Abstract
Mitigating oxidative stress-induced damage is critical to preserving neuronal function in diseased or injured brains. This study explores the mechanisms contributing to the neuroprotective effects of pigment epithelium-derived factor (PEDF) in cortical neurons. Cultured primary neurons are exposed to PEDF and H2O2 as well as inhibitors of phosphoinositide-3 kinase (PI3K) or extracellular signal-regulated kinase 1/2 (ERK1/2). Neuronal survival, cell death and levels of caspase 3, PEDF, phosphorylated ERK1/2, and Bcl-2 are measured. The data show cortical cultures release PEDF and that H2O2 treatment causes cell death, increases activated caspase 3 levels and decreases release of PEDF. Exogenous PEDF induces a dose-dependent increase in Bcl-2 expression and neuronal survival. Blocking Bcl-2 expression by siRNA reduced PEDF-induced increases in neuronal survival. Treating cortical cultures with PEDF 24 h before H2O2 exposure mitigates oxidant-induced decreases in neuronal survival, Bcl-2 expression, and phosphorylation of ERK1/2 and also reduces elevated caspase 3 level and activity. PEDF pretreatment’s effect on survival is blocked by inhibiting ERK or PI3K. However, only inhibition of ERK reduced the ability of PEDF to protect neurons from H2O2-induced Bcl-2 decrease and neuronal death. These data demonstrate PEDF- mediated neuroprotection against oxidant injury is largely mediated via ERK1/2 and Bcl-2 and suggest the utility of PEDF in preserving the viability of oxidatively challenged neurons.
Keywords: PEDF, oxidative stress, neurons, ERK, PI3K/Akt, apoptosis, neurotrophic
Introduction
Pigment epithelium-derived factor (PEDF) is a 50 kDa secreted multifunctional glycoprotein increasingly recognized for its neurotrophic properties (Yabe et al. 2010). PEDF is a member of the serpin superfamily of proteins but it lacks inhibitory properties against either serine or cysteine proteinases (Becerra et al. 1995; Tombran-Tink 2005; Tombran-Tink et al. 2005). PEDF is highly expressed in the retinal pigment epithelium (RPE) and has been implicated in vascular and neural retinal disorders (Barnstable and Tombran-Tink 2004; Becerra et al. 2004). In culture, PEDF exerts protective effects on RPE cells and retinal neurons via multiple mechanisms including extracellular signal-regulated kinase (ERK1/2) phosphorylation (Ho et al. 2006; Tsao et al. 2006) and inhibition of apoptosis-inducing factor nuclear translocation (Murakami et al. 2008).
However, PEDF expression is not confined to the retina; as it is found in serum, cerebrospinal fluid, and is widely expressed in the CNS (Kuncl et al. 2002; Singh et al. 1998; Tombran-Tink and Barnstable 2003b). PEDF has been shown to enhance survival and differentiation of neurons in culture as well as to induce neurite outgrowth (Gettins et al. 2002; Houenou et al. 1999). PEDF protects cerebellar granule cells from serum-deprivation and glutamate toxicity (Araki et al. 1998; Taniwaki et al. 1997). The protective effects of PEDF in these cells have been attributable to NF-κB activation and induction of both CRE- and NF-κB- dependent genes (Yabe et al. 2005; Yabe et al. 2001). In vivo, the neuroprotective function of PEDF against ischemic damage has been demonstrated in a rat middle cerebral artery occlusion model where infarct volume and degree of edema are significantly reduced in rats transfected to over-express the PEDF gene (Sanagi et al. 2008). However, the mechanisms whereby PEDF protects neurons from injury have not yet been fully elucidated.
Oxidative stress, caused by an imbalance between the generation and detoxification of reactive oxygen species, is a deleterious condition leading to cellular death in both the eye and brain. Oxidative stress is increasingly implicated as an important trigger for the development of neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease as well as brain injury (Aliev et al. 2002; Eghwrudjakpor and Allison 2010; Loh et al. 2006; Taupin 2010; Wang and Michaelis 2010). In the eye, PEDF has been shown to possess anti-oxidant properties. PEDF protects RPE from oxidant-mediated barrier dysfunction and prevents reactive oxygen species generation in retinal pericytes and endothelial cells exposed to oxidized low-density lipoprotein or high glucose, respectively (Banumathi et al. 2010; Ho et al. 2006; Zhang et al. 2008). The notion that the neuroprotective effects of PEDF in brain are also related to mitigating oxidative stress is supported by experiments in an in vitro model of Parkinson’s disease, showing that PEDF protects dopaminergic neurons against the neurotoxic effects of rotenone and 6-hydroxydopamine; two compounds that injure dopamine-producing neurons by oxidative mechanisms (Falk et al. 2009).
The objective of this study is to determine the mechanisms contributing to the neuroprotective effects of PEDF in cerebral cortical neurons. The ability of PEDF to promote neuronal survival, under basal and oxidant-stress conditions, and the signaling mechanisms that contribute to this neuroprotective effect are explored.
Materials and methods
2.1 Reagent preparation
All cell culture reagents and media were purchased from GIBCO/Invitrogen (Carlsbad, CA, USA). Recombinant PEDF (GF134) was obtained from Millipore (Temecula, CA) and reconstituted in sterile, deionized water, frozen until use and diluted in treatment media to attain the experimental doses (0 – 100 ng/ml). Neurons were treated with PEDF for 24 h. For experiments involving H2O2, neuronal cultures were pre-treated with PEDF with or without the kinase inhibitors for 24 h prior to exposure to H2O2. Cells were exposed to 50 μM H2O2, 5 μM U0126 (ERK1/2 inhibitor) or LY294002 (PI3K inhibitor) or dimethyl sulfoxide (DMSO, 0.5% final concentration) for 24 h.
2.2 Neuronal cell culture
Animal procedures were performed in accordance with the NIH “Guide for the Care and Use of Laboratory Animals” and Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee (IACUC) guidelines. Rat cerebral cortical cultures were prepared from cortices of 18-day gestation fetuses, as previously described (Reimann-Philipp et al. 2001). The cells were seeded on 6-well poly-L-lysine coated plates at a density of 3–5 X 105 cells per ml and incubated in Neurobasal medium containing B-27 supplement, antibiotic/antimycotic, glutamine (0.5 mM) and 5-fluoro-2′-deoxyuridine (20 μg/ml) to inhibit proliferation of glial cells. On day 5, fresh medium without 5-fluoro-2′-deoxyuridine was added. Neuronal cultures were used for experiments after 8–9 days in culture. Although no primary culture is 100% pure, we have previously characterized these cultures as > 90% neuronal (Grammas, et al. 1999). The neuronal identity of the cultures was confirmed using immunofluroescent labeling for beta-3 tubulin, a neuron-specific protein (Promega, Madison, WI) and glial fibrillary acidic protein (Promega).
2.3 Assessment of cell viability and cell death
Cell viability was determined using the MTT assay (Promega) as follows. Treatment medium was replaced with fresh treatment medium containing 25 μl/ml of the Cell Titer 96 Aqueous One Solution and incubated for 10 min at 37ºC after which optical density was measured at 490 nm using a microplate reader. The quantity of soluble formazan product, as measured by the amount of absorbance, was directly proportional to the number of viable cells. The optical density of untreated controls was normalized to 100%. The number of viable cells after treatment was determined by measuring optical density and expressing viability as percent of untreated controls. Caspase 3 activity was determined using the Caspase 3 Cellular Assay kit from Enzo Life Sciences (PA, USA). Apoptotic cell death was assayed by nucleosome ELISA using the Cell Death Detection ELISAPLUS kit (Roche, Mannheim, Germany) following the manufacturer’s protocols. Cell-death induced nucleosome release was expressed relative to control, untreated cells.
2.4 Detection of PEDF protein by ELISA
PEDF released into the supernatant was quantitated by indirect ELISA, using our previously published protocol (Sanchez et al. 2008; Tripathy et al. 2010). Supernatants (100 μl) collected from cerebral cortical cultures after various treatments were coated onto 96-well immulon 2HB (Fisher Scientific) flat bottom plates with sodium bicarbonate buffer (0.1 M) and incubated overnight at 4°C. Non-specific sites were blocked with 1% bovine serum albumin solution at 37°C for 45 min. The plates were incubated with primary antibody (ab14993; AbCam, Cambridge, MA, dilution 1:1000) in bicarbonate buffer and incubated at 37°C for 1 h followed by extensive washing to remove unbound antibody. Plates were then incubated with secondary antibody coupled with horseradish peroxidase (Bio-Rad, Hercules, CA 1:1,000 dilution) for 45 min at 37°C, in the dark. The reaction was developed by adding 200 μl/well of o-phenylene diamine H2O2 (Pierce, Chemicon, Temecula, CA, USA) for 20 min. Optical density was measured at 450 nm using a microplate ELISA reader (Bio-Rad, Hercules, CA). Samples were assayed in triplicate.
2.5 Western blot analysis
Total protein was extracted from neurons using lysis buffer (20 mM Tris-HCl pH 7.4, 50 mM NaCl, 0.5 % NP-40, 0.5% deoxycholate, 0.5% SDS, 1 mM EDTA, 1 μg/ml aprotinin) containing protease and phosphatase inhibitor cocktail tablets (Roche, Mannheim, Germany). Protein was determined by the Bradford method using Bio-Rad protein reagents. Equal amounts of protein were run on a 12% polyacrylamide gel, transferred on to a PVDF membrane, blocked with 5% milk solution (non-fat dry milk in Tris-buffered saline Tween-20) and immunoblotted with primary antibodies. The antibodies used included Bcl-2 (ab7973 1:200, Abcam), PEDF (07-280 1:300, Millipore) uncleaved form of caspase-3 (sc-7148 1:300, Santa Cruz Biotechnology), cleaved active form of caspase-3 (#9661 1:300), phosphorylated ERK1/2 (#4377, 1:300) and total ERK 1/2 (#4695, 1:300) from Cell Signaling (Danvers, MA). Primary antibody was followed by incubation with peroxidase- conjugated secondary antibodies. After extensive washing to remove unbound antibodies, membranes were developed with chemiluminescence reagents. Blots were later incubated with stripping buffer at 50°C for 1 h and re-probed with GAPDH (MAB374 1:1000, Chemicon). Band intensities were quantitified using Quantity One software (Bio-Rad) and graphically expressed as intensity units which reflects the average intensity over the area of the band. Densitometric measurements of bands were normalized to corresponding GAPDH levels. The control sample values were set to 100 and treatment values expressed as percent of control.
2.6 Bcl-2, ERK1 and ERK2 RNA silencing
Primary neuronal cultures were transfected with small interfering RNA (siRNA) for Bcl-2 (Sigma-Aldrich, St Louis, MO), ERK1, ERK2 (Santa Cruz Biotechnology, CA) or scrambled siRNA (negative control) on day 5 in culture following our previously published protocol (Tripathy and Grammas, 2009) with minor modifications. Transfection was carried out using culture media lacking antibiotics. Transfection reaction was prepared with 100 pmol/ml of specified siRNA or scrambled siRNA in 100 μl of Opti-MEM (Invitrogen, Carslbad, CA). Lipofectamine RNAiMAX (Invitrogen, Carslbad, CA) transfection reagent (6 μl) was diluted in 94 μl of OPTI-MEM. The siRNA mixture was slowly mixed with the lipofectamine and incubated for 20 min at room temperature to allow complex formation. The neuronal cultures were incubated with the siRNA lipofectamine complex for 6 h, changed to fresh media, and further incubated for 48 h. Target RNA silencing was detected by RT-PCR for Bcl-2, ERK1 and ERK2. Total RNA extracted from the cells treated with siRNA was reverse transcribed with specific primers and PCR products visualized on a 1.5% agarose gel using UV trans-illumination.
2.7 RT-PCR Analysis
Total RNA was extracted from neuronal cultures using the Trizol method (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions and then DNase treated. RNA (2 μg) was reverse transcribed using oligo (dT)15 primers (Promega) and amplified by PCR (5 min-95°C, 32 cycles of 30s-94°C, 30s-56°C, 45s-72°C and 5 min-72°C) using a Mastercycler system (Eppendorf). PCR reactions were performed for Bcl-2, ERK1 and ERK2 on each cDNA template along with the housekeeping gene GAPDH. Primers used for PCR are shown in Table 1. The PCR products were visualized on a 1.5% agarose gel using UV trans-illumination and quantified using Quantity One v. 4.6 (BioRad) software. Band densities were normalized to corresponding GAPDH levels. The controls were set to 100 and treatment sample values expressed relative to the control.
Table 1.
Primers used for reverse-transcription PCR
| Gene | Sequence | Amplicon Size |
|---|---|---|
| Bcl-2 | Fwd 5′ AGG GGG AAA CAC CAG AAT Rev 5′ TGG AAG GAG AAG ATG CCA G |
314 |
| ERK1 | Fwd 5′ GCT GAA TCA CAT CCT GGG TAT Rev 5′ AGA TCT GTA TCC TGG CTG GAA |
441 |
| ERK2 | Fwd 5′ GCC CGG AGA TGG TCC GC Rev 5′ ATG GTC TGG ATC TGC AAC A |
506 |
| GAPDH | Fwd 5′ ATG GGA AGC TGG TCA TCA AC Rev 5′ GGA TGC AGG GAT GAT GTT CT |
440 |
2.7 Statistical Analysis
Prism version 4.0 software (GraphPad Inc., San Diego CA) was used for graphical presentation and statistical analysis. Statistical analysis used included student’s t-test and one-way ANOVA followed by post hoc multiple comparison tests to compare different treatment groups. Data are presented as means + SEM of at least 3 independent experiments. Statistical significance was determined at p<0.05.
Results
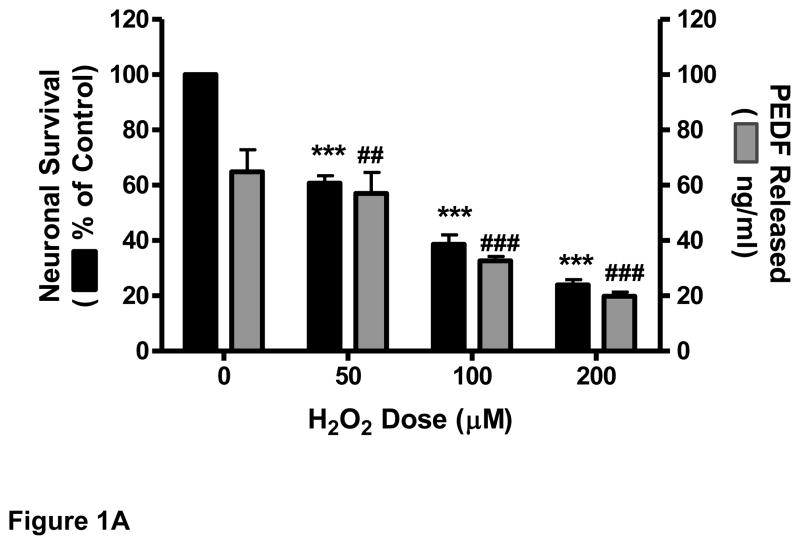
3.1 H2O2 reduced cell survival and decreased PEDF release in cortical neuronal cultures
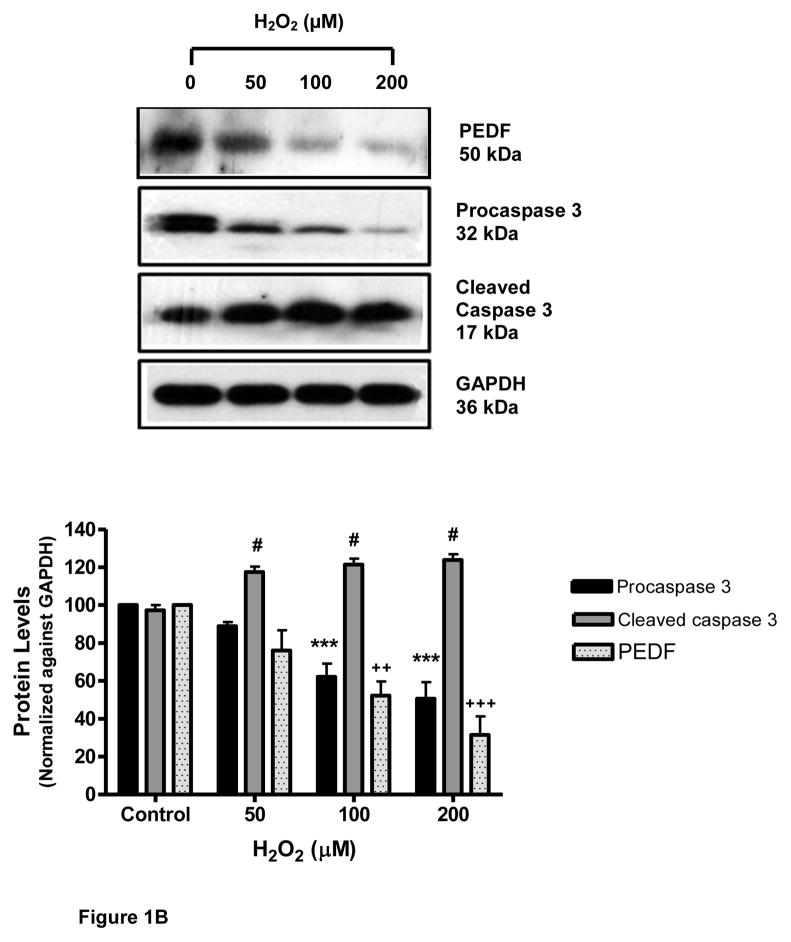
Primary cortical cultures were exposed to oxidative stress using increasing doses of H2O2. The data showed that treatment of neurons with H2O2 for 24 h caused a dose-dependent decrease in cell survival (Fig. 1A). This decrease (39.6%) was highly significant (p<0.001) at 50 μM H2O2. Addition of H2O2 also caused a decrease in the amount of immunodetectable PEDF released by the neurons into the supernatant. This decrease (30%) was significant (p<0.01) at 50 μM H2O2 and highly significant (p<0.001) at 100 μM (57%) (Fig.1A). Similarly, H2O2 treatment induced a dose-dependent decrease in the level of endogenous PEDF (50 kDa), and the uncleaved form of caspase 3 (32 kDa) while increasing the level of activated, cleaved caspase 3 (17 kDa) (Fig. 1B). A low level of caspase activation was detectable in control cultures.
Figure 1.
Eight-day old cortical neuron cultures were exposed to increasing concentrations of H2O2 for 24 h.
A. Cell survival was determined by MTT assay and PEDF release by ELISA.
***p<0.001; ##p<0.01, ###p<0.001 vs. corresponding control.
B. Levels of PEDF, procaspase 3 and cleaved caspase 3 determined by western blot analysis.
#p<0.05; ++p<0.01, +++p<0.001; ***p<0.001, vs. corresponding control.
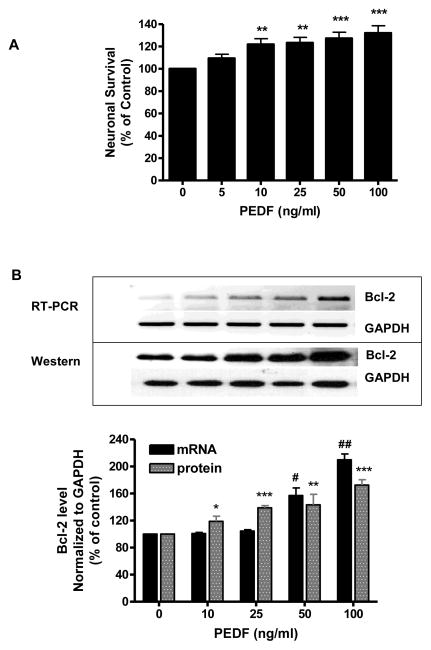
3.2 Exogenous PEDF enhanced survival of cultured neurons
To further explore direct PEDF effects on neuronal viability primary cortical cultures were exposed to increasing concentrations (5–100 ng/ml) of PEDF and neuronal viability assessed by MTT assay. The data showed that PEDF evoked a dose-dependent increase in cell survival starting at 10 ng/ml PEDF (p<0.01) (Fig. 2A). At 50 ng/ml PEDF caused a highly significant (p<0.001) increase in cell survival (Fig. 2A). In preliminary experiments we screened several signaling inhibitors to determine which pathway contributed to PEDF’s neurotrophic effect including, JNK II inhibitor (5 μM), p38 inhibitor (SB203580, 5 μM), ERK inhibitor (U0126, 5 μM), PI3K inhibitor (LY294002, 5 μM), NF-Kβ inhibitor (BAY 11-7085, 5 μM), dicoumarol (NF-kβ+JNK inhibitor, 25 μM) and the protein kinase C inhibitor bisindolylmaleimide (BSI, 5 μM). Our data indicated that neither dicoumarol nor SB2030580 significantly affected the neurotrophic effect of PEDF. Both BAY 11-7085 and BSI exhibited significant toxicity which resulted in a 40% decrease in neuronal survival. In contrast, inhibitors of the ERK and PI3K pathways alone did not affect neuroal survival but significantly (p<0.01 and p<0.05, respectively) blocked the increase in neuronal survival evoked by PEDF (Table 2). Thus, ERK and PI3K pathways were the focus of our additional experiments.
Figure 2.
Primary cultures of cortical neurons were treated with increasing concentrations of PEDF for 24 h.
A. Cell survival was determined by MTT assay.
**p<0.01, ***p<0.001 vs. 0 ng/ml PEDF.
B. Bcl-2 expression determined by RT-PCR (top) and western blot analysis (bottom)
*p<0.05, **p<0.01, ***p<0.001; #p<0.05, ##p<0.01 vs. corresponding control.
Table 2.
Inhibition of PEDF-mediated neurotrophic effects
| Treatment | Neuronal Survival (Mean+SEM) | Bcl-2 Protein Level |
|---|---|---|
| Control | 100.0 ± 0.0 | 100.0 ± 0.0 |
| PEDF (100 ng/ml) | 132.0 ± 6.6 *** | 113.4 ±3.4 ** |
| PEDF+ERK1/2 Inhibitor | 109.6 ± 8.1## | 79.3 ± 1.9### |
| PEDF+PI3/Akt Inhibitor | 111.2 ± 2.1# | 104.2 ± 1.5# |
Data are mean ± SEM from 3 separate experiments.
p<0.01,
p<0.001 vs. control;
p<0.05,
p<0.01,
p<0.001 vs. PEDF
3.3 PEDF increased Bcl-2 expression in neurons
The effect of PEDF on expression of the anti-apoptotic protein Bcl-2 was determined. Exposure of neuronal cultures to PEDF (10–100 ng/ml) for 24 h induced an increase in Bcl-2 expression at both message and protein levels (Fig. 2B). RT-PCR analysis showed significant increase in mRNA levels of the Bcl-2 gene (Fig. 2B) at 50 ng/ml PEDF (p<0.05). Western blot analysis of PEDF treated neurons showed an increase of Bcl-2 protein at 10 ng/ml (p<0.05) that was highly significant at 100 ng/ml (p<0.001) (Fig. 2B). Similar to the results obtained examining neuronal survival, treatment of cultures with inhibitors of ERK1/2 as well as PI3K/Akt significantly (p<0.001 and p<0.05, respectively) blocked the increase in Bcl-2 level evoked by PEDF (Table 2).
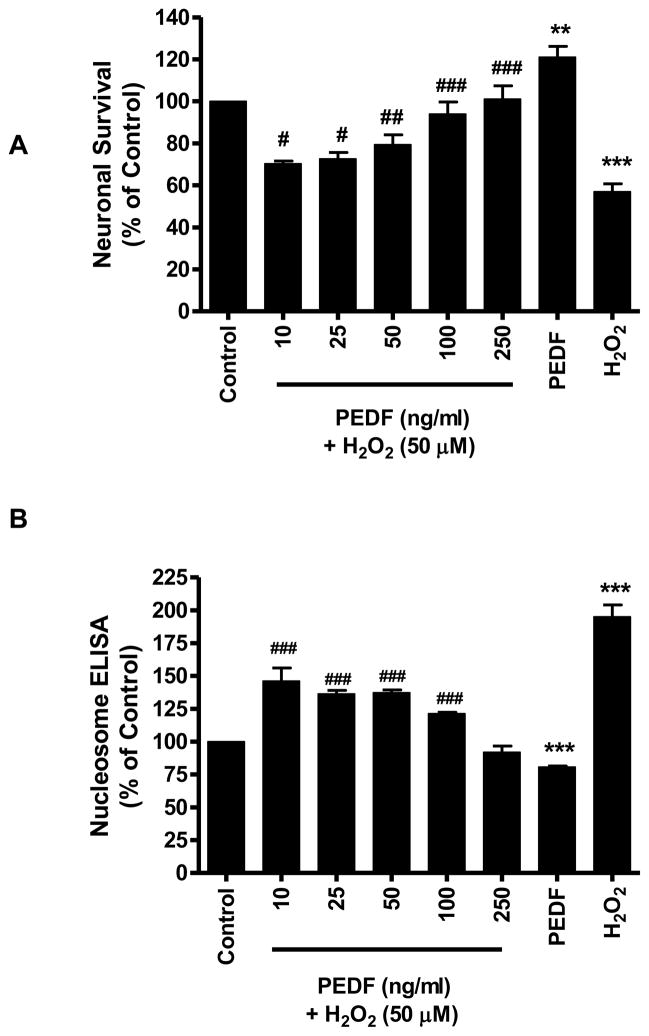
3.4 PEDF protected primary cortical neurons against H2O2
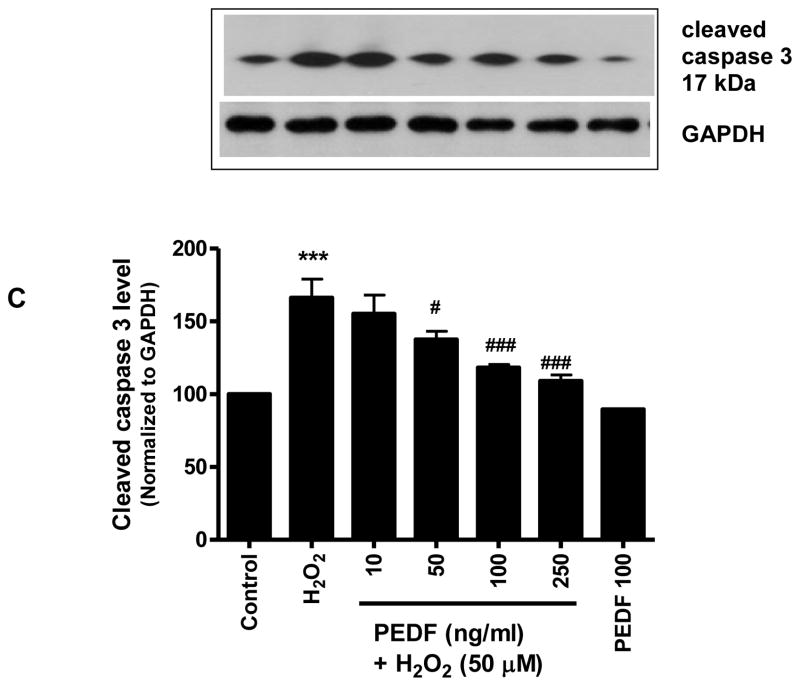
Based on results that showed a neurotrophic effect of exogenous PEDF on cerebral cortical cultures, the ability of PEDF to protect neurons challenged with the oxidant stressor H2O2 was investigated. Pre-treatment of neurons for 24 h with increasing doses of PEDF (10–250 ng/ml) enhanced survival of neuronal cultures subsequently exposed to H2O2. Cultures pretreated with 100 ng/ml PEDF demonstrated a significant (p<0.001) increase in survival compared to neurons exposed to H2O2 without pre-treatment (Fig.3A). Cell death induced by H2O2 was reduced by PEDF pretreatment as evidenced by the significant (p<0.001) reduction in nucleosome release from PEDF pretreated cells compared to cells treated with H2O2 alone (Fig. 3B). PEDF pretreatment of cultures with 100 and 250 ng/ml PEDF also showed a significant (p<0.001) decrease in the level of cleaved, activated form of caspase 3 compared to cells exposed to H2O2 alone (Fig 3C). In addition, PEDF pre-treatment prevented the increased caspase 3 activity induced by H2O2 (Table 3).
Figure 3.
Eight day old cortical neurons were treated with increasing dose of PEDF for 24 h and then exposed to H2O2 (50 μM, 24 h).
A. Neuronal survival was determined by MTT assay.
**p<0.01, ***p<0.001 vs. control; #p<0.05, ##p<0.01, ###p<0.001 vs. H2O2.
B. Cell death was determined by nucleosome ELISA.
***p<0.001 vs. control; ###p<0.001 vs. H2O2. C. The level of cleaved caspase 3 was determined by western blot analysis.
***p<0.001 vs. control; #p<0.05, ###p<0.001 vs. H2O2.
Table 3.
Effect of PEDF on caspase 3 activity
| Treatment | Caspase 3 Specific Activity (pmol/min/μg total protein) |
|---|---|
| Control | 35.8 ± 1.8 |
| H2O2 (50 μM) | 79.1 ± 4.8 *** |
| PEDF (100 ng/ml) | 34.5 ± 6.1 ### |
| H2O2+PEDF | 38.2 ± 3.3 ### |
Data are mean ± SEM of three experiments.
p<0.001 vs. control;
p<0.001 vs. H2O2
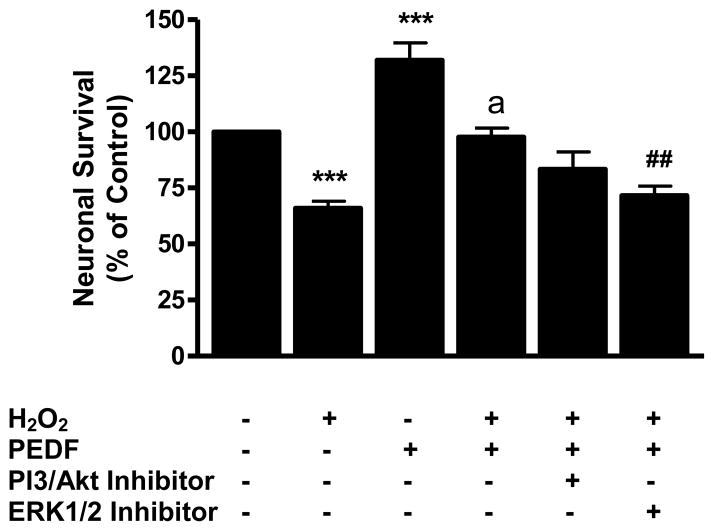
To probe the role of signaling pathways in the PEDF-mediated neuroprotection from oxidant injury, the PI3-K/Akt inhibitor LY294002 and the ERK 1/2 inhibitor U0126 were used. The data showed that incubation of cultures with the PI3K/Akt inhibitor did not significantly affect PEDF-mediated neuroprotection (Fig. 4). In contrast, incubation of neuronal cultures with the ERK 1/2 inhibitor U0126 significantly (p<0.01) reduced the ability of PEDF to protect neurons from H2O2-induced cell death (Fig. 4).
Figure 4.
Eight-day old neuron cultures were pre-treated with PEDF (100 ng/ml, 24 h) with or without a PI3/Akt or ERK 1/2 kinase inhibitor (5 μM) and exposed to H2O2 (50 μM) for 24 h. Cell survival was determined by MTT assay.
***p<0.001 vs. control; ap<0.001 vs. H2O2; ##p<0.01 vs. H2O2 + PEDF.
3.5 PEDF protection against H2O2 was associated with Bcl-2 induction
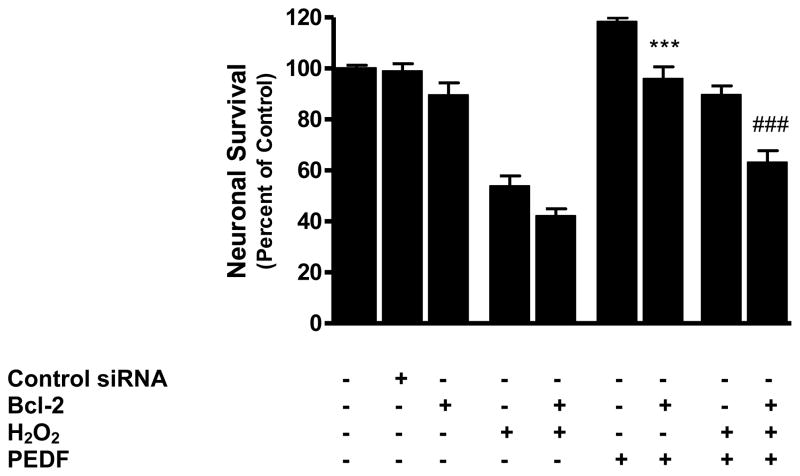
Since exogenous PEDF treatment caused an increase in the level of the anti-apoptotic protein Bcl-2, we determined whether blocking Bcl-2 would affect PEDF-mediated protection. Our results showed that blocking Bcl-2 using siRNA significantly reduced the increase in neuronal survival evoked by PEDF alone (p<0.001) and also reduced the protection imparted by PEDF against H2O2 (p<0.001) (Fig. 5).
Figure 5.
Cortical neurons were transfected with Bcl-2 siRNA (100 pmol, 6 h), pre-treated with PEDF (100 ng/ml, 24 h) and then exposed to H2O2 (50 μM, 24 h). Cell survival was then determined by MTT assay.
***p<0.001 vs. PEDF
###p<0.001 vs. H2O2 + PEDF
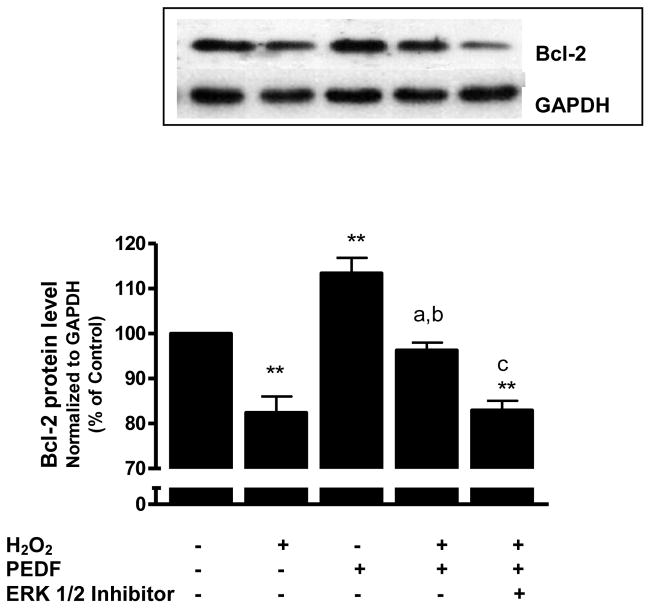
We also studied whether Bcl-2 expression was affected by PEDF under H2O2 stress. There was a reduction of Bcl-2 expression in neurons treated with 50 μM H2O2 for 24 h compared to untreated control cells (p<0.01). Pre-treatment of cultures with PEDF prior to H2O2 exposure blocked the decrease in Bcl-2 caused by H2O2 treatment (p<0.01). Addition of the ERK 1/2 inhibitor U0126 significantly (p<0.01) reduced the ability of PEDF to protect neurons from H2O2-mediated reduction in Bcl-2 (Fig. 6). Furthermore, the results showed that the increase in Bcl-2 expression caused by PEDF was significantly decreased by siRNA that blocked ERK1 (47%, p<0.01) or ERK2 (30%, p<0.01).
Figure 6.
Eight-day old neuron cultures were pre-treated with PEDF (100 ng/ml, 24 h) with or without ERK 1/2 kinase inhibitor (5 μM) and exposed to H2O2 (50 μM) for 24 h. Bcl-2 was determined by western blot.
**p<0.01 vs. control; ap<0.01 vs. H2O2; bp<0.01 vs. PEDF;
cp<0.01 vs. H2O2 + PEDF.
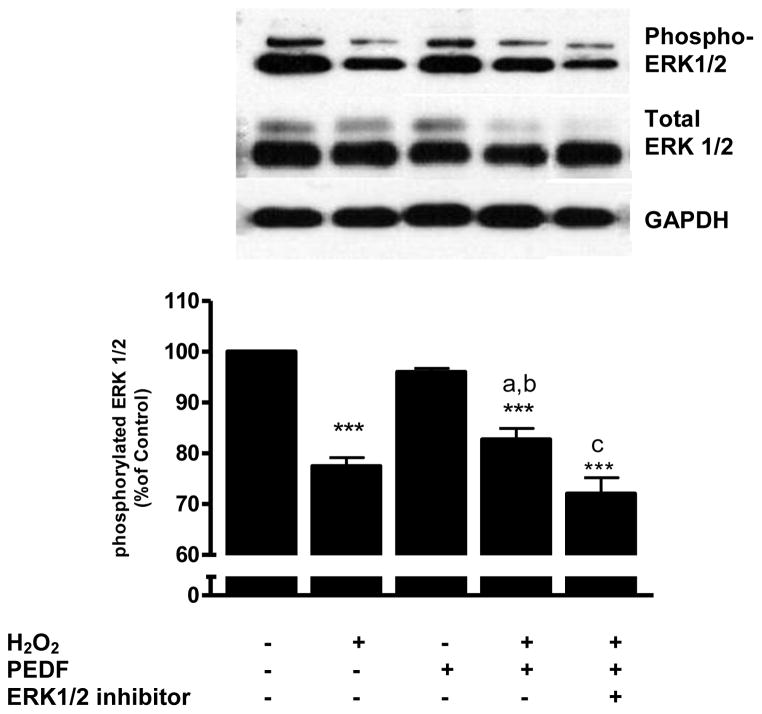
3.6 Levels of phosphorylated ERK 1/2 were modulated by PEDF and H2O2
Treatment of primary cortical neurons with H2O2 caused a significant (p<0.001) decline in the level of the phosphorylated form of p44/p42 (ERK 1/2) compared to control cells (Fig. 7). Exposure of neurons to PEDF alone did not alter the level of phosphorylated ERK 1/2. However, pretreatment of neurons with PEDF prior to H2O2 exposure resulted in a significantly (p<0.05) higher level of phosphorylated ERK 1/2 compared to that evoked by H2O2 alone. Incubation of PEDF pretreated cells with H2O2 together with the ERK 1/2 inhibitor blocked the effect of PEDF on phosphorylated ERK 1/2 (p<0.01; Fig. 7).
Figure 7.
Eight-day old cortical neuron cultures were pre-treated with PEDF (100 ng/ml, 24 h) with or without ERK 1/2 kinase inhibitor (5 μM) and exposed to H2O2 (50 μM) for 24 h. Levels of phosphorylated ERK1/2 and total ERK1/2 were determined by western blot.
***p<0.001 vs. control; ap<0.05 vs. H2O2; bp<0.001 vs. PEDF; cp<0.01 vs. H2O2 + PEDF.
Discussion
Neuronal degeneration, dysfunction and death are characteristics of brain injury and neurodegenerative diseases (Choonara et al. 2009). Although the precise mechanisms underlying neuronal loss in neurodegenerative diseases remain elusive, oxidative stress is increasingly implicated (Aliev et al. 2002). Oxidative stress, caused by an imbalance between the generation and detoxification of reactive oxygen species, is a deleterious condition leading to cellular death. Mitigating oxidative stress-induced damage is critical to preserving neuronal function in diseased or injured brains. In this study we demonstrate that pretreatment of cultured cortical neurons with exogenous PEDF protects cells against the neurotoxic effects of H2O2 exposure by several mechanisms including expression of the anti-apoptotic protein Bcl-2, reduction of activated caspase 3 level and activity, and activation of the ERK1/2 signaling pathway.
Apoptotic cell death is regulated by the antiapoptotic Bcl-2 family of proteins. Bcl-2 has been implicated in CNS functions such as brain development, neuronal process growth and regeneration, and adult hippocampal neurogenesis (Chiou et al. 2006; Korzhevskii et al. 2004; Perera et al. 2007; Seo et al. 2002). We have previously documented that treatment of neuronal cultures with the drug acetaminophen increases expression of Bcl-2 and that blocking drug-induced expression of Bcl-2 reduces the pro-survival effect of acetaminophen on cultured neurons (Tripathy and Grammas, 2009). In this light, we focused on the effect of PEDF on Bcl-2. Our study shows that PEDF evokes a dose-dependent increase in Bcl-2 in cultured cortical neurons and that blocking Bcl-2 reduces both the neurotrophic and neuroprotective effects of PEDF. Similar results have been reported in cultured retinal pericytes (Yamagishi et al. 2002) and cerebellar granule cells (Yabe et al. 2001). Bcl-2 has been shown to enhance neuronal survival in hippocampal neurons subjected to ischemic injury (Sasaki et al. 2006). Also, in PC-12 cells, Bcl-2 mitigates calcium entry and mitochondrial calcium overload through regulation of L-type calcium channels; effects that could contribute to maintenance of cell viability after injury (Diaz-Prieto et al. 2008). Thus, Bcl-2 expression may be a common pathway whereby drugs and proteins exert neurotrophic effects.
Treatment of neuronal cultures with PEDF alone does not increase ERK1/2 but does increase Bcl-2. It is possible that PEDF affects Bcl-2 through more than the ERK1/2 pathway. Indeed, we show that PEDF’s neurotrophic effect is mediated through both PI3K/Akt as well as ERK1/2 (Table 2). Therefore, the PEDF-mediated increase in Bcl-2 may involve PI3K/Akt, or potentially other signaling pathways. In this regard, although our initial experiments do not find a significant role for p38 and JNK in the PEDF-mediated neuroprotection observed the importance of other signaling pathways especially the large and diverse MAP kinase family, (Keshet and Seger, 2010) which includes the recently described neurotrophic effects of ERK5 (Obara and Nakahata, 2010), remains to be explored.
The regulation of neuronal cell death by intracellular signaling cascades is complex. Although little is known about PEDF’s signaling mechanisms in cortical neurons, in other cell types PEDF has been shown to activate several signaling pathways. PEDF triggers an intracellular cascade of signals leading to NF-κB activation and nuclear translocation as well as phosphorylation of p38 and other MAP kinases (Chen et al. 2006; Tombran-Tink and Barnstable 2003a). The pleiotropic signaling cascades activated by PEDF suggest its ability to mediate different functional outcomes in specific cell types. For example, in endothelial cells, the pro-apoptotic action of PEDF involves induction of PPAR-gamma which in turn increases p53 expression and activity (Ho et al. 2007). Also, PEDF prevents endothelial cell damage from oxidative stress induced by high glucose concentration through increased levels of reduced glutathione, increased superoxide dismutase activity and decreased caspase 3 activation (Banumathi et al. 2010). In the brain, PEDF inhibits cold-injury induced brain edema through suppression of NADPH oxidase via inhibition of Rac-1 activation (Jinnouchi et al. 2007). Our data indicate that inhibition of ERK 1/2 or PI3K/Akt prevents PEDF-mediated increase in neuronal survival. These data indicate that PEDF exerts its neurotrophic functions through multiple signaling cascades. This is consistent with work showing that in the brain upregulation of Bcl-2, in response to the anticonvulsant valproate, is mediated by ERK and PI3K (Creson et al. 2009). In contrast to the neurotrophic effect of PEDF on unstimulated neuronal cultures, the ability of PEDF to protect neurons against oxidative insult evoked by H2O2 is largely mediated through the ERK pathway as the ERK 1/2 inhibitor U0126 but not the PI3K/Akt inhibitor LY294002 significantly negates the neuroprotective effect of PEDF. The significance of the ERK pathway in PEDF-mediated protection against H2O2 is further demonstrated by experiments showing that blocking ERK1/2 negates the PEDF-mediated protection against H2O2 –induced decrease in Bcl-2 levels. Furthermore, levels of phosphorylated ERK1/2 closely reflect changes observed in Bcl-2 expression. Taken together, these results indicate that in cortical neurons PEDF exerts neurotrophic and neuroprotective functions through distinct but overlapping mechanisms.
In the current study we document expression of PEDF by cortical-derived neurons in culture. PEDF could be an important endogenous neurotrophic/neuroprotective factor. In this regard, we have previously documented that treatment of neuronal cultures with VEGF evokes a dose-dependent expression of PEDF (Sanchez et al., 2010). Increased expression of PEDF perivascular astrocytes has been shown to promote the proliferation of neural progenitor cells and the production of mature granule neurons in the adult hippocampus (Namba 2010). Thus, neuroprotective peptides such as PEDF derived from neurons and/or non-neuronal cell types may form an initial defense in response to brain injury. A study examining the expression pattern of PEDF in the CNS after injury finds PEDF levels are decreased 2 days after kainic acid exposure and then significantly elevated at 7 days post injury. PEDF protein and PEDF mRNA expression are co-localized with GFAP-positive reactive astrocytes 7 days after kainic acid exposure, suggesting that the increase in PEDF expression in injured brain may form part of a compensatory mechanism against neuronal degeneration (Sanagi et al. 2007). This idea is also supported by work demonstrating strong PEDF reactivity in brains of Alzheimer’s disease patients (Yamagishi et al. 2004).
Our data indicate that PEDF promotes neuronal survival and protects against oxidative stress by enhancing anti-apoptotic and/or pro-survival signaling in cortical neurons. Understanding how PEDF is regulated in neurons could suggest ways to bolster neuronal viability by enhancing endogenous neurotrophic proteins. Also, defining the mechanisms whereby PEDF exerts neuroprotection against oxidative stress could provide new insight into strategies for preserving neuronal function in neurological disorders such as AD where oxidative stress is a primary driver of neuronal injury.
H2O2 reduced cell survival and decreased PEDF release in cortical neuronal cultures.
Exogenous PEDF enhanced survival of cultured neurons.
PEDF increased Bcl-2 expression in neurons.
PEDF protected primary cortical neurons against H2O2.
PEDF protection against H2O2 was associated with Bcl-2 induction.
Levels of phosphorylated ERK 1/2 were modulated by PEDF and H2O2.
Acknowledgments
Sources of support: This work was supported in part by grants from the National Institutes of Health (AG15964, AG020569 and AG028367). Dr. Grammas is the recipient of the Mildred and Shirley Garrison Chair in Aging. The authors gratefully acknowledge the secretarial assistance of Terri Stahl.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aliev G, Smith MA, Seyidov D, Neal ML, Lamb BT, Nunomura A, Gasimov EK, Vinters HV, Perry G, LaManna JC, Friedland RP. The role of oxidative stress in the pathophysiology of cerebrovascular lesions in Alzheimer’s disease. Brain Pathol. 2002;12:21–35. doi: 10.1111/j.1750-3639.2002.tb00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Taniwaki T, Becerra SP, Chader GJ, Schwartz JP. Pigment epithelium-derived factor (PEDF) differentially protects immature but not mature cerebellar granule cells against apoptotic cell death. J Neurosci Res. 1998;53:7–15. doi: 10.1002/(SICI)1097-4547(19980701)53:1<7::AID-JNR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Banumathi E, Sheikpranbabu S, Haribalaganesh R, Gurunathan S. PEDF prevents reactive oxygen species generation and retinal endothelial cell damage at high glucose levels. Exp Eye Res. 2010;90:89–96. doi: 10.1016/j.exer.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retin Eye Res. 2004;23:561–577. doi: 10.1016/j.preteyeres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Becerra SP, Fariss RN, Wu YQ, Montuenga LM, Wong P, Pfeffer BA. Pigment epithelium-derived factor in the monkey retinal pigment epithelium and interphotoreceptor matrix: apical secretion and distribution. Exp Eye Res. 2004;78:223–234. doi: 10.1016/j.exer.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Becerra SP, Sagasti A, Spinella P, Notario V. Pigment epithelium-derived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop. J Biol Chem. 1995;270:25992–25999. doi: 10.1074/jbc.270.43.25992. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang SS, Barnstable CJ, Tombran-Tink J. PEDF induces apoptosis in human endothelial cells by activating p38 MAP kinase dependent cleavage of multiple caspases. Biochem Biophys Res Commun. 2006;348:1288–1295. doi: 10.1016/j.bbrc.2006.07.188. [DOI] [PubMed] [Google Scholar]
- Chiou SH, Ku HH, Tsai TH, Lin HL, Chen LH, Chien CS, Ho LL, Lee CH, Chang YL. Moclobemide upregulated Bcl-2 expression and induced neural stem cell differentiation into serotoninergic neuron via extracellular-regulated kinase pathway. Br J Pharmacol. 2006;148:587–598. doi: 10.1038/sj.bjp.0706766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choonara YE, Pillay V, du Toit LC, Modi G, Naidoo D, Ndesendo VM, Sibambo SR. Trends in the molecular pathogenesis and clinical therapeutics of common neurodegenerative disorders. Int J Mol Sci. 2009;10:2510–2557. doi: 10.3390/ijms10062510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creson TK, Yuan P, Manji HK, Chen G. Evidence for involvement of ERK, PI3K, and RSK in induction of Bcl-2 by valproate. J Mol Neurosci. 2009;37:123–134. doi: 10.1007/s12031-008-9122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Prieto N, Herrera-Peco I, de Diego AM, Ruiz-Nuno A, Gallego-Sandin S, Lopez MG, Garcia AG, Cano-Abad MF. Bcl2 mitigates Ca2+ entry and mitochondrial Ca2+ overload through downregulation of L-type Ca2+ channels in PC12 cells. Cell Calcium. 2008;44:339–352. doi: 10.1016/j.ceca.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Eghwrudjakpor PO, Allison AB. Oxidative stress following traumatic brain injury: enhancement of endogenous antioxidant defense systems and the promise of improved outcome. Niger J Med. 2010;19:14–21. doi: 10.4314/njm.v19i1.52466. [DOI] [PubMed] [Google Scholar]
- Falk T, Zhang S, Sherman SJ. Pigment epithelium derived factor (PEDF) is neuroprotective in two in vitro models of Parkinson’s disease. Neurosci Lett. 2009;458:49–52. doi: 10.1016/j.neulet.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Gettins PG, Simonovic M, Volz K. Pigment epithelium-derived factor (PEDF), a serpin with potent anti-angiogenic and neurite outgrowth-promoting properties. Biol Chem. 2002;383:1677–1682. doi: 10.1515/BC.2002.188. [DOI] [PubMed] [Google Scholar]
- Grammas P, Moore P, Weigel PH. Microvessels fom Alzheimer’s disease brains kill neurons in vitro. Am J Pathol. 1999;154:337–342. doi: 10.1016/S0002-9440(10)65280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Chen SL, Yang YC, Liao CL, Cheng HC, Tsao YP. PEDF induces p53-mediated apoptosis through PPAR gamma signaling in human umbilical vein endothelial cells. Cardiovasc Res. 2007;76:213–223. doi: 10.1016/j.cardiores.2007.06.032. [DOI] [PubMed] [Google Scholar]
- Ho TC, Yang YC, Cheng HC, Wu AC, Chen SL, Tsao YP. Pigment epithelium-derived factor protects retinal pigment epithelium from oxidant-mediated barrier dysfunction. Biochem Biophys Res Commun. 2006;342:372–378. doi: 10.1016/j.bbrc.2006.01.164. [DOI] [PubMed] [Google Scholar]
- Houenou LJ, D’Costa AP, Li L, Turgeon VL, Enyadike C, Alberdi E, Becerra SP. Pigment epithelium-derived factor promotes the survival and differentiation of developing spinal motor neurons. J Comp Neurol. 1999;412:506–514. [PubMed] [Google Scholar]
- Jinnouchi Y, Yamagishi S, Matsui T, Takenaka K, Yoshida Y, Nakamura K, Ueda S, Imaizumi T. Administration of pigment epithelium-derived factor (PEDF) inhibits cold injury-induced brain edema in mice. Brain Res. 2007;1167:92–100. doi: 10.1016/j.brainres.2007.04.088. [DOI] [PubMed] [Google Scholar]
- Keshet Y, Seger R. The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol. 2010;661:3–38. doi: 10.1007/978-1-60761-795-2_1. [DOI] [PubMed] [Google Scholar]
- Korzhevskii DE, Talantova OE, Pavlova NG. Expression of the bcl-2 protein in the developing human brain. Neurosci Behav Physiol. 2004;34:203–206. doi: 10.1023/b:neab.0000009216.72568.56. [DOI] [PubMed] [Google Scholar]
- Kuncl RW, Bilak MM, Bilak SR, Corse AM, Royal W, Becerra SP. Pigment epithelium-derived factor is elevated in CSF of patients with amyotrophic lateral sclerosis. J Neurochem. 2002;81:178–184. doi: 10.1046/j.1471-4159.2002.00813.x. [DOI] [PubMed] [Google Scholar]
- Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ. Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res. 2006;3:327–337. doi: 10.2174/156720506778249515. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Ikeda Y, Yonemitsu Y, Onimaru M, Nakagawa K, Kohno R, Miyazaki M, Hisatomi T, Nakamura M, Yabe T, Hasegawa M, Ishibashi T, Sueishi K. Inhibition of nuclear translocation of apoptosis-inducing factor is an essential mechanism of the neuroprotective activity of pigment epithelium-derived factor in a rat model of retinal degeneration. Am J Pathol. 2008;173:1326–1338. doi: 10.2353/ajpath.2008.080466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba TY, Gonda N, Ichikawa T, Sanagi E, Arikawa-Hirasawa H, Mochizuki S, Kohsaka S, Uchino Pigment epithelium-derived factor up-regulation induced by memantine, an N-methyl-D-aspartate receptor antagonist, is involved in increased proliferation of hippocampal progenitor cells. Neuroscience. 2010;167:372–383. doi: 10.1016/j.neuroscience.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Obara Y, Nakahata N. The signaling pathway leading to extracellular signaling regulated kinase 5 (ERK5) activation via G-proteins and ERK5-dependent neurotrophic effects. Mol Pharmacol. 2010;77:10–16. doi: 10.1124/mol.109.060236. [DOI] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackeim HA, Dwork AJ. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann-Philipp U, Ovase R, Weigel PH, Grammas P. Mechanisms of cell death in primary cortical neurons and PC12 cells. J Neurosci Res. 2001;64:654–660. doi: 10.1002/jnr.1119. [DOI] [PubMed] [Google Scholar]
- Sanagi T, Yabe T, Yamada H. Changes in pigment epithelium-derived factor expression following kainic acid induced cerebellar lesion in rat. Neurosci Lett. 2007;424:66–71. doi: 10.1016/j.neulet.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Sanagi T, Yabe T, Yamada H. Gene transfer of PEDF attenuates ischemic brain damage in the rat middle cerebral artery occlusion model. J Neurochem. 2008;106:1841–1854. doi: 10.1111/j.1471-4159.2008.05529.x. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Chiriva-Internati M, Grammas P. Transduction of PACAP38 protects primary cortical neurons from neurotoxic injury. Neurosci Lett. 2008;448:52–55. doi: 10.1016/j.neulet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Wadhwani S, Grammas P. Multiple neurotrophic effects of VEGF on cultured neurons. Neuropeptides. 2010;44:323–331. doi: 10.1016/j.npep.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Kitagawa K, Yagita Y, Sugiura S, Omura-Matsuoka E, Tanaka S, Matsushita K, Okano H, Tsujimoto Y, Hori M. Bcl2 enhances survival of newborn neurons in the normal and ischemic hippocampus. J Neurosci Res. 2006;84:1187–1196. doi: 10.1002/jnr.21036. [DOI] [PubMed] [Google Scholar]
- Seo JH, Rah JC, Choi SH, Shin JK, Min K, Kim HS, Park CH, Kim S, Kim EM, Lee SH, Lee S, Suh SW, Suh YH. Alpha-synuclein regulates neuronal survival via Bcl-2 family expression and PI3/Akt kinase pathway. FASEB J. 2002;16:1826–1828. doi: 10.1096/fj.02-0041fje. [DOI] [PubMed] [Google Scholar]
- Singh VK, Chader GJ, Rodriguez IR. Structural and comparative analysis of the mouse gene for pigment epithelium-derived factor (PEDF) Mol Vis. 1998;4:7. [PubMed] [Google Scholar]
- Taniwaki T, Hirashima N, Becerra SP, Chader GJ, Etcheberrigaray R, Schwartz JP. Pigment epithelium-derived factor protects cultured cerebellar granule cells against glutamate-induced neurotoxicity. J Neurochem. 1997;68:26–32. doi: 10.1046/j.1471-4159.1997.68010026.x. [DOI] [PubMed] [Google Scholar]
- Taupin P. A dual activity of ROS and oxidative stress on adult neurogenesis and Alzheimer’s disease. Cent Nerv Syst Agents Med Chem. 2010;10:16–21. doi: 10.2174/187152410790780172. [DOI] [PubMed] [Google Scholar]
- Tombran-Tink J. The neuroprotective and angiogenesis inhibitory serpin, PEDF: new insights into phylogeny, function, and signaling. Front Biosci. 2005;10:2131–2149. doi: 10.2741/1686. [DOI] [PubMed] [Google Scholar]
- Tombran-Tink J, Aparicio S, Xu X, Tink AR, Lara N, Sawant S, Barnstable CJ, Zhang SS. PEDF and the serpins: phylogeny, sequence conservation, and functional domains. J Struct Biol. 2005;151:130–150. doi: 10.1016/j.jsb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Tombran-Tink J, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nat Rev Neurosci. 2003a;4:628–636. doi: 10.1038/nrn1176. [DOI] [PubMed] [Google Scholar]
- Tombran-Tink J, Barnstable CJ. Therapeutic prospects for PEDF: more than a promising angiogenesis inhibitor. Trends Mol Med. 2003b;9:244–250. doi: 10.1016/s1471-4914(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Tripathy D, Grammas P. Acetaminophen inhibits neuronal inflammation and protects neurons from oxidative stress. J Neuroinflammation. 2009;6:10. doi: 10.1186/1742-2094-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy D, Thirumangalakudi L, Grammas P. RANTES upregulation in the Alzheimer’s disease brain: a possible neuroprotective role. Neurobiol Aging. 2010;31:8–16. doi: 10.1016/j.neurobiolaging.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao YP, Ho TC, Chen SL, Cheng HC. Pigment epithelium-derived factor inhibits oxidative stress-induced cell death by activation of extracellular signal-regulated kinases in cultured retinal pigment epithelial cells. Life Sci. 2006;79:545–550. doi: 10.1016/j.lfs.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe T, Kanemitsu K, Sanagi T, Schwartz JP, Yamada H. Pigment epithelium-derived factor induces pro-survival genes through cyclic AMP-responsive element binding protein and nuclear factor kappa B activation in rat cultured cerebellar granule cells: Implication for its neuroprotective effect. Neuroscience. 2005;133:691–700. doi: 10.1016/j.neuroscience.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Yabe T, Sanagi T, Yamada H. The neuroprotective role of PEDF: implication for the therapy of neurological disorders. Curr Mol Med. 2010;10:259–266. doi: 10.2174/156652410791065354. [DOI] [PubMed] [Google Scholar]
- Yabe T, Wilson D, Schwartz JP. NFkappaB activation is required for the neuroprotective effects of pigment epithelium-derived factor (PEDF) on cerebellar granule neurons. J Biol Chem. 2001;276:43313–43319. doi: 10.1074/jbc.M107831200. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Inagaki Y, Amano S, Okamoto T, Takeuchi M, Makita Z. Pigment epithelium-derived factor protects cultured retinal pericytes from advanced glycation end product-induced injury through its antioxidative properties. Biochem Biophys Res Commun. 2002;296:877–882. doi: 10.1016/s0006-291x(02)00940-3. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Inagaki Y, Takeuchi M, Sasaki N. Is pigment epithelium-derived factor level in cerebrospinal fluid a promising biomarker for early diagnosis of Alzheimer’s disease? Med Hypotheses. 2004;63:115–117. doi: 10.1016/j.mehy.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Wang JJ, Dashti A, Wilson K, Zou MH, Szweda L, Ma JX, Lyons TJ. Pigment epithelium-derived factor mitigates inflammation and oxidative stress in retinal pericytes exposed to oxidized low-density lipoprotein. J Mol Endocrinol. 2008;41:135–143. doi: 10.1677/JME-08-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]