different seed cluster image volumes. FSL’s ‘fslstats’ utility was then used to record the number of voxels within the seed cluster that corresponded to the respective target mask. The numbers of voxels were then normalized to the corresponding target mask [(number of voxels in seed cluster/number of voxels in the corresponding target mask) × 100] in order to control for differences in target mask size (i.e. cortical region size).
Analysis of variance (ANOVA) was used to compare the normalized number of voxels that were connected to the six different cortical masks. Bonferroni post-hoc analyses were performed when significant differences were observed.
Results
Demographic and CRF data are shown in Table 1. Although this study is not investigating sex differences related to CRF, we did observe a significant sex difference for height, weight, VO2 peak, total time on treadmill, 1-minute HR recovery and SES. Male participants demonstrated significantly higher values for height [F(1, 24) = 22.11 for P < 0.0005], weight [F(1, 24) = 6.32 for P < 0.019], VO2 peak [F(1, 24) = 17.34 for P < 0.0005], total time on treadmill [F(1, 24) = 20.41 for P < 0.0005], and 1-minute HR recovery [F(1, 24) = 9.86 for P = 0.004]. Additionally, the female participants demonstrated significantly higher ISP values [F(1, 24) = 7.94 for P = 0.01], which is representative of a lower SES on the ISP scale.
Table 1.
Demographic data and fitness test scores.
| Subjects | Age | SES | Height (m) |
Weight (kg) |
VO2 Peak (ml/kg/min) |
Time On Treadmill (sec) |
Heart Rate Recovery (% Peak HR) |
|---|---|---|---|---|---|---|---|
| n = 26 | 64.8 (2.8) |
23.1 (9.2) |
1.69 (0.09) |
73.8 (13.2) |
35.3 (11.7) |
1139.3 (307.3) |
13.2 (4.4) |
| Female n = 14 |
65.6 (2.8) |
27.3† (8.4) |
1.64 (0.06) |
68.3 (11.4) |
28.4 (6.5) |
950.1 (250.1) |
11.0 (4.2) |
| Male n =12 |
63.8 (2.6) |
18.3 (7.9) |
1.75*** (0.07) |
80.2* (12.7) |
43.3*** (11.4) |
1359.9*** (205.1) |
15.7** (3.3) |
Abbreviations: SES = Socioeconomic Status; m = meters; kg = kilograms; ml = milliliters; min = minute; s = seconds; HR = heart rate. Note: values are means and values in parentheses are S.D.
P = 0.010,
P = 0.019,
P < 0.005,
P < 0.0005
Table 2 presents the partial correlations between the independent variables while controlling for sex. SES demonstrated a significant inverse relationship with VO2 peak (r = −0.402, p < 0.05) and total time on treadmill (r = −0.391, p < 0.05), which reflects a positive relationship as the ISP scale is inverted. VO2 peak showed a significant positive correlation with total time on treadmill (r = 0.774, p < 0.005). Finally, a trend towards significance was observed between VO2 peak and 1-minute HR recovery (r = 0.322, p = 0.058).
Table 2.
Partial correlations between the independent variables after controlling for sex.
| Variables | Age | SES | VO2 Peak (ml/kg/min) |
Time On Treadmill (sec) |
Heart Rate Recovery (% Peak HR) |
Composite- CRF |
|---|---|---|---|---|---|---|
| Age | - | −0.029 | 0.0002 | −0.156 | 0.298 | 0.088 |
| SES | - | - | −0.402* | −0.391* | 0.134 | −0.257 |
| VO2 Peak (ml/kg/min) | - | - | - | 0.774** | 0.322† | - |
| Time On Treadmill (sec) | - | - | - | - | 0.197 | - |
Abbreviations: SES = Socioeconomic Status; m = meters; kg = kilograms; ml = milliliters; min = minute; s = seconds; HR = heart rate. Note: r values are displayed.
P < 0.05,
P ≤ 0.005,
P = 0.058.
White Matter Microstructural Integrity and Aerobic Fitness
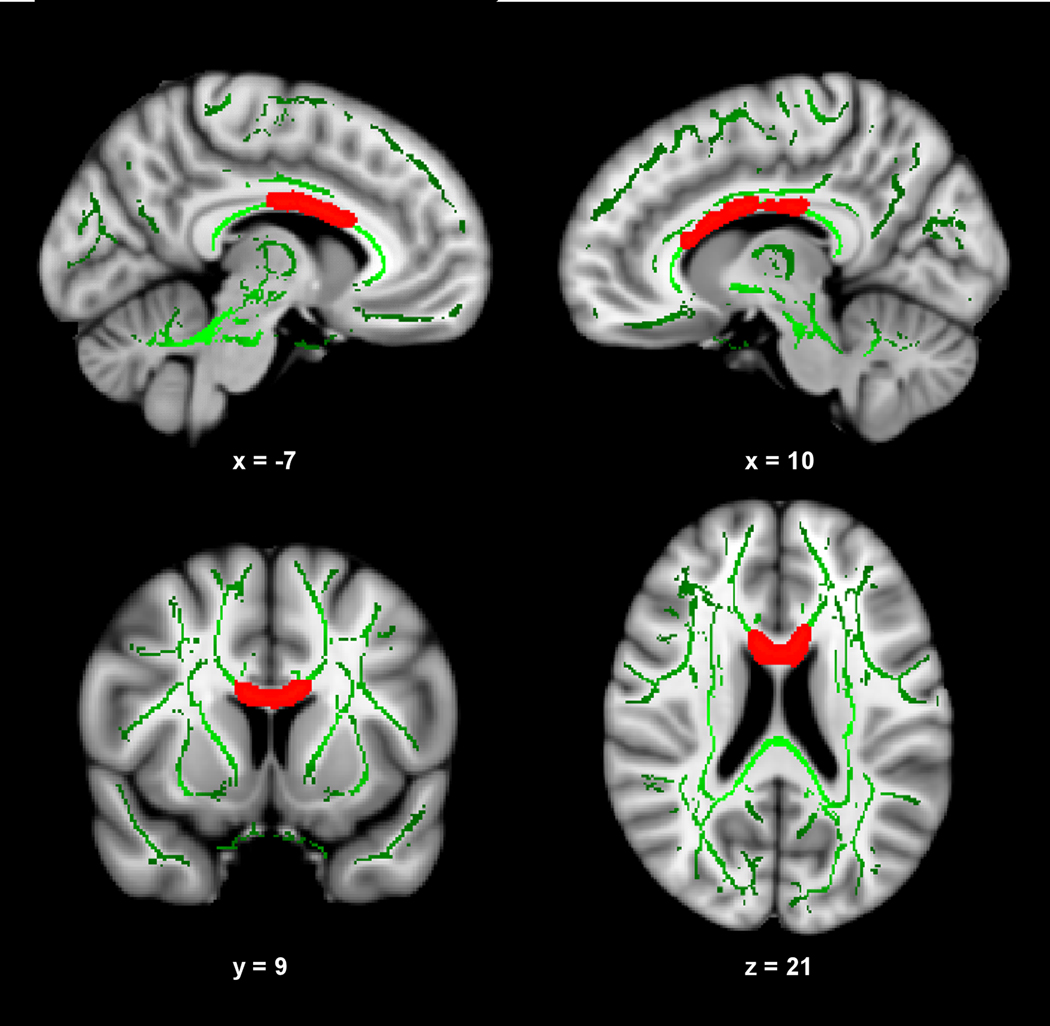
Figure 1 presents the results of the voxelwise multiple regression analysis between CRF and FA. A positive correlation (shown in red) was observed between composite-CRF and FA in a large portion of the body of the corpus callosum that extended into posterior portions of the genu. The subsequent ROI-based analysis revealed that VO2 peak (r = 0.458 p = 0.009) and total time on treadmill (r = 0.452 p = 0.010) contributed significantly to explaining the variance in mean FA in this region. One minute HR recovery showed a trend toward significance (r = 0.300 p = 0.068). Scatter plots illustrating these relationships are present in Figure 2.
Fig. 1.
CRF is positively correlated with FA in the corpus callosum. Slices highlight the positive correlation observed in the body of the corpus callosum, and extending into the genu (right side of panel), after controlling for age, sex, and socioeconomic status. The anatomic underlay used for illustration is the MNI152 T1-weighted 1mm brain. The registered average FA skeleton is represented in green. The numbers below each slice represent the respective x, y, and z coordinates in MNI space.
Fig. 2.
The relationship between individual measures of CRF and FA. Regression plots show relationships between each of the three objective measures of fitness (VO2 peak, total time on treadmill, and 1-minute heart rate recovery) and FA in the corpus callosum cluster identified in the DTI voxelwise analysis.
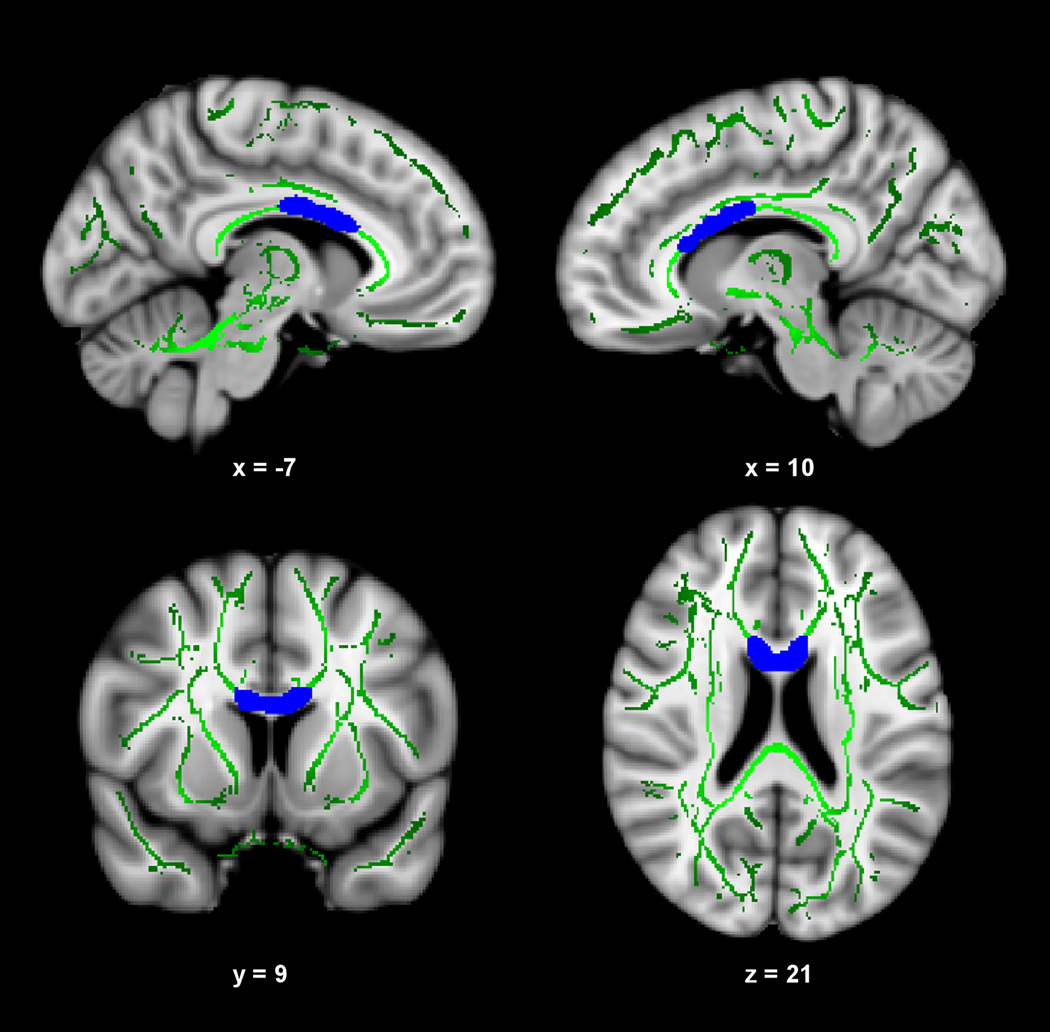
Figure 3 presents the results of the voxelwise multiple regression analysis between CRF and DR. A negative correlation (shown in blue) was observed between composite-CRF and DR in a large portion of the body of the corpus callosum that extended into posterior portions of the genu. The subsequent ROI-based analysis revealed that VO2 peak (r = −0.379 p = 0.028) and total time on treadmill (r = −0.373, p = 0.030) were significant correlates with DR in this region. One minute HR recovery did not contribute significantly to DR (r = −0.240, p = 0.119). Scatter plots illustrating these relationships are present in Figure 4.
Fig. 3.
CRF is negatively correlated with DR in the corpus callosum. The anatomic underlay and registered average FA skeleton are described in the Fig. 1 legend. The panel displays results after controlling for age, sex, and socioeconomic status.
Fig. 4.
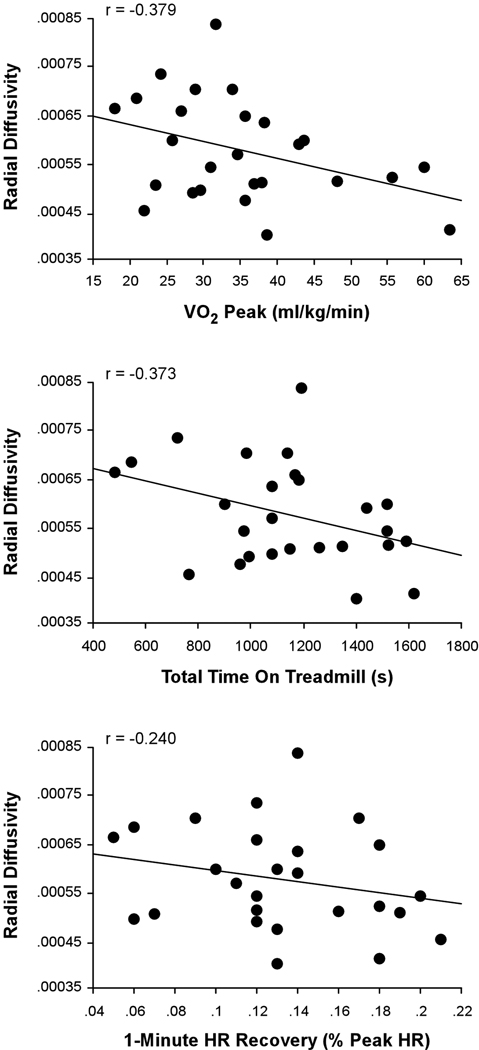
The relationship between individual measures of CRF and DR. Regression plots showing relationships between each of the three objective fitness measures (VO2 peak, total time on treadmill, and 1-minute heart rate recovery) and DR in the corpus callosum cluster identified in the DTI voxelwise analysis.
In contrast to the robust relationship between CRF and DR, the voxelwise multiple regression analysis revealed no voxels showing a significant relationship between CRF scores and either DA, or MD. The voxel p values closest to significance for DA and MD were p = 0.391 and p = 0.100, respectively.
DTI Tractography
Figure 5A presents the results from the hard segmentation of the seed cluster in a single, representative subject. The topography observed is consistent with that reported using comprehensive fiber tractography in the corpus callosum (Hofer and Frahm, 2006). Figure 5B presents a histogram plot of the normalized number of voxels within the significant corpus callosum cluster that were connected to each cortical mask. A significant effect for target mask was observed, F(5, 21) = 33.79, P < 0.0005. Post-hoc comparisons using Bonferroni correction indicated that the normalized percentage of voxels corresponding to the PrM cortex (M = 0.427, SD = 0.204) was significantly greater than the normalized percentage of voxels corresponding to the FP (M = 0.051, SD = 0.036), SFG (M = 0.215, SD = 0.204), PrCG (M = 0.150, SD = 0.100), and PoCG (M = 0.040, SD = 0.035) (all P’s < 0.0005). The difference in the normalized percentage of voxels between the PrM cortex and the MFG did not reach significance (M = 0.246, SD = 0.212, P = 0.17). Both the PoCG and FP demonstrated a significantly lower normalized percentage of voxels when compared to the MFG, SFG, PrM, and PrCG (all P’s ≤ 0.01).
Fig. 5.
Connectivity patterns of corpus callosum voxels with cortical target masks. (A) Results of the hard segmentation from a sagittal slice of a single representative subject using six target masks [frontal pole (FP) = cyan, superior frontal gyrus (SFG) = magenta, middle frontal gyrus (MFG) = yellow, premotor cortex (PrM) = blue, precentral gyrus (PrCG) = green, postcentral gyrus (PoCG) = red). The anatomic underlay used for illustration is the MNI152 T1-weighted 1mm brain. (B) Mean normalized number of voxels (expressed as a percentage of the total number of voxels in the target mask) connected to each of the six different cortical masks.
Discussion
The present study represents the first comprehensive exploration of relationships between CRF and WM microstructural integrity, exploring multiple fitness measures (VO2 peak, total time on treadmill, and 1-minute HR recovery) and DTI metrics (FA/MD/DR/DA and tractography). Voxelwise analyses across the brain’s WM indicated a positive correlation between FA and composite-CRF in a large portion of the corpus callosum. Subsequent analyses demonstrated a negative correlation between composite-CRF and DR (but no relationship with MD or DA) in the same region of the corpus callosum. These findings could not be explained by age, sex, or socioeconomic status (SES). Tractography results indicated that the significant corpus callosum cluster contained fibers interconnecting a network of homologous prefrontal cortex regions, most prominently involving those linked with motor planning. The details of these findings and their implications are discussed below.
We observed a positive correlation between a comprehensive measure of CRF (which included VO2 peak, total treadmill exercise time, and 1-minute HR recovery) and FA across the majority of the body of the corpus callosum, and extending into posterior portions of the genu. As previously noted, Marks et al. (2010) reported a moderate positive correlation in a middle portion of the left cingulum (at an uncorrected threshold of P < 0.05 appropriate for their ROI analyses). In contrast, our voxelwise analyses were intended to identify potential correlates of a comprehensive measure of CRF across the brain’s WM. Because this method requires stringent corrections for multiple comparisons, regions less strongly associated with VO2 peak would be excluded from our results. We thus do not conclude that the corpus callosum is the only region likely to be associated with VO2 peak, but rather that this region shows a very strong statistical relationship with VO2 peak.
Subsequent analyses demonstrated that exercise tolerance (as measured by total time on treadmill), in addition to VO2 peak, was significantly correlated with WM integrity in the corpus callosum. These findings may have clinical implications as both exercise tolerance and peak VO2 have been shown to decline precipitously with age (Astrand, 1960; Fleg et al., 2005; Jackson et al., 1995; Jackson et al., 1996; Kostis et al., 1982; Ogawa et al., 1992). Some of the limiting factors responsible for these CRF declines are increases in total peripheral resistance (Wiebe et al., 1999) and reductions in sympathetic output (Seals et al., 1994), cardiac output (Fleg et al., 1995; Wiebe et al., 1999), and fat free mass (Rosen et al., 1998). However, it is important to note that adaptations to chronic exercise training have been shown to be comparable in seniors and young adults (Stratton et al., 1994), suggesting that aerobic training intervention strategies could potentially have an indirect impact on preserving WM integrity with age.
Joint consideration of the other major components of the diffusion tensor showed that the positive correlation between composite-CRF and FA in the corpus callosum was primarily characterized by a negative correlation between DR and composite-CRF, in the absence of significant correlations with either MD or DA. The pattern of increased DR, in the absence of MD or DA alterations, has been observed in animal studies of experimentally induced myelin loss (Song et al., 2002). Our results thus suggest that CRF may benefit myelin integrity, even in the absence of any detectable larger-scale tissue changes. Although the mechanisms contributing to a potential fitness-myelin relationship are still unknown, the electrical activity of cortical neurons may play a significant role because axonal myelination by oligodendrocytes appears to be triggered by neural activity (Bradl and Lassmann, 2010; Gyllensten and Malmfors, 1963; Omlin, 1997).
Other factors that have been linked with CRF improvements in animal models that may have contributed to the WM benefits we observed include activation of neurotrophic factors (Ding et al., 2006), alteration of the expression of various gene transcripts (Molteni et al., 2002), and increased vascularization (Ding et al., 2006). For example, rodents exposed to various degrees of treadmill or wheel running show both angiogenic and cerebral blood volume increases in motor regions when compared to inactive rodents (Ding et al., 2006; Isaacs et al., 1992; Swain et al., 2003). In the present study, participants were screened for factors known to negatively affect WM such as uncontrolled hypercholesterolemia, hypertension and diabetes, and no visible WM hyperintensities were observed on T2 images, thus minimizing the influence of macrostructural WM change on our results. Nevertheless, microscopic angiogenic benefits associated with CRF would be expected to contribute to the fitness-related WM microstructural integrity benefits we observed.
Interestingly, if the electrical activity of cortical neurons contributes to fitness-related WM integrity benefits, then we would expect those benefits to be present in tracts connecting neural regions involved in coordinating movement. Our tractography results are consistent with this possibility. Specifically, portions of the corpus callosum associated with composite-CRF included those interconnecting frontal regions associated with high-level motor planning and voluntary motor function. The strongest link between composite-CRF and WM integrity was observed in a portion of the corpus callosum that interconnects homologous regions of the PrM cortex. The PrM cortex is situated immediately rostral to motor cortex and is comprised of both lateral (premotor proper) and medial (supplementary motor area; SMA) portions of Brodmann area 6. Both regions of PrM cortex have been linked with motor planning (Deiber et al., 1998; Gerardin et al., 2000; Grafton et al., 1996; Rao et al., 1993; Stephan et al., 1995). In particular, SMA has been strongly linked with mental rehearsal of motor sequences (Rao et al., 1993), a process thought to be critical for skilled movement (Boschert et al., 1983; Eccles, 1982; Roland et al., 1980).
Tractography results suggest that potential CRF benefits on anatomical connectivity may not be limited to motor systems. Instead, CRF was also positively associated with WM anatomical connections between homologous, non-motor pre-frontal regions. In particular, the number of corpus callosum seed voxels interconnecting the superior frontal gyri (SFG) and middle frontal gyri (MFG) were significantly higher than those interconnecting homologous somatosensory or frontal polar cortices (although not as high as those inter-connecting PrM cortex). Both the MFG and the SFG contribute to a range of high-level cognitive functions including working memory (D'Esposito et al., 1998; D'Esposito et al., 1999), task switching (Dove et al., 2000; Gold et al., 2010b; Kim et al., 2011a; Ravizza and Carter, 2008) and inhibitory control (Kerns et al., 2004; Kim et al., 2010; Kim et al., 2011b; MacDonald et al., 2000). While we consider these results encouraging, the lack of relationship observed between CRF and WM integrity in tracts connecting somatosensory cortices and frontopolar cortices may also suggest anatomically-based constraints on beneficial effects of CRF.
The location of CRF-related effects within the corpus callosum may be of special relevance to the study of aging given that this tract is especially vulnerable to age-related declines (Bennett et al., 2010; Sullivan and Pfefferbaum, 2006). As the brain’s main commissural tract system, the corpus callosum enables sensory, motor and cognitive integration between the hemispheres (Gazzaniga, 1995). For example, in the motor domain, corpus callosum plays a significant role in bi-manual motor performance (Bonzano et al., 2008; Johansen-Berg et al., 2007; Serrien et al., 2001). Additionally, some studies have shown that the corpus callosum may be responsible for transferring inhibitory signals to the opposing hemisphere during, and prior to, motor execution (Jeeves and Silver, 1988; Tanaka et al., 1990). Our results raise the possibility that interhemispheric communication related to motor planning and execution may be improved through CRF and its positive relationship with WM microstructure in the corpus callosum.
The present study has several caveats that highlight areas for future work in this field. First, the cross-sectional nature of our study limits the ability to draw causal conclusions about effects of CRF on WM integrity. The relationship observed in the present study between CRF and WM integrity serves to justify future intervention studies to determine if improved fitness boosts WM integrity. Second, longitudinal designs are required to determine if high CRF and WM integrity attenuate age-related declines in motor and cognitive function. Third, our study focused exclusively on DTI analyses. Future work should combine DTI with other imaging measures to gain a more complete understanding of the relationships between CRF, brain structure and brain function. For example, future studies should collect DTI and fMRI data to understand if WM gains result in more efficient neural recruitment. In addition, as a number of animal studies have demonstrated, exercise can have a significant impact on cortical perfusion secondary to angiogenic changes. Future studies should explore this issue through the collection of DTI, FLAIR and perfusion images.
In conclusion, our results demonstrate that CRF is positively correlated with FA in the corpus callosum in seniors. The observed CRF-FA relationship was primarily driven by a negative correlation between CRF and radial diffusivity, raising the possibility that CRF may attenuate age-related declines in myelin integrity in the corpus callosum. Results from tractography analyses suggest that portions of the corpus callosum most strongly correlated with WM integrity were those interconnecting homologous premotor cortex regions involved in motor planning. These findings motivate future longitudinal studies aimed to determine if increasing levels of physical activity and improvement in CRF attenuates age-related declines in WM integrity.
Acknowledgments
This study was supported by NIH Grant AG03303, NSF Grant BCS 0814302, and an internal grant from the University of Kentucky’s Clinical Research Development and Operations Center’s (CR-DOC). We thank CR-DOC’s Functional Assessment and Body Composition Core Laboratory for collecting all fitness-related measures. We also thank Dr. David K. Powell, Sara E. Cilles, and Doug E. Long for their assistance in data analysis, recruiting, scanning, and testing participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl. 1960;49:1–92. [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003a;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003b;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010:378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzano L, Tacchino A, Roccatagliata L, Abbruzzese G, Mancardi GL, Bove M. Callosal contributions to simultaneous bimanual finger movements. J Neurosci. 2008;28:3227–3233. doi: 10.1523/JNEUROSCI.4076-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- Boschert J, Hink RF, Deecke L. Finger movement versus toe movement-related potentials: further evidence for supplementary motor area (SMA) participation prior to voluntary action. Exp Brain Res. 1983;52:73–80. doi: 10.1007/BF00237151. [DOI] [PubMed] [Google Scholar]
- Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Backman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: Region-specific patterns of diffusivity. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Behrens TE, Altmann DR, Orrell RW, Howard RS, Johansen-Berg H, Miller DH, Matthews PM, Thompson AJ. Probabilistic diffusion tractography: a potential tool to assess the rate of disease progression in amyotrophic lateral sclerosis. Brain. 2006;129:1859–1871. doi: 10.1093/brain/awl100. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- Concha L, Gross DW, Wheatley BM, Beaulieu C. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage. 2006;32:1090–1099. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- D'Amore S, Mora S. Gender-specific prediction of cardiac disease: importance of risk factors and exercise variables. Cardiol Rev. 2006;14:281–285. doi: 10.1097/01.crd.0000244460.25429.c8. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Ibanez V, Honda M, Sadato N, Raman R, Hallett M. Cerebral processes related to visuomotor imagery and generation of simple finger movements studied with positron emission tomography. Neuroimage. 1998;7:73–85. doi: 10.1006/nimg.1997.0314. [DOI] [PubMed] [Google Scholar]
- Ding YH, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res. 2006;3:15–23. doi: 10.2174/156720206775541787. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Eccles JC. The initiation of voluntary movements by the supplementary motor area. Arch Psychiatr Nervenkr. 1982;231:423–441. doi: 10.1007/BF00342722. [DOI] [PubMed] [Google Scholar]
- Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med. 1988;319:1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- Fleg JL, O'Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol. 1995;78:890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Principles of human brain organization derived from split-brain studies. Neuron. 1995;14:217–228. doi: 10.1016/0896-6273(95)90280-5. [DOI] [PubMed] [Google Scholar]
- Gerardin E, Sirigu A, Lehericy S, Poline JB, Gaymard B, Marsault C, Agid Y, Le Bihan D. Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex. 2000;10:1093–1104. doi: 10.1093/cercor/10.11.1093. [DOI] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Andersen AH, Smith CD. Alterations in multiple measures of white matter integrity in normal women at high risk for Alzheimer's disease. Neuroimage. 2010a;52:1487–1494. doi: 10.1016/j.neuroimage.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2010b;31:512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Exp Brain Res. 1996;112:103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Gulati M, Arnsdorf MF, Shaw LJ, Pandey DK, Thisted RA, Lauderdale DS, Wicklund RH, Al-Hani AJ, Black HR. Prognostic value of the duke treadmill score in asymptomatic women. Am J Cardiol. 2005;96:369–375. doi: 10.1016/j.amjcard.2005.03.078. [DOI] [PubMed] [Google Scholar]
- Gulati M, Shaw LJ, Thisted RA, Black HR, Bairey Merz CN, Arnsdorf MF. Heart rate response to exercise stress testing in asymptomatic women: the st. James women take heart project. Circulation. 2010;122:130–137. doi: 10.1161/CIRCULATIONAHA.110.939249. [DOI] [PubMed] [Google Scholar]
- Gyllensten L, Malmfors T. Myelinization of the optic nerve and its dependence on visual function--a quantitative investigation in mice. J Embryol Exp Morphol. 1963;11:255–266. [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Social Class and Mental Illness. New York: John Wiley & Sons; 1958. [Google Scholar]
- Hsich E, Gorodeski EZ, Starling RC, Blackstone EH, Ishwaran H, Lauer MS. Importance of treadmill exercise time as an initial prognostic screening tool in patients with systolic left ventricular dysfunction. Circulation. 2009;119:3189–3197. doi: 10.1161/CIRCULATIONAHA.109.848382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12:110–119. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- Jackson AS, Beard EF, Wier LT, Ross RM, Stuteville JE, Blair SN. Changes in aerobic power of men, ages 25–70 yr. Med Sci Sports Exerc. 1995;27:113–120. [PubMed] [Google Scholar]
- Jackson AS, Wier LT, Ayers GW, Beard EF, Stuteville JE, Blair SN. Changes in aerobic power of women, ages 20–64 yr. Med Sci Sports Exerc. 1996;28:884–891. doi: 10.1097/00005768-199607000-00016. [DOI] [PubMed] [Google Scholar]
- Jeeves MA, Silver PH. The formation of finger grip during prehension in an acallosal patient. Neuropsychologia. 1988;26:153–159. doi: 10.1016/0028-3932(88)90038-3. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage. 2007;36 Suppl 2:T16–T21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kim C, Chung C, Kim J. Multiple cognitive control mechanisms associated with the nature of conflict. Neurosci Lett. 2010;476:156–160. doi: 10.1016/j.neulet.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Kim C, Johnson NF, Cilles SE, Gold BT. Common and distinct mechanisms of cognitive flexibility in prefrontal cortex. J Neurosci. 2011a;31:4771–4779. doi: 10.1523/JNEUROSCI.5923-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Kroger JK, Kim J. A functional dissociation of conflict processing within anterior cingulate cortex. Hum Brain Mapp. 2011b;32:304–312. doi: 10.1002/hbm.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55:1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA: The Journal Of The American Medical Association. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- Kostis JB, Moreyra AE, Amendo MT, Di Pietro J, Cosgrove N, Kuo PT. The effect of age on heart rate in subjects free of heart disease. Studies by ambulatory electrocardiography and maximal exercise stress test. Circulation. 1982;65:141–145. doi: 10.1161/01.cir.65.1.141. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Laukkanen JA, Kurl S, Salonen R, Rauramaa R, Salonen JT. The predictive value of cardiorespiratory fitness for cardiovascular events in men with various risk profiles: a prospective population-based cohort study. European Heart Journal. 2004;25:1428–1437. doi: 10.1016/j.ehj.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mark DB, Hlatky MA, Harrell FE, Jr, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med. 1987;106:793–800. doi: 10.7326/0003-4819-106-6-793. [DOI] [PubMed] [Google Scholar]
- Michaelides AP, Andrikopoulos GK, Antoniades C, Soulis D, Tzeis S, Hatzistamatiou E, Tzannos K, Fourlas C, Seferlis C, Stefanadis CI. Duration of treadmill exercise testing combined with QRS score predicts adverse cardiac outcome at long-term follow-up. Coron Artery Dis. 2009;20:337–342. doi: 10.1097/MCA.0b013e32832c4589. [DOI] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Myers J, Arena R, Dewey F, Bensimhon D, Abella J, Hsu L, Chase P, Guazzi M, Peberdy MA. A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. Am Heart J. 2008;156:1177–1183. doi: 10.1016/j.ahj.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–1398. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Spina RJ, Martin WH, 3rd, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- Omlin FX. Optic disc and optic nerve of the blind cape mole-rat (Georychus capensis): a proposed model for naturally occurring reactive gliosis. Brain Res Bull. 1997;44:627–632. doi: 10.1016/s0361-9230(97)00283-9. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LD, et al. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–2318. doi: 10.1212/wnl.43.11.2311. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Carter CS. Shifting set about task switching: behavioral and neural evidence for distinct forms of cognitive flexibility. Neuropsychologia. 2008;46:2924–2935. doi: 10.1016/j.neuropsychologia.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- Rosen MJ, Sorkin JD, Goldberg AP, Hagberg JM, Katzel LI. Predictors of age-associated decline in maximal aerobic capacity: a comparison of four statistical models. J Appl Physiol. 1998;84:2163–2170. doi: 10.1152/jappl.1998.84.6.2163. [DOI] [PubMed] [Google Scholar]
- Seals DR, Taylor JA, Ng AV, Esler MD. Exercise and aging: autonomic control of the circulation. Med Sci Sports Exerc. 1994;26:568–576. [PubMed] [Google Scholar]
- Sen PN, Basser PJ. A model for diffusion in white matter in the brain. Biophys J. 2005;89:2927–2938. doi: 10.1529/biophysj.105.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien DJ, Nirkko AC, Wiesendanger M. Role of the corpus callosum in bimanual coordination: a comparison of patients with congenital and acquired callosal damage. Eur J Neurosci. 2001;14:1897–1905. doi: 10.1046/j.0953-816x.2001.01798.x. [DOI] [PubMed] [Google Scholar]
- Shaw LJ, Peterson ED, Shaw LK, Kesler KL, DeLong ER, Harrell FE, Jr, Muhlbaier LH, Mark DB. Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation. 1998;98:1622–1630. doi: 10.1161/01.cir.98.16.1622. [DOI] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Andersen AH, Powell DA, Lovell MA, Xiong S, Gold BT. White matter diffusion alterations in normal women at risk of Alzheimer' disease. Neurobiol Aging. 2010;31:1122–1131. doi: 10.1016/j.neurobiolaging.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol. 1995;73:373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, Abrass IB. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation. 1994;89:1648–1655. doi: 10.1161/01.cir.89.4.1648. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Le TQ, Armstrong RC, Cross AH, Song SK. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage. 2006;32:1195–1204. doi: 10.1016/j.neuroimage.2006.04.212. [DOI] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Iwasa H, Yoshida M. Diagonistic dyspraxia: case report and movement-related potentials. Neurology. 1990;40:657–661. doi: 10.1212/wnl.40.4.657. [DOI] [PubMed] [Google Scholar]
- Thompson WR, Gordon NF, Pescatello LS, editors. American College of Sports Medicine's Guidelines for Exercise Testing and Prescription. 8th ed. Philadelphia: Lippincott Williams and Wilkins; 2010. [Google Scholar]
- van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: the FINE Study. Neurology. 2004;63:2316–2321. doi: 10.1212/01.wnl.0000147474.29994.35. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation. 2001;104:1911–1916. [PubMed] [Google Scholar]
- Wiebe CG, Gledhill N, Jamnik VK, Ferguson S. Exercise cardiac function in young through elderly endurance trained women. Med Sci Sports Exerc. 1999;31:684–691. doi: 10.1097/00005768-199905000-00010. [DOI] [PubMed] [Google Scholar]